Abstract
Many pharmacological and psychological approaches have been found efficacious in patients with generalized anxiety disorder (GAD), but many treatment-seeking patients will not respond and others will relapse despite continuing with interventions that initially had beneficial effects. Other patients will respond but then stop treatment early because of untoward effects such as sexual dysfunction, drowsiness, and weight gain. There is much scope for the development of novel approaches that could have greater overall effectiveness or acceptability than currently available interventions or that have particular effectiveness in specific clinical subgroups. ‘Experimental medicine’ studies in healthy volunteers model disease states and represent a proof-of-concept approach for the development of novel therapeutic interventions: they determine whether to proceed to pivotal efficacy studies and so can reduce delays in translating innovations into clinical practice. Investigations in healthy volunteers challenged with the inhalation of air ‘enriched’ with 7.5% carbon dioxide (CO2) indicate this technique provides a validated and robust experimental medicine model, mirroring the subjective, autonomic, and cognitive features of GAD. The anxiety response during CO2 challenge probably involves both central noradrenergic neurotransmission and effects on acid-base sensitive receptors and so may stimulate development of novel agents targeted at central chemosensors. Increasing awareness of the potential role of altered cytokine balance in anxiety and the interplay of cytokines with monoaminergic mechanisms may also encourage the investigation of novel agents with modulating effects on immunological profiles. Although seemingly disparate, these two approaches to treatment development may pivot on a shared mechanism in exerting anxiolytic-like effects through pharmacological effects on acid-sensing ion channels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Generalized anxiety disorder (GAD) is a common and impairing condition for which currently available pharmacological and psychological treatments are not ideal, having suboptimal efficacy and acceptability problems in both short-term and long-term treatment. |
‘Experimental medicine’ studies in healthy volunteers provide useful proof-of-concept approaches in the development of novel pharmacological and psyschological interventions. Two promising avenues include the development of novel agents targeted at central chemosensors or at modulating immunological responses. |
Investigations in healthy volunteers challenged with the inhalation of air ‘enriched’ with 7.5% carbon dioxide (CO2) indicate this technique provides a validated and robust experimental medicine model, mirroring the subjective, autonomic, and cognitive features of GAD. |
1 Current Diagnosis and Pharmacological Treatment of Generalized Anxiety Disorder
Generalized anxiety disorder (GAD) is the most common impairing anxiety disorder, with an estimated 12-month prevalence of 1.7–3.4% (being more prevalent in individuals aged ≥65 years) [1]. Diagnosis currently rests on establishing the presence of psychological and physical anxiety symptoms for at least 6 months, the symptoms not being understandable as arising from another disorder. In the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) criteria [2], particular emphasis is given to tension, worry, and apprehension, whereas the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria [3] emphasize multiple and uncontrollable worries. Previous versions of DSM diagnostic criteria (based on the presence of symptoms for 1 month) had low inter-rater reliability [4, 5], but the requirements of a 6-month duration and perception of uncontrollable worry enhanced the reliability of diagnosis [6]. Whilst reliability has increased, some concerns remain about diagnostic validity, including the distinction from depression and the necessary symptom severity threshold [6, 7]. This suggests a more dimensional approach based on worry, distress, and associated symptoms could be beneficial in delineating the condition [8, 9]. In current research settings, symptom severity tends to be assessed through observer-rated scales. Despite inherent limitations, including non-specificity for GAD [10], the Hamilton Rating Scale for Anxiety (HAMA) [11] remains widely regarded as the ‘gold standard’: a HAMA score of <9 corresponds to symptom remission, a score of ≥24 corresponds to anxiety symptoms of at least moderate intensity [12], and scores are positively correlated with symptom-rated disability [13].
Evidence-based treatment guidelines summarise what is known about the efficacy, tolerability, and clinical roles of currently available pharmacological and psychological treatments. Selective serotonin reuptake inhibitors (SSRIs) have become first-line pharmacological treatments in patients with GAD based on efficacy and tolerability in randomized controlled trials (RCTs) [14]. Despite high placebo response rates, only around one-half of patients exposed to active medication will enter symptom remission at the end of acute treatment [15]. Coexisting depressive symptoms of mild-moderate intensity often diminish with treatment, even with anxiolytic drugs with no proven efficacy in major depression [16, 17]. An early onset of clinical effect (measured by a ≥20% reduction in symptom severity after 2 weeks) predicts a greater likelihood of treatment response [18, 19]. There is much uncertainty about the relative efficacy and tolerability of differing pharmacological treatments [20].
As GAD tends to be either episodic in nature or waxing and waning in intensity over many years [21], long-term treatment is usually required. Relapse-prevention studies support the long-term efficacy of a range of pharmacological treatments, including some SSRIs (escitalopram, paroxetine) [22, 23], the serotonin-noradrenaline reuptake inhibitors (SNRIs) duloxetine and venlafaxine [24–26], the anxiolytic drug pregabalin [27], the antipsychotic drug quetiapine [28], and the novel antidepressant agomelatine [29].
Despite challenging problems, including risks of tolerance and dependence,benzodiazepine anxiolytics are still often employed in routine clinical practice when treating patients with GAD [30]. There is a potential role for judicious prescribing of benzodiazepines in some patients, for example in short-term treatment (up to 4 weeks) whilst waiting for an SSRI to become effective, and in longer-term treatment, when persistently distressed and severely symptomatic patients have not responded to an SSRI, SNRI, pregabalin, the 5-HT1A partial agonist buspirone, or to psychological interventions [31]. Some of the pharmacological properties of the ‘multi-modal’ antidepressant vortioxetine and its effects in pre-clinical animal models together suggest potential anxiolytic effects in clinical samples [32]. A meta-analysis of RCTs found that vortioxetine can reduce anxiety symptoms in depressed patients [33]. A pooled analysis of RCTs in patients with major depressive episodes found no significant difference between vortioxetine and placebo in treatment-emergent anxiety (unlike many antidepressants) [34]. However, placebo-controlled trials of acute treatment in patients with GAD have produced inconsistent findings [35], despite clear efficacy in preventing relapse [36]. These differential findings suggest it is possible to delineate more clearly the position of current and potential novel pharmacological treatments in the differing phases of clinical management in GAD.
