Abstract
A once-daily, fixed-dose combination of memantine extended-release (ER)/donepezil 28/10 mg (Namzaric™) is available in the USA for patients with moderate to severe Alzheimer’s disease (AD) on stable memantine and donepezil therapy. The fixed-dose formulation is bioequivalent to coadministration of the individual drugs. In a 24-week, phase III trial in patients with moderate to severe AD, addition of memantine ER 28 mg once daily to stable cholinesterase inhibitor (ChEI) therapy was more effective than add-on placebo on measures of cognition, global clinical status, dementia behaviour and semantic processing ability, although between-group differences on a measure of daily function did not significantly differ. In subgroup analyses in donepezil-treated patients, add-on memantine ER was more effective than add-on placebo on measures of cognition, dementia behaviour and semantic processing, although there were no significant between-group differences on measures of global clinical status and daily function. Memantine ER plus ChEI combination therapy was generally well tolerated in the phase III trial, with diarrhoea, dizziness and influenza occurring at least twice as often with add-on memantine ER as add-on placebo in donepezil-treated patients. Thus, memantine ER plus donepezil combination therapy is an effective and well tolerated treatment option for patients with moderate to severe AD. The fixed-dose combination is potentially more convenient than coadministration of the individual agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fixed-dose combination of an N-methyl-d-aspartate receptor antagonist (memantine) and a cholinesterase inhibitor (donepezil) |
Bioequivalent to coadministered memantine and donepezil |
Potentially more convenient, once-daily regimen for patients with moderate to severe AD on stable memantine and donepezil therapy |
In donepezil-treated patients with moderate to severe AD, add-on memantine ER is more effective than add-on placebo on measures of cognition, dementia behaviour and semantic processing |
Memantine ER plus donepezil is generally well tolerated |
1 Introduction
Alzheimer’s disease (AD) is the most common type of dementia, accounting for ≈60–80 % of cases [1]. Currently, four drugs are approved by the US FDA for AD treatment, including three cholinesterase inhibitors (ChEIs) [i.e. galantamine and rivastigmine (both for use in mild to moderate AD), and donepezil (for use in mild to severe AD)] and one N-methyl-d-aspartate (NMDA) receptor antagonist [i.e. memantine (for use in moderate to severe AD)] [2]. Although there is no evidence for any of these drugs having disease-modifying effects, their use can mitigate AD symptoms relating to decline in cognition, behaviour and activities of daily living in the moderate or severe stages of disease, and may delay nursing home care in patients with end-stage disease [2].
In patients with moderate to severe AD, combination therapy with memantine and a ChEI is considered the standard of care, with memantine typically being initiated after patients are stabilized on ChEI therapy [2]. Although randomized trial findings have been mixed regarding the efficacy of combining an immediate-release (IR) formulation of memantine with donepezil in patients with moderate to severe AD [3, 4], post hoc and pooled analyses that included these trials found evidence of additive benefits with memantine plus donepezil combination therapy across several clinical domains [5]. Furthermore, meta-analyses of randomized trials support the use of memantine plus ChEI combination therapy in AD [6, 7], and long-term observational studies have provided real-world clinical evidence of its superiority over ChEI monotherapy with regard to slowing cognitive and functional decline [8] and delaying nursing home admission [9].
A once-daily, extended-release (ER) formulation of memantine 28 mg, approved for the treatment of moderate to severe AD in the USA in 2010, has the potential to provide a lower pill burden and more convenient dosage regimen than memantine IR (10 mg twice daily) [10]. More recently, fixed-dose combination memantine ER/donepezil (Namzaric™) 14/10 and 28/10 mg capsules with once-daily administration have become available in the USA for the treatment of moderate to severe AD in patients previously on stable memantine and donepezil therapy [11]. This article provides an overview of the pharmacological properties and clinical data relevant to the use of memantine ER/donepezil in patients with moderate to severe AD.
2 Pharmacodynamic Properties
The pharmacodynamic properties of both memantine and donepezil are well established and have previously been described in detail [10, 12, 13]. These two drugs are postulated to act via different, interconnected pathological pathways in AD [11, 12].
