Abstract
Memantine is an uncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist that is a well-established treatment option for moderate to severe dementia of the Alzheimer’s type, either alone or in combination with cholinesterase inhibitors. The immediate-release (IR) formulations of memantine (tablets and oral solution) have been available in numerous countries, including the USA, for more than a decade and are administered orally twice daily at a maximum recommended total daily dosage of 20 mg/day. The memantine extended-release (ER) (Namenda XR®) 28 mg once-daily capsule formulation was approved in the USA in 2010 and became available more recently. The potential advantages of memantine ER over the IR formulation include a more convenient dosage regimen and lower pill burden that may improve adherence to therapy; also, memantine ER capsules may be opened and the contents sprinkled on applesauce for patients who have difficulty swallowing. Memantine ER provides a higher total daily dosage than the recommended memantine IR regimen and pharmacokinetic data indicate greater exposure with the ER formulation, but the clinical implications of this are unclear, as the two formulations have not been assessed in a comparative clinical trial. The efficacy of memantine ER 28 mg once daily was demonstrated in a large, multinational, phase III trial, which showed that the addition of memantine ER to ongoing oral cholinesterase inhibitors improved key outcomes compared with cholinesterase inhibitor monotherapy, including measures of cognition and global status, which were the co-primary endpoints of the study. The most common adverse events were headache, diarrhoea and dizziness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Memantine has a well-established efficacy and tolerability profile in the management of dementia of the Alzheimer’s type, as monotherapy or when added to cholinesterase inhibitors |
An ER formulation of memantine (28 mg once daily), alone or with cholinesterase inhibitors, is approved in the USA for moderate to severe Alzheimer’s disease; a fixed-dose combination of memantine ER plus donepezil was also recently approved |
Memantine ER (28 mg once daily) provides a more convenient dosage regimen that may improve adherence, while also allowing for an increased total daily dosage, compared with the immediate-release formulation (10 mg twice daily) |
Memantine ER (28 mg once daily) added to ongoing cholinesterase inhibitor treatment improves most key efficacy outcomes, including measures of cognition and global status, and is well tolerated in patients with moderate to severe Alzheimer’s disease, as shown in a large, multinational, phase III trial |
1 Introduction
Dementia of the Alzheimer’s type (Alzheimer’s disease) is the most common type of dementia, accounting for ≈60–80 % of cases [1]. In the USA in 2014, an estimated 5.2 million people had Alzheimer’s disease (diagnosed or undiagnosed), including ≈5 million Americans ≥65 years of age [1]. The greatest risk factor for Alzheimer’s disease is advanced age, and the number of cases in the USA and worldwide is projected to increase markedly in coming years because of global population aging [1, 2]. Interestingly, although demographics will drive an increase in the overall number of dementia cases, there appears to be a declining prevalence of dementia in US and European elderly populations, possibly related to factors such as higher levels of education and reduced rates of stroke [3]. Early symptoms of Alzheimer’s disease include difficulty remembering recent conversations, names or events, as well as apathy and depression; later symptoms include impaired communication, disorientation, confusion, poor judgment, behaviour changes and, ultimately, difficulty speaking, swallowing and walking [1]. In addition to morbidity and mortality, Alzheimer’s disease is associated with a substantial caregiver and societal economic burden [1, 2].
Pharmacological treatment options in Alzheimer’s disease include cholinesterase inhibitors and memantine, although research in the development of new agents is ongoing [4]. Memantine is an uncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist that is approved for the treatment of moderate to severe Alzheimer’s disease, either alone or in combination with cholinesterase inhibitors [5, 6]. The immediate-release (IR) tablet and solution formulations of memantine have been available in numerous countries, including the USA, for more than a decade and are administered orally twice daily at a maximum recommended total daily dosage of 20 mg/day [5, 6].
Several randomized, double-blind, placebo-controlled trials evaluated the efficacy of memantine IR in patients with moderate to severe Alzheimer’s disease, as monotherapy [7–9] and/or in combination with the cholinesterase inhibitor donepezil [9, 10]. Most of these data have been reviewed previously in some detail [5]. Noteworthy are findings from two ≈6-month trials that evaluated memantine IR as monotherapy (study MRZ-90001-9605) [7] and in combination with ongoing donepezil (study MEM-MD-02) [10]. In both studies, memantine IR was statistically superior to placebo on measures of function (a co-primary endpoint [7, 10]) and cognition (a co-primary [10] or secondary [7] endpoint). In MEM-MD-02 the addition of memantine IR to donepezil was also associated with significantly better outcomes on measures of behaviour and global change [10]. More recent findings from the 1-year, randomized, double-blind DOMINO trial in donepezil-treated patients with moderate to severe Alzheimer’s disease showed statistically significant benefits on measures of cognition and function (co-primary endpoints) for continued donepezil compared with discontinued donepezil (with or without add-on memantine IR) and for memantine IR versus add-on placebo (with or without donepezil discontinuation) [9]. With high and disproportionate withdrawal in placebo arms, the added benefits of combination therapy with memantine IR added to baseline donepezil, which were apparent in the first 30 weeks, were not observed by end of trial [11]. A pooled analysis of data from the Memantine Clinical Trial Program and reassessment of DOMINO data showed that adding memantine to donepezil was associated with benefits across multiple clinical domains [12].