Together, the sometimes disappointing effects of current medications in relieving anxiety symptoms and the many associated tolerability concerns provide the imperative to develop novel pharmacological treatments [37]. There is room for development of interventions with an earlier onset of effect (within a few days), greater overall effectiveness, and enhanced effectiveness, whilst avoiding untoward effects such as sedation, weight gain, sexual dysfunction [38], and risks of tolerance and dependence [37]. This article provides a mechanistic rationale for the possible ‘repurposing’ of familiar medications (including amiloride and certain analgesics) as potential anxiolytic treatments and highlights a potentially productive route to novel psychotropic drug development based on insights from an experimental medicine model of GAD that involves inhalation of air with an increased proportion of carbon dioxide (CO2).
2 Experimental Medicine Approaches in Anxiety Disorders
Confirming the potential benefit of novel treatments in the necessary large RCTs is time consuming and costly, and novel psychotropic drug development is now often regarded as ‘high risk’, with many biotechnology and pharmaceutical companies reducing investment in neuroscience [39]. There is scope for refining animal models of anxiety disorders and for improving methods for establishing likely anxiolytic properties of novel compounds [40], but successful development of novel anxiolytics may depend upon a refined biomarker approach combining genetic, cognitive, and neuroimaging measures [41]. Delays are typically prolonged before empirical innovations translate into clinical practice [42], but ‘experimental medicine’ studies in healthy volunteers can be a useful proof-of-concept approach to determine whether encouragement is sufficient to proceed to necessary pivotal efficacy studies.
Table 1 lists the necessary criteria [43, 44] for an experimental medicine model. A range of such models have been used to support the investigation and development of treatments in anxiety disorders, including lactate infusion and cholecystokinin challenge in panic disorder, the Trier Social Stress Test in social phobia, oxytocin administration and attachment priming in separation anxiety disorder, and threat of unpredictable shock or CO2 inhalation in panic disorder and GAD. Experimental approaches tend to focus on the physiological, pharmacological, or psychological induction of anxiety symptoms [44] and have some limitations. For example, evaluating the effects of novel compounds in drug-induced anxiety can be complicated by drug interactions, and the effects of physiological or psychological challenges are influenced by individual resilience. Anxiety induction following CO2 inhalation is the most comprehensively investigated approach as an experimental medicine model of GAD.
2.1 Carbon Dioxide (CO2) Inhalation
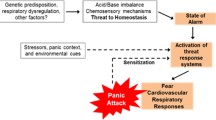
Inhalation of air ‘enriched’ with a higher than normal proportion of CO2 is one of the most frequent experimental approaches in the investigation of induced anxiety, though studies have employed variable procedures, altering the CO2 concentration, the duration of inhalation, the population sample, and the range of outcome measures. Brief inhalation of air with high concentrations of CO2 (such as single vital capacity inhalations of 35% CO2) is associated with the experience of acute severe anxiety, which often includes a panic attack [45]. By contrast, 20-min inhalation of 7.0–7.5% CO2 can induce subjective and autonomic responses and neurocognitive changes that resemble the features of generalised anxiety; increases in heart rate and systolic blood pressure are observed reliably, but an increase in diastolic blood pressure is less often seen, and induction of panic is unusual [44].
The mechanisms underlying the provocation of anxiety by CO2 challenge are not established fully [46, 47], though genetic factors may be important in CO2 hypersensitivity [48, 49]. Inhalation of air enriched with a high proportion (35%) of CO2 is associated with increased cortisol secretion [50, 51], but whether the cortisol response is specific to CO2 challenge or occurs through a more general response to other procedural aspects is unclear [44]. The role of disturbed respiratory physiology in panic attack induction following CO2 inhalation has not been clarified, but experimentally induced panic attacks are associated with low end-tidal CO2 and high ventilation variance at baseline [52].
Serotonergic mechanisms may influence the panic response to CO2 challenge. Tryptophan depletion alone does not induce panic [53] but does enhance the panic response to CO2 inhalation [54], and administration of the serotonin precursor L-5-hydroxytryptophan reduces the panic response [55]. The associations of increased subjective anxiety, heart rate, and blood pressure in healthy volunteers after 35% CO2 challenge also suggest a shared noradrenergic-mediated mechanism underlying CO2 sensitivity [56]. Changes in CO2 saturation may act upon pH- or CO2-dependent chemoreceptors within the locus coeruleus (LC) and increase the release of noradrenaline, as 5% CO2 increases the LC neuronal firing rate in rat brain slices [57]. This CO2-induced noradrenaline release may mediate autonomic and subjective features of anxiety through projections to centers involved in cardiovascular control and the limbic system, and the endocrine response may be mediated by altered noradrenergic input into the paraventricular nucleus, enhancing the release of corticotrophin-releasing factor and anti-diuretic hormone, so triggering cortisol secretion. Whilst noradrenaline is important in mediating anxiety provoked by 35% CO2 challenge, additional mechanisms must be involved because drugs that affect noradrenergic function have little effect on subjective responses to CO2 [58].
CO2 reactivity in mice is linked to chemosensors within the amygdala [59]. The most well-characterized chemosensor is the acid-sensing ion channel 1 (ASIC-1a), a voltage-insensitive H+-gated cation channel, highly expressed in the amgydala, dentate gyrus, cortex, striatum, and nucleus accumbens [60]. ASICs are pH sensitive and able to detect small reductions in brain pH (acidosis), such as that arising from inhalation of an acidic gas (including CO2) [61]. Inhalation of 2–20% CO2 elicits normal mouse fear behavior in the presence of fully functioning ASIC-1a chemosensors, but pharmacological blockade or elimination of ASIC-1a in knockout mice impairs fear responses to CO2, whereas subsequent amygdala-localised re-expression restores fear behaviour [62, 63]. Other chemosensor structures include orexin neurones in the hypothalamus, serotonergic neurones in the medullary raphe [63], T-cell death-associated gene-8 receptors in the subfornical organ, and hypoxia-sensitive chemosensory neurones in the periaqueductal gray [46]. Findings in animal models may not translate to humans, and perturbed chemosensor activity may not fully explain the physiological effects of CO2 challenge that are not understandable solely in terms of CO2-provoked alterations in noradrenergic activity.