Memantine is an uncompetitive (open-channel), voltage-dependent, NMDA receptor antagonist that binds preferentially to NMDA receptor-operated cation channels with low to moderate affinity [10–12]. Although the pathophysiology of AD has not been fully characterized, it is thought that constant low-level stimulation of NMDA receptors by glutamate and amyloid-β creates a decrease in the post-synaptic membrane potential, which removes the magnesium blockade of the NMDA receptor channels (present under normal, resting, physiological conditions) and increases the continuous flow of calcium ions into the post-synaptic neuron [12]. Memantine is thought to reduce the pathological ‘background noise’ of dysfunctional glutamate signalling in AD by blocking NMDA receptor channels during the prolonged periods of moderate stimulation, unlike the weaker binding magnesium ions. In the presence of transiently high glutamate levels (i.e. a physiological signal), memantine dissociates from the NMDA receptor to allow for normal neurotransmission. As well as facilitating normal glutamate signalling, memantine may prevent neuronal damage caused by the excitotoxicity of excessive glutamate stimulation [12].
Donepezil is a potent, selective, uncompetitive, reversible inhibitor of acetylcholinesterase that is thought to enhance cholinergic function by increasing acetylcholine levels in the central nervous system [11, 13]. Cholinergic neurodegeneration is hypothesized to cause decreased levels of acetylcholine and weakened signalling, which contributes to memory impairment and cognitive decline in AD [12].
In preclinical studies, the combination of memantine plus donepezil significantly improved spatial memory in a mouse model with cognitive deficits, with combination treatment providing improvements in measures of spatial memory that were greater than those observed with memantine or donepezil alone [12]. These findings indicate that the complementary mechanisms of action of memantine and donepezil may have the potential to provide greater clinical benefits than either drug alone [12].
There were no clinically relevant pharmacodynamic interactions between memantine and donepezil [11]. When coadministered, memantine does not affect the acetylcholinesterase inhibitory properties of donepezil. The adverse event profile of memantine IR plus donepezil was generally similar to that of donepezil monotherapy in patients with moderate to severe AD [11].
3 Pharmacokinetic Properties
After oral administration, memantine and donepezil have linear pharmacokinetics over their respective therapeutic dosage ranges [11]. During once-daily administration of memantine ER 28 mg, the memantine peak plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) from time zero to 24 h are 48 and 33 % higher, respectively, than during twice-daily administration of memantine IR 10 mg. Following multiple-dose administration of memantine ER, Cmax is reached after ≈9–12 h, while donepezil has 100 % relative oral bioavailability and reaches Cmax within 3–4 h. Donepezil reaches steady-state concentrations within 15 days of multiple-dose administration. The mean volume of distribution of memantine is 9–11 L/kg and the steady-state volume of distribution of donepezil is 12–16 L/kg. Plasma protein binding is 45 % for memantine and ≈96 % for donepezil [11].
The fixed-dose combination of memantine ER/donepezil 28/10 mg was bioequivalent to coadministration of corresponding doses of the separately available formulations of memantine ER plus donepezil in healthy adults under fasting conditions (Table 1) [14]. In this single-dose crossover study, the pharmacokinetic parameters were not significantly different (Table 1) and mean plasma concentration-time curves for memantine and donepezil were generally similar between the fixed-dose combination and coadministered agents. In a second crossover study in healthy adults, memantine and donepezil exposure (Cmax and AUC) from a single dose of memantine ER/donepezil 28/10 mg did not significantly differ between administration in the fasted and fed state or between when the capsule was swallowed intact and when the capsule contents were consumed after being sprinkled on applesauce [14].
Memantine undergoes partial hepatic metabolism; however, cytochrome 450 (CYP) isoenzymes are not significantly involved [11]. Memantine has three primary metabolites, each with minimal NMDA receptor antagonistic activity. Approximately 48 % of an administered memantine dose is excreted as unchanged drug in the urine, with renal clearance involving active tubular secretion and pH-dependent tubular reabsorption. Clearance of memantine is reduced under alkaline urine conditions (i.e. pH 8), which may be caused by diet, comorbid conditions (e.g. severe urinary tract infections) or drugs. Memantine has an elimination half-life (t½) of ≈60–80 h [11].
Donepezil is extensively metabolized to four major metabolites (including two with cholinesterase inhibitory activity) and multiple minor metabolites, with metabolism by CYP2D6, CYP3A4 and glucuronidation [11]. After administration of radiolabelled donepezil, ≈57 and 15 % of the dose was recovered in the urine and faeces over 10 days; ≈17 % of the dose recovered in the urine was unchanged drug. Donepezil has a mean apparent plasma clearance of 0.13–0.19 L/h/kg and an elimination t½ of ≈70 h [11].
Based on available evidence, no dosage adjustments for memantine ER/donepezil are recommended in patients with mild or moderate renal or hepatic impairment [11]. However, a lower dosage of memantine ER/donepezil 14/10 mg once daily is recommended in patients with severe renal impairment (Sect. 6). The pharmacokinetics of memantine ER/donepezil have not been studied in patients with severe hepatic impairment. Memantine exposure and clearance do not appear to be affected to a clinically relevant extent by gender (after taking into account differences in bodyweight) and age, respectively. Donepezil clearance does not appear to be affected to a clinically relevant extent by age, gender or race (i.e. Japanese vs. Caucasian patients), although it does appear to be affected by bodyweight, with an almost twofold increase in clearance over the bodyweight range of 50–110 kg [11].
The pharmacokinetic properties of memantine and donepezil were not affected when these drugs were coadministered [11]. Based on in vitro studies, memantine is not expected to affect the pharmacokinetic properties of drugs that are metabolized by CYP isoenzymes, and given that memantine undergoes predominantly renal excretion, drugs that are CYP substrates and/or inhibitors are not expected to affect the pharmacokinetics of memantine. As alkaline urine conditions could potentially increase plasma memantine concentrations, memantine ER/donepezil should be used with caution in patients receiving drugs that may cause urine alkalinity (e.g. sodium bicarbonate and carbonic anhydrase inhibitors) [11].
At clinically relevant donepezil concentrations in vitro, there was little or no evidence for direct inhibition of CYP2B6, CYP2C8 or CYP2C19 isoenzymes [11]. It is unknown whether donepezil has the potential to cause enzyme induction. However, in vitro studies suggest that the metabolism of donepezil is reduced by inhibitors of CYP3A4 (e.g. ketoconazole) and CYP2D6 (e.g. quinidine), while inducers of CYP3A4 (e.g. phenytoin and dexamethasone) could increase donepezil elimination. In a population pharmacokinetic analysis of patients with AD, the clearance of donepezil was reduced by ≈17 % during coadministration of donepezil 10 or 23 mg with a known CYP2D6 inhibitor [11].
4 Therapeutic Efficacy
The efficacy of memantine ER/donepezil 28/10 mg once daily has not been evaluated in phase III trials in patients with moderate to severe AD. However, the efficacy of memantine ER 28 mg once daily versus placebo as add-on therapy to ChEIs was established in this patient population in a 24-week, randomized, double-blind, multinational, phase III trial (MEM-MD-50 [15]; Sect. 4.1), which has been reviewed in detail previously [10]. Additionally, prespecified subgroup analyses in donepezil-treated patients from MEM-MD-50 (Sect. 4.1.1) [16] and post hoc pooled analyses of randomized trials that included MEM-MD-50 (Sect. 4.2) [17, 18] have evaluated the efficacy of mematine plus donepezil combination therapy in patients with moderate to severe AD.
4.1 MEM-MD-50 Trial
Patients in MEM-MD-50 (n = 676) were aged ≥50 years with a clinical diagnosis of probable AD and a Mini-Mental State Examination (MMSE) score of 3–14 at baseline. Patients had been receiving stable ChEI therapy for ≥3 months; 69 % of patients were receiving donepezil (mean dosage 7.9 mg/day), 21 % were receiving galantamine (mean dosage 13.5 mg/day) and 11 % were receiving rivastigmine (mean dosage 6.8 mg/day). At baseline, patients had a mean age of 76 years, a mean MMSE score of 10.8 and 72 % of patients were female. Memantine ER was initiated at 7 mg once daily and titrated weekly (by 7 mg/day) to a target dosage of 28 mg once daily by the start of week 4; patients were required to tolerate a minimum dosage of 21 mg/day by week 8 to continue study treatment. At the end of the study, the mean daily dose of memantine ER was 27.0 mg and 92 % of patients were receiving the maximum daily dose of 28 mg. At week 24, the coprimary endpoints were the baseline-to-endpoint change on the Severe Impairment Battery (SIB) [assessment of cognition] and endpoint score on the Clinician’s Interview-Based Impression of Change Plus Caregiver Input (CIBIC-Plus) [assessment of global clinical status]. Primary efficacy analyses used data from the intent-to-treat (ITT) population with last observation carried forward (LOCF) [15].