3 Low-Dose CO2 Inhalation as an Experimental Medicine Model of Generalized Anxiety
Low-dose (7.5%) but prolonged (20 min) CO2 inhalation was first found to induce anxiety in a double-blind, placebo-controlled trial involving healthy volunteers: when compared with normal (placebo) air inhalation, CO2 inhalation increased heart rate and blood pressure and heightened subjective anxiety [64]. A subsequent single-blind, placebo-controlled study in healthy volunteers found that, when compared with air, inhalation of 7% CO2 increased respiratory rate, minute volume, and end-tidal CO2; skin conductance and subjective feelings of anxiety. A sub-group that experienced marked anxiety underwent a second identical inhalation with good test–retest repeatability. However, the study findings highlighted potential limitations of the model: 30% of participants were ‘non-responders’ and 10% of participants experienced significant anxiety during (placebo) air inhalation [65]. Rechallenge of 7.5% CO2 can reliably induce dysfunction in neuropsychological mechanisms that characterize (unchallenged) trait anxious populations and patients with GAD, e.g., hypervigilance/alertness [66], poor attention control (increased distractibility), and selective processing of environmental threat [67]. Although inhalation challenges with <15% CO2 provoke significantly more panic attacks in patients with panic disorder than in healthy controls [44], it is uncertain whether altered sensitivity to ‘low-dose’ CO2 inhalation is also seen in patients with GAD. A small single-blind, randomized, cross-over study that employed a repeated 20-min 7.5% inhalation in medication-free patients with GAD found that CO2 inhalation increased subjective anxiety and systolic blood pressure when compared with air inhalation; qualitative assessment of participants’ experiences found they resembled their previous symptoms of generalised anxiety—more closely for physiological than for cognitive symptoms [68].
4 Effects of Current and Potential Pharmacological Treatments on CO2 Inhalation
The effectiveness of psychotropic medication in attenuating CO2-evoked anxiety has been assessed repeatedly, with variable findings. In general, acute benzodiazepine administration reduces subjective CO2-provoked anxiety but has little impact on the physiological response. Administration of lorazepam 2 mg attenuated subjective anxiety (with no accompanying change in autonomic measures) when compared with placebo in healthy participants undergoing 20-min 7.5% CO2 inhalation [69]. These findings were replicated when lorazepam was employed as a control in studies using the same inhalation procedure to assess novel anxiolytic compounds [70, 71]. Alprazolam 1 mg and the partial benzodiazepine receptor antagonist zolpidem 5 mg both attenuated subjective anxiety in healthy volunteers after 20 min of 7.5% CO2 inhalation [72]. However, a subsequent double-blind, placebo-controlled crossover study that investigated dose–response relationships with lorazepam—and used the same experimental paradigm and measures—found no attenuation of subjective or autonomic responses [73].
Administration of various SSRIs, the SNRI venlafaxine, tricyclic antidepressants, or the monoamine oxidase inhibitor toloxatone can attenuate the panic response to CO2 challenge [44]. As SSRIs often take 4 weeks to exert sustained therapeutic effects in GAD, prolonged drug administration may be needed to generate valid results. A study involving 3 min of 5% CO2 inhalation in individuals ‘at high risk of panic disorder’ found that 2-week escitalopram administration had no effect on self-report or autonomic indicators of anxiety [74]. By contrast, investigations in patients with panic disorder found that 12 weeks of treatment with different SSRIs or SNRIs reduced subjective anxiety following 5 and 7% CO2 challenge when compared with baseline inhalation before treatment [75].
Studies involving administration of SSRIs, SNRIs, or beta-blockers in healthy volunteers using a 20-min 7.5% CO2 challenge have generated variable findings. As with benzodiazepines, SSRIs or SNRIs have a limited effect on physiological responses to CO2 challenge. Placebo-controlled administration of the SSRI paroxetine for 21 days (10 mg titrated to 20 mg after day 8) reduced subjective anxiety [69]. A placebo-controlled investigation of 3-week administration of either venlafaxine 150 mg or the anxiolytic pregabalin 200 mg found no significant effect on ratings of subjective anxiety or autonomic response in either the venlafaxine or the pregabalin group [76]. The beta-blocker propranolol 40 mg had no attenuating effect on self-report anxiety in healthy volunteers undergoing 20 min of 7.5% CO2 inhalation [77], which accords with its lack of efficacy in anxiety disorders [78].
The CO2 inhalation experimental model may be useful for signalling the potential anxiolytic efficacy of novel therapeutic agents in proof-of-concept studies, including psychological interventions [79]. The model has already been employed in investigations of the CRF1 receptor antagonist R317573 [70] and the NK1 receptor antagonists vestipitant and vofopitant [80]. Studies with compounds that target chemosensory mechanisms could inform the development of anxiolytics with a novel mechanism of action, for example, targeting the ASIC with amiloride, which has been found to have neuroprotective effects [81]. Other potentially fruitful areas include evaluating the effects of orexin receptor antagonists, which can attenuate anxiety-like responses to CO2 challenge in rats [82], and targeting the carbonic anhydrase enzyme, responsible for conversion of CO2 to carbonic acid and thence to hydrogen and bicarbonate ions [46], as acetazolamide (a carbonic anhydrase inhibitor) has been associated with reduced intensity of anxiety and breathlessness during ventilation in patients with panic disorder [83].
5 Cytokines and Anxiety Disorders
Cytokines are soluble bioactive mediators released by various cell types in the periphery (e.g., monocytes and macrophages) and centrally (e.g., microglia and astrocytes). Cytokine production is dependent upon type 1 helper cells (Th1), which generally mediate a pro-inflammatory cellular immune response, and type 2 helper cells (Th2), which enhance humoral immune reactions. The balance between Th1 and Th2 cytokines is an important determinant of inflammation. Pro-inflammatory cytokines include tumour necrosis factor (TNF)-α and interferon (IFN)-γ: they enhance the immune response and speed the elimination of intracellular pathogens. Anti-inflammatory cytokines include interleukin (IL)-4 and IL-10: they enhance phagocytosis of extracellular pathogens, facilitate tissue repair, and attenuate synthesis of pro-inflammatory cytokines [84]. Within the central nervous system (CNS), microglia predominantly secrete Th1 cytokines, and astroglia predominately secrete Th2 cytokines [85, 86].