Memantine ER was more effective than placebo when added to stable ChEI therapy for 24 weeks with regard to the SIB (cognition) and CIBIC-Plus (global clinical status) coprimary endpoints in patients with moderate to severe AD [15]. At week 24, add-on memantine ER recipients had significantly (p < 0.01) greater improvements than add-on placebo recipients in the mean change in SIB scores from baseline (2.7 vs. 0.3 points; assessed in 332 and 327 patients, respectively) and mean endpoint CIBIC-Plus scores (3.8 vs. 4.1 points; assessed in 333 and 328 patients, respectively). In sensitivity analyses of observed cases, add-on memantine ER recipients had significantly (p < 0.05) greater improvements in terms of the change in SIB at weeks 12, 18 and 24 and CIBIC-Plus score at week 12 than add-on placebo recipients [15].
The addition of memantine ER to ChEI therapy was also associated with significantly (p < 0.01) greater baseline-to-endpoint improvements than add-on placebo on the Neuropsychiatric Inventory (NPI) [assessment of dementia behavioural disturbance] and verbal fluency test (VFT) [assessment of semantic processing ability] [15]. At week 24, the least-squares mean (LSM) between-group differences for the baseline-to-endpoint changes in NPI and VFT scores were –2.7 points (95 % CI –4.45 to –0.8) and +0.5 points (95 % CI +0.2 to +0.9), respectively, in favour of add-on memantine ER. However, the LSM between-group difference for the baseline-to-endpoint change on the 19-item Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL19) [assessment of daily functional ability] did not reach statistical significance [15].
In exploratory post hoc analysis of the caregiver distress component on the NPI (NPI-D), add-on memantine ER provided significantly (p < 0.05) greater improvements than add-on placebo on items regarding agitation and delusions at week 12 (LSM differences of −0.27 and −0.23, respectively), and agitation and night time behaviour at week 24 (LSM differences of −0.29 and −0.24, respectively) [abstract presentation] [19]. These findings suggest that the addition of memantine ER to stable ChEI background therapy may decrease caregiver burden that accompanies the neuropsychiatric symptoms of AD, in particular those associated with agitation, delusions and night time behaviours [19].
4.1.1 Subgroup Analyses in Donepezil-Treated Patients
The efficacy of add-on memantine ER 28 mg once daily in donepezil-treated patients with moderate to severe AD was evaluated in prespecified subgroup analyses of MEM-MD-50 data (abstract presentation) [16]. The prospectively defined efficacy assessments were conducted in the donepezil-treated subgroup of the ITT population (n = 456) using the LOCF approach [16]. Subgroup analyses in rivastigmine- or galantamine-treated patients have not been reported.
As in the overall population, memantine ER plus donepezil recipients (n = 232) had significantly (p < 0.01) greater improvements in baseline-to-endpoint changes at week 24 on the SIB, NPI and VFT than placebo plus donepezil recipients (n = 224), with LSM between-group differences of 3.2, 2.8 and 0.9 points, respectively, while the baseline-to-endpoint change on the ADCS-ADL19 showed no significant between-group difference [16]. On the CIBIC-Plus, improvements or no change from baseline were estimated in 75 % of memantine ER plus donepezil recipients versus 72 % of placebo plus donepezil recipients; however, in contrast to the overall population, the overall CIBIC-Plus score distribution in donepezil-treated patients did not significantly differ between treatment groups [16].
In post hoc sensitivity analyses of observed cases (using the mixed-effects model with repeated measures approach), memantine ER plus donepezil was associated with a significant (p < 0.05) advantage over placebo plus donepezil at all study visits on the SIB, CIBIC-Plus, NPI and VFT, but not on the ADCS-ADL19 scale [16]. Post hoc area under the curve (AUC) analyses supported these findings, with significant (p < 0.05) cumulative improvements in the week 0–24 AUCs on the SIB, CIBIC-Plus, NPI and VFT with memantine ER plus donepezil versus placebo plus donepezil, ranging from 100 % (for SIB) to 151 % (for CIBIC-Plus), although the week 0–24 AUC for the ADCS-ADL19 did not significantly differ between groups [16].
4.2 Pooled Analyses
The cumulative efficacy of memantine plus donepezil combination therapy was compared with that of placebo or memantine or donepezil monotherapy in post hoc pooled analyses of data from four 6-month, randomized, double-blind trials in patients with moderate to severe AD (n = 1408), including the MEM-MD-50 trial (Sect. 4.1) and three trials with memantine IR [17]. In these trials, patients received memantine (either IR 10 mg twice daily or ER 28 mg once daily) or placebo on a background of stable donepezil, any ChEI or no ChEI therapy. In the pooled analyses, data from patients who received a ChEI other than donepezil (i.e. galantamine or rivastigmine) were excluded to limit heterogeneity [17].
In week 0–24 AUC analyses, memantine plus donepezil recipients (n = 429) had significantly (p < 0.05) greater cumulative improvements than placebo only (n = 281), memantine only (n = 289) or placebo plus donepezil (n = 418) recipients on the SIB, NPI, CIBIC-Plus and a four-domain composite index (4D-CI; with equal weighting for SIB, NPI, CIBIC-Plus and ADCS-ADL19 outcome measures) [17]. Over 24 weeks, memantine plus donepezil was the only treatment that provided significant (p < 0.01) cumulative improvements from baseline on the CIBIC-Plus. In the last study interval (i.e. weeks 18–24), memantine plus donepezil was the only treatment that continued to provide significant (p < 0.0001) cumulative benefits on the SIB and NPI. Furthermore, significant (p < 0.01) cumulative benefits on the 4D-CI continued to be observed in the last treatment interval for memantine plus donepezil and placebo plus donepezil recipients [17].
Across 24 weeks’ treatment, memantine plus donepezil was associated with significant (p < 0.001) relative cumulative AUC improvements over placebo on the ADCS-ADL19, CIBIC-Plus, SIB, NPI and 4D-CI, ranging from 104 % (for ADCS-ADL19) to 459 % (for 4D-CI) [17]. In a comparison of absolute AUC improvements in the memantine plus donepezil versus placebo groups with the sum of improvements in each of the monotherapy groups (i.e. memantine only and placebo plus donepezil), there was no significant treatment interaction between memantine and donepezil, indicating that the clinical efficacy of memantine plus donepezil was additive rather than synergistic [17].
In further pooled analyses of data from a phase III trial with memantine IR [3] and donepezil-treated patients from the MEM-MD-50 trial (Sect. 4.1) [n = 828], memantine plus donepezil was associated with significantly (p < 0.05) greater improvements than placebo plus donepezil on several activities of daily living items (including grooming, conversing, finding belongings and eating) in patients with severe or lower moderate stage AD (i.e. with a baseline MMSE score of 1, 8 or 11) regardless of donepezil pretreatment duration (abstract presentation) [18]. The effects of memantine and donepezil on activities of daily living were mainly observed in patients with moderate disease, while memantine effects were evident in those with severe AD, indicating that memantine plus donepezil provides complementary benefits across moderate and severe stages of AD [18].
5 Tolerability
Once-daily memantine ER as add-on therapy to ChEIs was generally well tolerated in the 24-week, MEM-MD-50 trial discussed in Sect. 4.1. Treatment-emergent adverse events (TEAEs) were reported in 62.8 and 63.9 % of add-on memantine ER and add-on placebo recipients, with TEAEs resulting in discontinuation in 9.9 and 6.3 % of patients; the most common reason for discontinuation of add-on memantine ER was dizziness (1.5 vs. 0 % with add-on placebo) [15].
The adverse event profile for memantine ER was generally similar to that of placebo when added to stable ChEI therapy [15]. The most frequently reported TEAEs with add-on memantine ER (i.e. in ≥5 % of patients) were fall (5.6 vs. 7.8 % with add-on placebo), urinary tract infection (5.6 vs. 7.2 %), headache (5.6 vs. 5.1 %) and diarrhoea (5.0 vs. 3.9 %). TEAEs that occurred with twofold or higher incidence with add-on memantine ER than add-on placebo were dizziness (4.7 vs. 1.5 %), depression (3.2 vs. 1.5 %), bodyweight increase (3.2 vs. 0.9 %), constipation (2.9 vs. 1.2 %), somnolence (2.9 vs. 1.2 %), back pain (2.6 vs. 0.6 %) and abdominal pain (2.1 vs. 0.6 %), while add-on placebo was associated with twofold or higher incidences than add-on memantine ER of bodyweight decrease (3.3 vs. 1.5 %), irritability (2.4 vs. 1.2 %) and cough (2.4 vs. 0.9 %). Low haemoglobin was the only potentially clinically significant laboratory abnormality that occurred in more than twice as many add-on memantine ER than add-on placebo recipients (2.4 vs. 1.1 %) [15].