Cytokine signalling is involved in neurochemical, neuroendocrine, and behavioral processes, and a delicate balance of pro- and anti-inflammatory cytokines may be needed for optimal neuropsychological functioning [84, 87–91]. This balance is influenced by the proportions of activated microglia (excess Th1) and astroglia (excess Th2) and by the interplay between activated T cells and CNS glutamate levels [88, 92–94]. Th1–Th2 imbalance can influence tryptophan metabolism by shifting tryptophan catabolism towards kynurenine (instead of towards serotonin) and kynurenine catabolism towards either microglia quinolinic acid (Th1-response mediated) or astroglial kynurenic acid (Th2-response mediated) [95, 96]. Early reports of immune disturbance in depressed patients [97, 98] encouraged extensive investigations of disturbances of cytokines in major depression [86]. Current evidence suggests a Th1-predominant immunophenotype that shifts kynurenine catabolism towards microglial quinolinic acid in major depressive disorder, in contrast to a Th2-predominant immunophenotype that shifts kynurenine catabolism towards astroglial kynurenic acid in schizophrenia [86, 88, 94].
An anxiety-specific effect on inflammatory activity in clinically anxious individuals [99] has been described, but there is a need for deeper understanding of the potential role of specific cytokines and immune balance in anxiety states and different anxiety disorders [100]. A meta-analysis of six studies found no overall difference in TNF-α and IL-6 levels between patients with obsessive-compulsive disorder (OCD) and healthy controls [101]. Post-traumatic stress disorder (PTSD) has been associated with increased levels of IL-6, IL-1β, TNF- α, and IFN-γ [102]. Findings in panic disorder have been mixed [103–106], but agoraphobia has been found to be associated with evidence of chronic low-grade systemic inflammation [107]. An investigation of lipopolysaccharide (LPS)-stimulated cytokine profile in patients with OCD or social anxiety disorder suggested that leukocytes of patients with OCD (but not socially anxious patients) produced less IL-6 than matched controls [108]. The findings of a longitudinal study in patients with GAD suggest that observed increased C-reactive protein levels are probably attributable to body mass index and medication use [109]. Another study in patients with GAD indicated deficiencies in Th1 and Th2 cytokines following T-cell activation when compared with controls, though Th1:Th2 ratios were not examined [110]. A recent case–control study comparing pro-inflammatory to anti-inflammatory cytokine ratios found significantly higher ratios of TNF-α/IL10, TNF-α/IL4, IFN-γ/IL10, and IFN-γ/IL4 in patients with GAD than in matched controls [111].
6 Selective Cyclooxygenase-2 Inhibitors in Reducing Depressive and Anxiety Symptoms
Pre-clinical, post-marketing, and treatment studies with traditional non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and ibuprofen have suggested beneficial effects in animal models of depression [112, 113], and ibuprofen had anxiolytic-like effects in an animal model of PTSD [114]. The mechanism underlying potential anxiolytic effects is uncertain, but ibuprofen inhibits both the activity and the inflammation-induced expression of ASICs in nocioceptors in rodents [115], possibly by decreasing the maximal proton-induced current and by slowing down ASIC desensitization [116]. NSAIDs have also been found to be beneficial in reducing depressive symptoms in patients with osteoarthritis [117] and in enhancing the effects of antidepressants in depressed patients [118]. However, not all evidence is supportive [119, 120], and concomitant use of NSAIDs can increase the risk of gastrointestinal adverse effects with SSRI and SNRI antidepressants [121].
Selective cyclooxygenase (COX-2) inhibitors (such as celecoxib) are a type of NSAID that target the enzyme COX-2, thereby inhibiting prostaglandin-E2 (PGE2) production and cytokine production; they have a lower risk of associated peptic ulceration than traditional NSAIDs. Placebo-controlled studies have suggested that celecoxib augments the response to antidepressant (reboxetine, fluoxetine) drugs [122, 123] and reduces depressive symptoms when given in monotherapy to patients with physical illnesses [124, 125] (brucellosis, breast cancer). Subsequent meta-analyses provide further support for the beneficial effects of celecoxib in antidepressant augmentation [126, 127].
Additional support for the potential role of COX-2 inhibitors in reducing depressive and anxiety symptoms comes from a series of studies with curcumin, the principal curcuminoid in the spice turmeric, which can downregulate COX-2 expression and PGE2 synthesis, so representing a ‘natural’ COX-2 inhibitor [128]. Randomized, placebo-controlled antidepressant augmentation studies [129, 130] and randomised placebo-controlled monotherapy studies [131–133] have together shown that curcumin has the potential to reduce depressive and/or anxiety symptoms in depressed patients. However, whether these effects of curcumin are related to its COX-2-inhibitory properties or to other pharmacological properties, including enhanced monoaminergic neurotransmission and mitochondrial protection and reduced cortisol- and quinolate-induced neurotoxicity and oxidative and nitrosative damage, is unclear [128].
7 Conclusions
GAD is a common and impairing medical condition associated with increased physical and psychological comorbidity and increased mortality, some of which may be related to states of chronic peripheral or central inflammation. Current pharmacological and psychological treatments are less than ideal, having suboptimal short-term and long-term effectiveness and significant acceptability concerns. There is a persistent need to develop new treatment approaches with enhanced efficacy and improved tolerability. ‘Experimental medicine’ studies performed in healthy volunteers with novel pharmacological compounds or innovative psychological or neuromodulatory interventions can represent a useful proof-of-concept approach to determine whether pharmaceutical, biotechnology, or medical device companies should proceed to pivotal but more time-consuming and costly formal efficacy studies. The experimental medicine approach may therefore reduce the typically prolonged delays before treatment innovations are licensed by regulators for use in clinical samples and adopted into routine clinical practice. For GAD, a body of investigations challenging healthy volunteers through inhalation of air ‘enriched’ with 7.5% CO2 suggest this technique provides a robust experimental medicine model that mirrors the subjective, autonomic, and cognitive features of the clinical condition and can be used to accompany other experimental models (e.g., threat of unexpected shock, and worry-induction procedures) to comprehensively evaluate novel treatment targets.
The anxiety response during CO2 challenge probably depends upon perturbations in central noradrenergic neurotransmission and other mechanisms involving acid-base sensitive receptors. Two promising but seemingly rather disparate pharmacological approaches in the development of novel anxiolytics include the development of novel agents that target central chemosensors (e.g., drugs with properties similar to amiloride or acetazolamide) or modulate immunological mechanisms (e.g., compounds with similarities to ibuprofen or celecoxib), though there may be a common mechanism in exerting anxiolytic-like effects through shared activities on acid-sensing ion channels [59]. Enhanced awareness of the potential roles of altered cytokine balance in anxiety, and its potential interplay with monoaminergic and chemosensor mechanisms, may together encourage the development of novel anxiolytic agents.
References
Wittchen H-U, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–79.
World Health Organization. International statistical classification of diseases and related health problems, 10th revision (ICD-10). Geneva: WHO; 1993.
American Psychiatric Association. Desk reference to the diagnostic criteria from DSM-5. Washington, DC: APA; 2013.
Di Nardo PA, O’Brien GT, Barlow DH, et al. Reliability of DSM-III anxiety disorder categories using a new structured interview. Arch Gen Psychiatry. 1983;40:1070–4.
Mannuzza S, Fyer AJ, Martin LY, et al. Reliability of anxiety assessment. I. Diagnostic agreement. Arch Gen Psychiatry. 1989;46:1093–101.
Brown TA, Di Nardo PA, Lehman CL, Campbell LA. Reliability of DSM-IV anxiety and mood disorders: implications for the classification of emotional disorders. J Abnorm Psychol. 2001;110:49–58.
Brown TA, Chorpita BA, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol. 1998;107:179–92.
Gordon D, Heimberg RG. Reliability and validity of DSM-IV generalized anxiety disorder features. J Anxiety Disord. 2011;25:813–21.
Rutter LA, Brown TA. Reliability and validity of the dimensional features of generalized anxiety disorder. J Anxiety Disord. 2015;29:1–6.
Koerner N, Antony MM, Dugas MJ. Limitations of the Hamilton Anxiety Rating Scale as a primary outcome measure in randomized, controlled trials of treatments for generalized anxiety disorder. Am J Psychiatry. 2010;167:103–4.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5.
Bandelow B, Baldwin DS, Dolberg OT, Andersen HF, Stein DJ. What is the threshold for symptomatic response and remission for major depressive disorder, panic disorder, social anxiety disorder, and generalized anxiety disorder? J Clin Psychiatry. 2006;67:1428–34.
Stein DJ, Bandelow B, Dolberg OT, Andersen HF, Baldwin DS. Anxiety symptom severity and functional recovery or relapse. Ann Clin Psychiatry. 2009;21:81–8.
Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28:403–39.
Baldwin DS, Huusom AKT, Maehlum E. Escitalopram and paroxetine in the treatment of generalised anxiety disorder: randomised, placebo-controlled, double-blind study. Br J Psychiatry. 2006;189:262–72.
Stein DJ, Baldwin DS, Baldinetti F, et al. Efficacy of pregabalin in depressive symptoms associated with generalized anxiety disorder: a pooled analysis of 6 studies. Eur Neuropsychopharmacol. 2008;18:422–30.
Baldwin DS, den Boer JA, Lyndon G, et al. Efficacy and safety of pregabalin in generalised anxiety disorder: a critical review of the literature. J Psychopharmacol. 2015;29:1047–60.
Baldwin DS, Stein DJ, Olberg OT, Bandelow B. How long should a trial of escitalopram treatment be in patients with major depressive disorder, generalised anxiety disorder or social anxiety disorder? An exploration of the randomised controlled trial database. Hum Psychopharmacol. 2009;24:269–75.
Baldwin DS, Schweizer E, Xu Y, et al. Does early improvement predict endpoint response in patients with generalized anxiety disorder (GAD) treated with pregabalin or venlafaxine XR? Eur Neuropsychopharmacol. 2012;22:137–42.
Baldwin DS, Woods R, Lawson R, et al. Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ. 2011;342:d1199.
Angst J, Gamma A, Baldwin DS, et al. The generalized anxiety spectrum: prevalence, onset, course and outcome. Eur Arch Psychiatry Clin Neurosci. 2009;259:37–45.
Allgulander C, Florea I, Huusom AK. Prevention of relapse in generalized anxiety disorder by escitalopram treatment. Int J Neuropsychopharmacol. 2006;9:495–505.
Stocchi F, Nordera G, Jokinen RH, et al. Efficacy and tolerability of paroxetine for the long-term treatment of generalized anxiety disorder. J Clin Psychiatry. 2003;64:250–8.
Davidson JR, Wittchen H-U, Llorca PM, et al. Duloxetine treatment for relapse prevention in adults with generalized anxiety disorder: a double-blind placebo-controlled trial. Eur Neuropsychopharmacol. 2008;18:673–81.
Hackett D, et al. Relapse prevention in patients with generalised anxiety disorder (GAD) by treatment with venlafaxine. In: Poster presented at THE 1st international forum on mood and anxiety disorders, Monte Carlo, November 2000 http://www.aimgroup.it/2000/ifmad/POSTER20.htm. Accessed 6 July 2010.
Rickels K, Etemad B, Khalid-Khan S, et al. Time to relapse after 6 and 12 months’ treatment of generalized anxiety disorder with venlafaxine extended release. Arch Gen Psychiatry. 2010;67:1274–81.
Feltner D, Wittchen H-U, Kavoussi R, et al. Long-term efficacy of pregabalin in generalized anxiety disorder. Int Clin Psychopharmacol. 2008;23:18–28.
Katzmann MA, Brawman-Mintzer O, Reyes EB, et al. Extended release quetiapine fumarate (quetiapine XR) monotherapy as maintenance treatment for generalized anxiety disorder: a long-term, randomized, placebo-controlled trial. Int Clin Psychopharmacol. 2011;26:11–24.
Stein DJ, Ahokas A, Albarran C, et al. Agomelatine prevents relapse in generalized anxiety disorder: a 6-month randomized, double-blind, placebo-controlled discontinuation study. J Clin Psychiatry. 2012;73:1002–8.
Baldwin DS, Allgulander C, Bandelow B, et al. An international survey of reported prescribing practice in the treatment of patients with generalised anxiety disorder. World J Biol Psychiatry. 2012;13:510–6.
Baldwin DS, Aitchison K, Bateson A, et al. Benzodiazepines: risks and benefits. A reconsideration. J Psychopharmacol. 2013;27:967–71.
Baldwin DS, Hanumanthaiah VB. Vortioxetine in the treatment of major depressive disorder. Future Neurol. 2015;01:79–89.
Baldwin DS, Florea I, Jacobsen PL, et al. A meta-analysis of the efficacy of vortioxetine in patients with major depressive disorder (MDD) and high levels of anxiety symptoms. J Affective Disord. 2016;206:140–50.
Baldwin DS, Chrones L, Florea I, Nielsen R, Nomikos GG, Palo W, Reines E. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30:242–52.