Serious adverse events occurred in 8.2 and 6.3 % of add-on memantine ER and add-on placebo recipients, with the most frequent serious adverse events being fall (0.6 vs. 1.5 %) and urinary tract infections (0.6 vs. 0.9 %) [15]. In the memantine ER group, pneumonia, cerebrovascular accident and syncope were the only other serious adverse events that were reported by more than one patient [each reported in two patients (0.6 %) vs. no patients in the placebo group]. Four patients (1.2 %) in the memantine ER group and five patients (1.5 %) in the placebo group died; none of the deaths in the memantine ER group were considered related or possibly related to treatment [15].
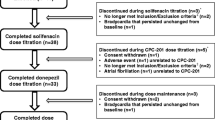
In subgroup analyses of donepezil-treated patients, 61.9 and 59.9 % of memantine ER plus donepezil and placebo plus donepezil recipients reported TEAEs [16]. Diarrhoea, dizziness, fall, headache, influenza and urinary tract infection were the most frequently reported TEAEs with memantine ER plus donepezil (Fig. 1), with diarrhoea, dizziness and influenza occurring with at least twofold higher incidence in the memantine ER plus donepezil than the placebo plus donepezil group [16].
Treatment-emergent adverse events occurring with a ≥5 % incidence with memantine extended-release or placebo in donepezil-treated patients with moderate to severe Alzheimer’s disease in a phase III trial [16]. DON donepezil, MEM ER memantine extended-release, PL placebo
6 Dosage and Administration of Memantine ER/Donepezil
In the USA, oral memantine ER/donepezil is indicated for the treatment of moderate to severe dementia of the Alzheimer’s type in patients receiving stable therapy with memantine (IR 10 mg twice daily or ER 28 mg once daily) plus donepezil 10 mg/day [11]. These patients can be switched to one memantine ER/donepezil 28/10 mg capsule daily (in the evening); patients with severe renal impairment receiving stable therapy with memantine (IR 5 mg twice daily or ER 14 mg once daily) plus donepezil 10 mg/day can be switched to one memantine ER/donepezil 14/10 mg capsule daily. Memantine ER/donepezil can be taken with or without food; the capsules should be taken intact or they may be opened and the entire contents sprinkled on applesauce and then consumed [11]. Local prescribing information should be consulted for more detailed information regarding contraindications, warnings and precautions, drug interactions and use in special patient populations.
7 Place of Memantine ER/Donepezil in the Management of Alzheimer’s Disease
Active management of AD, which includes appropriate use of available medications, can improve patient quality of life through all stages of the disease [1]. Although there is no cure for AD, effective pharmacological treatment may mitigate symptoms and reduce clinical decline [2]. Among the agents available to treat AD, there is no strong evidence to support choosing one drug over another, with treatment choice generally being based on tolerability, ease of use and cost [20]. Memantine and donepezil are both well established and widely used treatment options that have been used in the management of AD for many years [10, 13]; moreover, combination therapy with memantine plus ChEI is considered the standard of care in patients with moderate to severe AD (Sect. 1) [2]. For patients with moderate to severe AD on stable memantine and donepezil therapy, the recently available fixed-dose combination of memantine ER/donepezil 28/10 mg with once-daily administration may provide a more convenient dosage regimen. In healthy adults, this fixed-dose combination had bioequivalence to coadministration of corresponding doses of memantine ER plus donepezil (Sect. 3).