Pae C-U, Wang SM, Han C, et al. Vortioxetine, a multimodal antidepressant for generalized anxiety disorder: a systematic review and meta-analysis. J Psychiatric Res. 2015;64:88–98.
Baldwin DS, Loft H, Florea I. Lu AA21004, a multimodal psychotropic agent, in the prevention of relapse in adult patients with generalized anxiety disorder. Int Clin Psychopharmacol. 2012;27:197–207.
Baldwin DS, Brandish EK. Pharmacological treatment of anxiety disorders. In: Emmelkamp P, Ehring T, editors. The wiley handbook of anxiety disorders, vol. 2. Chichester: Wiley; 2014. p. 865–82.
Baldwin DS, Manson C, Nowak C. Impact of antidepressant drugs on sexual function and satisfaction. CNS Drugs. 2015;29:905–13.
Insel TR, Voon V, Nye JS, et al. Innovative solutions to novel drug development in mental health. Neurosci Biobehav Rev. 2013;37:2438–44.
Haller J, Aliczki M, Gyimesine Pelczer K. Classical and novel approaches to the preclinical testing of anxiolytics: a critical evaluation. Neurosci Biobehav Rev. 2013;37:2318–30.
Insel TR. The NIMH experimental medicine initiative. World Psychiatry. 2015;14:151–3.
Hanney SR, Castle-Clarke S, Grant J, et al. How long does biomedical research take? Studying the time taken between biomedical and health research and its translation into products, policy, and practice. Health Res Policy Syst. 2015;13:1.
Guttmacher LB, Murphy DL, Insel TR. Pharmacologic models of anxiety. Compr Psychiatry. 1983;24:321–6.
Bailey J, Dawson GR, Dourish CT, Nutt DJ. Validating the inhalation of 7.5% CO(2) in healthy volunteers as a human experimental medicine: a model of generalized anxiety disorder (GAD). J Psychopharmacol. 2011;25:1192–8.
Van den Hout MA, Griez E. Panic symptoms after inhalation of carbon dioxide. Br J Psychiatry. 1984;144:503–7.
Leibold NK, van den Hove DLA, Esquivel G, De Cort K, Goossens L, Strackx E, et al. The brain acid-base homeostasis and serotonin: a perspective on the use of carbon dioxide as human and rodent experimental model of panic. Progress Neurobiol. 2015;129:58–78.
Vollmer LL, Strawn JR, Sah R. Acid-base dysregulation and chemosensory mechanisms in panic disorder: a translational update. Transl Psychiatr. 2015;5:e572.
Battaglia M, Ogliari A, Harris J, Spatola CAM, Pesenti-Gritti P, Reichborn-Kjennerud T, et al. A genetic study of the acute anxious response to carbon dioxide stimulation in man. J Psychiatric Res. 2007;2007(41):906–17.
Battaglia M, Pesenti-Gritti P, Spatola CAM, Ogliari A, Tambs K. A twin study of the common vulnerability between heightened sensitivity to hypercapnia and panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:586–93.
Argyropoulos SV, Bailey JE, Hood SD, Kendrick AH, Rich AS, Laszlo G, et al. Inhalation of 35% CO2 results in activation of the HPA axis in healthy volunteers. Psychoneuroendocrinol. 2002;27:715–29.
Kaye J, Buchanan F, Kendrick A, Johnson P, Lowry C, Bailey J, et al. Acute carbon dioxide exposure in healthy adults: evaluation of a novel means of investigating the stress response. J Neuroendocrinol. 2004;16:256–64.
Papp LA, Martinez JM, Klein DF, Coplan JD, Norman RG, Cole R, et al. Respiratory psychophysiology of panic disorder: three respiratory challenges in 98 subjects. Am J Psychiatr. 1997;154:1557–65.
Goddard AW, Sholomskas DE, Walton KE, Augeri FM, Charney DS, Heninger GR, et al. Effects of tryptophan depletion in panic disorders. Biol Psychiatr. 1994;36:775–7.
Schruers K, Klaassen T, Pols H, Overbeek T, Deutz NEP, Griez E. Effects of tryptophan depletion on carbon dioxide provoked panic in panic disorder patients. Psychiatr Res. 2000;93:179–87.
Schruers K, van Diest R, Overbeek T, Griez E. Acute L-5-hydroxytryptophan administration inhibits carbon dioxide-induced panic in panic disorder patients. Psychiatr Res. 2002;113:237–43.
Bailey JE, Argyropoulos SV, Lightman SL, Nutt DJ. Does the brain noradrenaline network mediate the effects of the CO2 challenge? J Psychopharmacol. 2003;17:252–9.
Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatr Clin N Am. 2009;32:549–75.
Pinkney V, Bamford S, Baldwin DS, Munafo MR, Garner M. The effects of duloxetine on subjective, autonomic and neurocognitive response to 7.5% carbon dioxide challenge. Eur Neuropsychopharmacol. 2014;24:S579.
Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–21.
Wemmie JA. Neurobiology of panic and pH chemosensation in the brain. Dialogue Clin Neurosci. 2011;13:475–83.
Sherwood TW, Frey EN, Askwith CC. Structure and activity of the acid-sensing ion channels. Am J Physiol Cell Physiol. 2012;303:C699–710.
Lin SH, Sun WH, Chen CC. Genetic exploration of the role of acid-sensing ion channels. Neuropharmacol. 2015;94:99–118.
Wang WG, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511:433–50.
Bailey JE, Argyropoulos SV, Kendrick AH, Nutt DJ. Behavioral and cardiovascular effects of 7.5% CO2 in human volunteers. Depress Anxiety. 2005;21:18–25.
Poma SZ, Milleri S, Squassante L, Nucci G, Bani M, Perini GI, Merlo-Pich E. Characterization of a 7% carbon dioxide (CO2) inhalation paradigm to evoke anxiety symptoms in healthy subjects. J Psychopharmacol. 2005;19:494–503.
Garner MJ, Attwood A, Baldwin DS, Munafo MR. Inhalation of 7.5% carbon dioxide increases alerting and orienting attention network function. Psychopharmacology. 2012;223:67–73.
Garner MJ, Attwood D, Baldwin DS, et al. Inhalation of 7.5% carbon dioxide increases threat processing in humans. Neuropsychopharmacology. 2011;36:1557–62.
Seddon K, Morris K, Bailey J, Potokar J, Rich A, Wilson S, Bettica P, Nutt DJ. Effects of 7.5% CO2 challenge in generalized anxiety disorder. J Psychopharmacol. 2011;25:43–51.
Bailey JE, Kendrick A, Diaper A, Potokar JP, Nutt DJ. A validation of the 7.5% CO2 model of GAD using paroxetine and lorazepam in healthy volunteers. J Psychopharmacol. 2007;21:42–9.
Bailey JE, Papadopoulos A, Diaper A, Phillips S, Schmidt ME, van der Ark P, et al. Preliminary evidence of anxiolytic effects of the CRF1 receptor antagonist R317573 in the 7.5% CO2 proof-of-concept experimental model of human anxiety. J Psychopharmacol. 2011;25:1199–206.
Gomes de Oliveira DC, Chagas MHN, Garcia LV, Crippa JAS, Zuardi AW. Oxytocin interference in the effects induced by inhalation of 7.5% CO2 in healthy volunteers. Hum Psychopharmacol. 2012;27:378–85.
Bailey JE, Papadopoulos A, Seddon K, Nutt DJ. A comparison of the effects of a subtype selective and non-selective benzodiazepine receptor agonist in two CO2 models of experimental human anxiety. J Psychopharmacol. 2009;23:117–22.
Diaper A, Papadopoulos A, Rich AS, Dawson GR, Dourish CT, Nutt DJ, Bailey JE. The effect of a clinically effective and non-effective dose of lorazepam on 7.5% CO2-induced anxiety. Hum Psychopharmacol. 2012;27:540–8.
Coryell W, Rickels H. Effects of escitalopram on anxiety and respiratory responses to carbon dioxide inhalation in subjects at high risk for panic disorder a placebo-controlled, crossover study. J Clin Psychopharmacol. 2009;29:174–8.
Gorman JM, Martinez J, Coplan JD, Kent J, Kleber M. The effect of successful treatment on the emotional and physiological response to carbon dioxide inhalation in patients with panic disorder. Biol Psychiatr. 2004;56:862–7.
Diaper A, Osman-Hicks V, Rich A, Craig K, Dourish C, Dawson G, et al. Evaluation of the effects of venlafaxine and pregabalin on the carbon dioxide inhalation models of generalised anxiety disorder and panic. J Psychopharmacol. 2013;27:135–45.
Papadopoulos A, Rich A, Nutt DJ, Bailey JE. The effects of single dose anxiolytic medication on the CO2 models of anxiety: differentiation of subjective and objective measures. J Psychopharmacol. 2010;24:649–56.
Steenen SA, van Wijk AJ, van der Heijden GJ, van Westrhenen R, de Lange J, de Jongh A. Propranolol for the treatment of anxiety disorders: systematic review and meta-analysis. J Psychopharmacol. 2016;30:128–39.
Ainsworth B, Marshall JE, Meron D, Baldwin DS, Chadwick P, Munafò MR, Garner M. Evaluating psychological interventions in a novel experimental human model of anxiety. J Psychiatr Res. 2015;63:117–22.
Poma SZ, Merlo-Pich E, Bettica P, Bani M, Fina P, Ziviani L, Milleri S. Anxiolytic effects of vestipitant in a sub-group of healthy volunteers known to be sensitive to CO2 challenge. J Psychopharmacol. 2014;28:491–7.
Arun T, Tomassini V, Sbardella E, de Ruiter MB, Matthews L, Leite MI, et al. Targeting ASIC1 in primary progressive multiple sclerosis: evidence of neuroprotection with amiloride. 2013. Brain 136:106–15.
Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacol. 2012;37:1911–22.
Gorman JM, Papp LA, Coplan J, Martinez J, Liebowitz MP, Klein DF. The effect of acetazolamide on ventilation in panic disorder patients. Am J Psychiatry. 1993;150:1480–4.
Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157:683–94.
Müller N, Schwarz MJ. Immune system and schizophrenia. Curr Immunol Rev. 2010;6:213–20.
Müller N, Myint AM, Schwarz MJ. Kynurenine pathway in schizophrenia: pathophysiological and therapeutic aspects. Curr Pharm Des. 2011;17:130–6.
Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17:69–85.
Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatr. 2007;12:988–1000.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56.
Loftis JM, Huckans M, Morasco BJ. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol Dis. 2010;37:519–33.
Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–38.
Schwarz MJ. Cytokines, neurophysiology, neuropsychology, and psychiatric symptoms. Dialogues Clin Neurosci. 2003;5:139–53.
Schwarz MJ, Krönig H, Riedel M, Dehning S, Douhet A, Spellmann I, et al. IL-2 and IL-4 polymorphisms as candidate genes in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256:72–6.
Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflamm. 2013;10:43. doi:10.1186/1742-2094-10-43.
Steiner J, Bogerts B, Sarnyai Z, Walter M, Gos T, Bernstein HG, et al. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J Biol Psychiatry. 2012;13:482–92.
Müller N, Schwarz MJ. Immunological aspects of depressive disorders. Der Nervenarzt. 2007;78:1261–73.
Maes M, Bosmans E, Suy E, Vandervorst C, De Jonckheere C, Raus J. Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiol. 1990;24:115–20.
Maes M, Bosmans E, Suy E, Vandervorst C, DeJonckheere C, Raus J. Depression-related disturbances in mitogen-induced lymphocyte responses and interleukin-1 beta and soluble interleukin-2 receptor production. Acta Psychiatr Scand. 1991;84:379–86.
O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, et al. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun. 2010;24:1074–7.
Hou R, Baldwin DS. A neuroimmunological perspective on anxiety disorders. Hum Psychopharmacol. 2012;27:6–14.
Gray SM, Bloch MH. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr Psychiatry Rep. 2012;14:220–8.
Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–12.
Weizman R, Laor N, Wiener Z, Wolmer L, Bessler H. Cytokine production in panic disorder patients. Clin Neuropharmacol. 1999;22:107–9.
Brambilla F, Bellodi L, Perna G, Bertani A, Panerai A, Sacerdote P. Plasma interleukin-1 beta concentrations in panic disorder. Psychiatry Res. 1994;54:135–42.
van Duinen MA, Schruers KR, Griez EJ, Maes M. Neuroimmunological parameters in panic disorder. Acta Neuropsychiatr. 2004;16:94–100.
Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anx. 2009;26:447–55.
Wagner EN, Wagner JT, Glaus J, Vandeleur CL, Castelao E, Strippoli MF, et al. Evidence for chronic low-grade systemic inflammation in individuals with agoraphobia from a population-based prospective study. PLoS ONE. 2015;10(4):e0123757. doi:10.1371/journal.pone.0123757.