In MEM-MD-50, a 24-week, phase III trial in patients with moderate to severe AD (Sect. 4.1), the addition of memantine ER 28 mg once daily to stable ChEI therapy provided significantly greater improvements than add-on placebo on several measures of efficacy, including SIB (cognition) and CIBIC-Plus (global clinical status) [coprimary endpoints]. In subgroup analyses in donepezil-treated patients (Sect. 4.1.1), add-on memantine ER for 24 weeks was significantly more effective than add-on placebo on the SIB, NPI (dementia behaviour) and VFT (semantic processing), although the between-group difference on the CIBIC-Plus and ADCS-ADL19 (daily functioning) was not significant. Post hoc AUC analyses of subgroup data revealed cumulative improvements on the SIB, CIBIC-Plus, NPI and VFT that significantly favoured add-on memantine ER over add-on placebo. Subsequent post hoc pooled analyses of four randomized trials that included MEM-MD-50 (Sect. 4.2) confirmed that memantine (i.e. IR 10 mg twice daily or ER 28 mg once daily) plus donepezil for 24 weeks provided cumulative benefits on most clinical domains, with the combination therapy having additive (rather than synergistic) clinical efficacy.
Memantine ER plus ChEI therapy for 24 weeks was generally well tolerated in patients with moderate to severe AD, with an adverse event profile that was largely similar to that of placebo plus ChEI (Sect. 5). In subgroup analyses in donepezil-treated patients, adverse events that occurred with at least twofold higher incidence with add-on memantine ER than add-on placebo were diarrhoea, dizziness and influenza.
One of the limitations of the MEM-MD-50 trial include its lack of an active control, such that a direct comparison of the efficacy, tolerability or treatment adherence of add-on memantine ER with that of memantine IR with standard dosing was not conducted [15]. Furthermore, the efficacy and tolerability of the fixed-dose combination of memantine ER/donepezil 28/10 mg has not been evaluated in a randomized trial in patients with moderate to severe AD. Further studies are needed to address these issues.
Adherence to medication plays an important role in effective AD management, reducing the emergence of symptoms and unwanted dementia behaviours (e.g. delusions, agitation and sleep/wake disorders) that are often associated with progressive disease [2]. In particular, increased treatment persistence (i.e. the length of time on drug therapy) is associated with significantly slower rates of cognitive and functional decline in patients with AD [21]. Adherence to oral AD medications is generally low, being estimated at 58 % of patients in one study [22]. The chronic nature of AD and its associated memory loss may negatively influence treatment adherence, which may also be affected by regimen complexity, dosing convenience, management of adverse effects and treatment affordability [22]. In retrospective analyses, coadministration of AD medications was the most significant predictor of persistence, with addition of memantine to donepezil having a particularly significant and positive effect [23]. As administration of these two drugs can increase pill burden, the once-daily memantine ER/donepezil fixed-dose combination may potentially improve patient adherence by reducing pill burden and simplifying medication management [14], which may ultimately lead to increased treatment benefits in patients with moderate to severe AD.
Given the recent FDA approval of memantine ER/donepezil for moderate to severe AD, there are no published data regarding the cost effectiveness of the fixed-dose combination in this indication. However, a cost utility analysis based on MEM-MD-50 data has indicated that memantine ER plus ChEI combination therapy was more effective and less costly than ChEI monotherapy from a societal and healthcare payer perspective over a 3-year time horizon in the USA [24].
In conclusion, combination therapy with memantine ER plus donepezil is an effective and generally well tolerated treatment option in patients with moderate to severe AD, with additive cumulative benefits across multiple clinical domains over 6 months’ treatment. The once-daily, fixed-dose combination of memantine ER/donepezil 28/10 mg is bioequivalent to coadministration of corresponding doses of the individual drugs, and represents a potentially more convenient treatment option for patients with moderate to severe AD on stable memantine and donepezil therapy.
Data selection sources:
Relevant medical literature (including published and unpublished data) on memantine extended-release/donepezil was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 14 October 2015], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Namzaric, memantine, donepezil, drug combinations, combin*, co-admin*, concurrent, Alzheimer.
Study selection: Studies in patients with Alzheimer’s disease who received memantine extended-release plus donepezil. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Association Alzheimer’s. 2014 Alzheimer’s disease facts and figures. Alzheimers Dement. 2014;10(2):e47–92.
Atri A. Effective pharmacological management of Alzheimer’s disease. Am J Manag Care. 2011;17(Suppl 13):S346–55.
Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–24.
Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366(10):893–903.
Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and memantine clinical trial program. J Prev Alzheimers Dis. 2015;2(3):165–71.
Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN Guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889–98.
Atri A, Molinuevo JL, Lemming O, et al. Memantine in patients with Alzheimer’s disease receiving donepezil: new analyses of efficacy and safety for combination therapy. Alzheimers Res Ther. 2013;5(1):[article no. 6].
Atri A, Shaughnessy LW, Locascio JJ, et al. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209–21.
Lopez OL, Becker JT, Wahed AS, et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80(6):600–7.
Plosker GL. Memantine extended release (28 mg once daily): a review of its use in Alzheimer’s disease. Drugs. 2015;75(8):887–97.
Forest Pharmaceuticals Inc. Namzaric™ (memantine hydrochloride extended-release and donepezil hydrochloride) capsules, for oral use: US prescribing information. 2014. http://www.fda.gov. Accessed 14 Oct 2015.
Parsons CG, Danysz W, Dekundy A, et al. Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res. 2013;24(3):358–69.
Jelic V, Darreh-Shori T. Donepezil: a review of pharmacological characteristics and role in the management of Alzheimer disease. Clin Med Insights Ther. 2010;2:771–88.
Boinpally R, Chen L, Zukin SR, et al. A novel once-daily fixed-dose combination of memantine extended release and donepezil for the treatment of moderate to severe Alzheimer’s disease: two phase I studies in healthy volunteers. Clin Drug Investig. 2015;35(7):427–35.
Grossberg GT, Manes F, Allegri RF, et al. The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs. 2013;27(6):469–78.
Grossberg GT, Alva G, Hendrix S, et al. Efficacy and tolerability of memantine extended release added to stable donepezil regimen in individuals with moderate to severe Alzheimer’s disease: subset analysis of a randomized clinical trial [abstract no. P1-370 plus poster]. Alzheimers Dement. 2014;10(4 Suppl):P450.
Atri A, Hendrix SB, Pejovic V, et al. Cumulative, additive benefits of memantine-donepezil combination over component monotherapies in moderate to severe Alzheimer’s dementia: a pooled area under the curve analysis. Alzheimers Res Ther. 2015;7(1):[article no. 28].
Hendrix S, Ellison N, Otcheretko V. Complementary benefit of memantine and donepezil on activities of daily living by disease stage when used together: post hoc analysis of two trials in moderate to severe Alzheimer’s disease [abstract no. 2710 plus poster]. In: Alzheimer’s Association International Conference. 2015.
Atri A, Hendrix S, Ellison N, et al. Caregiver distress related to neuropsychiatric symptoms is reduced with extended-release memantine-cholinesterase inhibitor combination in patients with moderate to severe Alzheimer’s disease [abstract no. 4636 plus poster]. In: Alzheimer’s Association International Conference. 2015.
Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148(5):370–8.
Rountree SD, Chan W, Pavlik VN, et al. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimers Res Ther. 2009;1(2):[article no. 7].
Borah B, Sacco P, Zarotsky V. Predictors of adherence among Alzheimer’s disease patients receiving oral therapy. Curr Med Res Opin. 2010;26(8):1957–65.
Brewer L, Bennett K, McGreevy C, et al. A population-based study of dosing and persistence with anti-dementia medications. Eur J Clin Pharmacol. 2013;69(7):1467–75.
Saint-Laurent Thibault C, Stillman IO, Chen S, et al. Cost-utility analysis of memantine extended release added to cholinesterase inhibitors compared to cholinesterase inhibitor monotherapy for the treatment of moderate-to-severe dementia of the Alzheimer’s type in the United States. J Med Econ. 2015. doi:10.3111/13696998.2015.1063501.
Acknowledgments
During the peer review process, the manufacturer of memantine ER/donepezil was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Sarah Greig is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: S. Gauthier, McGill Center for Studies in Aging, Alzheimer Disease and Related Disorders Unit, Douglas Mental Health University Institute, Montreal, QC, Canada; V. John, Department of Neurology, University of California Los Angeles, Los Angeles, CA, USA.
Rights and permissions
About this article
Cite this article
Greig, S.L. Memantine ER/Donepezil: A Review in Alzheimer’s Disease. CNS Drugs 29, 963–970 (2015). https://doi.org/10.1007/s40263-015-0287-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-015-0287-2