Fluitman S, Denys D, Vulink N, Schutters S, Heijnen C, Westenberg H. Lipopolysaccharide-induced cytokine production in obsessive-compulsive disorder and generalized social anxiety disorder. Psychiatr Res. 2010;178:313–6.
Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol Med. 2012;42:2641–50.
Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araújo-Lima CF, et al. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol. 2010;229:212–8.
Hou R, Garner M, Holmes C, Osmond C, Teeling J, Lau L, Baldwin DS. Peripheral inflammatory cytokines and immune balance in generalised anxiety disorder: case-controlled study. Brain Behav Immun. 2017. doi:10.1016/j.bbi.2017.01.021 (Epub 1 Feb 2017).
Wang Y, Yang F, Liu YF, Gao F, Jiang W. Acetylsalicylic acid as an augmentation agent in fluoxetine treatment resistant depressive rats. Neurosci Lett. 2011;499:74–9.
Bhatt S, Kilambi P, Patel P, Patel N, Panchal A, Shah G, Goswami S. Beneficial effect of aspirin against interferon-α-2b-induced depressive behavior in Sprague Dawley rats. Clin Exp Pharmacol Physiol. 2016;43(12):1208–15. doi:10.1111/1440-1681.12660.
Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder. Kor J Physiol Pharmacol. 2016;20:357–66.
Voilley N, de Weille J, Mamet J, Lazdunski MJ. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. Neurosci. 2001;21:8026–33.
Dorofeeva NA, Barygin OI, Staruschenko A, Bolshakov KV, Magazanik LG. Mechanisms of non-steroid anti-inflammatory drugs action on ASICs expressed in hippocampal interneurons. J Neurochem. 2008;106:429–41.
Iyengar RL, Gandhi S, Aneja A, Thorpe K, Razzouk L, Greenberg J, et al. NSAIDs are associated with lower depression scores in patients with osteoarthritis. Am J Med. 2013;126:1017.e11-8. doi:10.1016/j.amjmed.2013.02.037 (Epub 2013 Aug 29).
Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol. 2006;21:227–31.
Fields C, Drye L, Vaidya V, Lyketsos C, ADAPT Research Group. Celecoxib or naproxen treatment does not benefit depressive symptoms in persons age 70 and older: findings from a randomized controlled trial. Am J Geriatr Psychiatry. 2012;20:505–13.
Ghanizadeh A, Hedayati A. Augmentation of citalopram with aspirin for treating major depressive disorder, a double blind randomized placebo controlled clinical trial. Antiinflamm Antiallergy Agents Med Chem. 2014;13:108–11.
Anglin R, Yuan Y, Moayyedi P, Tse F, Armstrong D, Leontiadis GI. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;10:811–9.
Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–4.
Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, Mohebbi-Rasa S, Raznahan M, Kamalipour A. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. 2009;26:607–11.
Jafari S, Ashrafizadeh SG, Zeinoddini A, Rasoulinejad M, Entezari P, Seddighi S, Akhondzadeh S. Celecoxib for the treatment of mild-to-moderate depression due to acute brucellosis: a double-blind, placebo-controlled, randomized trial. J Clin Pharm Ther. 2015;40:441–6.
Mohammadinejad P, Arya P, Esfandbod M, Kaviani A, Najafi M, Kashani L, Zeinoddini A, Emami SA, Akhondzadeh S. Celecoxib versus diclofenac in mild to moderate depression management among breast cancer patients: a double-blind, placebo-controlled, randomized trial. Ann Pharmacother. 2015;49:953–61.
Na KS, Lee KJ, Lee JS, Cho YS, Jung HY. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:79–85.
Faridhosseini F, Sadeghi R, Farid L, Pourgholami M. Celecoxib: a new augmentation strategy for depressive mood episodes. A systematic review and meta-analysis of randomized placebo-controlled trials. Hum Psychopharmacol. 2014;29:216–23.
Lopresti AL, Hood SD, Drummond PD. Multiple antidepressant potential modes of action of curcumin: a review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J Psychopharmacol. 2012;26:1512–24.
Bergman J, Miodownik C, Bersudsky Y, Sokolik S, Lerner PP, Kreinin A, Polakiewicz J, Lerner V. Curcumin as an add-on to antidepressive treatment: a randomized, double-blind, placebo-controlled, pilot clinical study. Clin Neuropharmacol. 2013;36:73–7.
Yu JJ, Pei LB, Zhang Y, Wen ZY, Yang JL. Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. J Clin Psychopharmacol. 2015;35:406–10.
Lopresti AL, Maes M, Maker GL, Hood SD, Drummond PD. Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J Affect Disord. 2014;167:368–75.
Esmaily H, Sahebkar A, Iranshahi M, Ganjali S, Mohammadi A, Ferns G, Ghayour-Mobarhan M. An investigation of the effects of curcumin on anxiety and depression in obese individuals: a randomized controlled trial. Chin Integr Med. 2015;21:332–8.
Lopresti AL, Drummond PD. Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: a randomised, double-blind, placebo-controlled study. J Affect Disord. 2016;207:188–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Over his academic career, DSB has held research grants from the following pharmaceutical and biotechnology companies: Bristol-Myers Squibb, Cephalon, Eli Lilly Ltd, GlaxoSmithKline, H. Lundbeck A/S, Pierre Fabre, Pfizer Ltd, Roche, and Vernalis Ltd. He has served on advisory boards hosted by Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly Ltd, GlaxoSmithKline, Grunenthal, H. Lundbeck A/S, Pierre Fabre, and Pfizer Ltd. He is a past President of Depression Alliance and a current Medical Patron of Anxiety UK. RG, NH, RH, and MG have no potential conflicts of interest.
Funding
RG and NH are National Institute for Health Research (NIHR) Academic Clinical Fellows with supportive grants from the Research Management Committee of the Faculty of Medicine at the University of Southampton. No specific funding was sought or received for the preparation of this review.
Rights and permissions
About this article
Cite this article
Baldwin, D.S., Hou, R., Gordon, R. et al. Pharmacotherapy in Generalized Anxiety Disorder: Novel Experimental Medicine Models and Emerging Drug Targets. CNS Drugs 31, 307–317 (2017). https://doi.org/10.1007/s40263-017-0423-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-017-0423-2