An extended-release (ER) formulation of memantine (Namenda XR®)—28 mg capsules for once-daily administration—is approved in the USA for the treatment of moderate to severe dementia of the Alzheimer’s type [13]. Lower dosages of memantine ER (7, 14 and 21 mg capsules) are also available, primarily for the initial dosage titration period. Memantine ER provides a higher total daily dosage than the recommended memantine IR regimen, but the clinical implications of this are unclear, as they have not been assessed in a comparative trial [13]. This article reviews the pharmacology, clinical efficacy and tolerability profile of memantine ER (28 mg once daily) capsules available in the USA. A discussion of the lower-dose memantine ER (20 mg once daily) formulation available in the EU [14] is outside the scope of the article.
2 Pharmacodynamic Properties
The pathophysiology of Alzheimer’s disease is complex and has not been fully elucidated, but dysregulation of glutamate signalling and its resulting excitotoxicity at NMDA receptors, which play an important role in cognition and memory, appear to be contributing factors [15, 16]. As a low-to-moderate affinity, uncompetitive (open-channel), voltage-dependent NMDA receptor antagonist that binds preferentially to the NMDA receptor-operated cation channels, memantine is thought to modulate the pathological effects of glutamate in Alzheimer’s disease, thereby facilitating normal physiological function [13, 16–19]. NMDA receptor channels are blocked by Mg2+ ions under normal, resting, physiological conditions, and a strong transient glutamate synaptic signal depolarises the post-synaptic membrane, removing the voltage-dependent blockade by Mg2+ ions, opening the NMDA channel and allowing the flow of Ca2+ ions into the post-synaptic neuron [19]. However, in Alzheimer’s disease it is thought that the presence of glutamate and amyloid-beta produces a low-level stimulation that decreases the membrane potential, thereby relieving the Mg2+ channel block and increasing the continuous flow of Ca2+ ions into the post-synaptic neuron, resulting in a `background noise’ of stimulation at rest. Under such pathological conditions, memantine (unlike the weaker Mg2+ ions) continues to block the NMDA receptor channel, thereby reducing the `background noise’ of dysfunctional glutamate signalling. When high levels of glutamate are transiently present (i.e. when a physiological signal arrives), memantine dissociates from the NMDA receptor and allows normal neurotransmission. In addition to facilitating normal glutamate signalling processes, memantine also appears to protect neurons from the excitotoxicity of excessive glutamate stimulation [19]. The role of glutamate neurotransmission in Alzheimer’s disease and the pharmacodynamic properties of memantine have been reviewed previously in some detail [5, 15, 16, 19].
In vitro studies indicate that memantine also has antagonistic effects at the serotonin 5-HT3 receptor, which it binds to with a similar potency as for the NMDA receptor [13]. In addition, it has a more modest antagonistic effect at nicotinic acetylcholine receptors, to which it binds at a potency of one-sixth to one-tenth that for the NMDA receptor. It has low to negligible affinity for GABA, benzodiazepine, dopamine, adrenergic, histamine and glycine receptors and for voltage-dependent Ca2+, Na+ and K+ channels. Memantine does not affect the reversible inhibition of acetylcholinesterase by donepezil, galantamine or tacrine in vitro [13].
3 Pharmacokinetic Properties
3.1 Absorption and Distribution
In a comparative pharmacokinetic study, steady-state values for peak plasma memantine concentration (Cmax) and area under the plasma concentration-time curve (AUC) from time 0–24 h were 48 and 33 % higher, respectively, for memantine ER 28 mg once daily than with memantine IR 10 mg twice daily [13]. Time to achieve Cmax was 9–12 h after administration of memantine ER. There was no difference in exposure to memantine (Cmax or AUC) when memantine ER was administered with or without food or when the capsule was swallowed intact compared with when the capsule contents were taken after being sprinkled on applesauce. The mean volume of distribution of memantine is 9–11 L/kg and plasma protein binding is 45 % [13].
3.2 Metabolism and Elimination
Although memantine undergoes partial hepatic metabolism, cytochrome P450 (CYP) isoenzymes do not play a significant role [13]. There are three main metabolites (the N-glucuronide conjugate, 6-hydroxy memantine and 1-nitroso-deaminated memantine), which have only minimal NMDA receptor antagonistic activity. Approximately 48 % of an administered dose of memantine is eliminated in the urine unchanged. Renal clearance involves active tubular secretion moderated by pH-dependent tubular reabsorption. The clearance of memantine is reduced by ≈80 % under alkaline urine conditions at pH 8, which may have clinical implications in patients with urinary tract infections (UTIs). The elimination half-life (t½) of memantine is ≈60–80 h.
3.3 Special Patient Populations
Compared with healthy volunteers with normal renal function [creatinine clearance (CLCR) >80 mL/min], mean memantine AUC from time 0–∞ increased by 4, 60 and 115 %, respectively, and mean t½ increased by 18, 41 and 95 %, respectively, in subjects with mild (CLCR >50 to 80 mL/min), moderate (CLCR 30–49 mL/min) or severe (CLCR 5–29 mL/min) renal impairment in a pharmacokinetic study involving single-dose administration of memantine IR 20 mg [13]. For patients with severe renal impairment, a lower dosage of memantine ER 14 mg once daily is recommended (Sect. 6).
Memantine pharmacokinetics were also evaluated following single-dose administration of memantine IR 20 mg in healthy volunteers and subjects with moderate hepatic impairment (Child-Pugh class B) [13]. There was no between-group difference in exposure to memantine (Cmax or AUC), although t½ was ≈16 % longer in those with moderate hepatic impairment compared with healthy subjects with normal hepatic function.
3.4 Drug Interactions
In general, memantine has a low propensity for drug interactions [13]. When administered concomitantly with donepezil, there was no effect on the pharmacokinetics of either drug. In addition, memantine did not affect the pharmacokinetics of bupropion or warfarin when coadministered with each of these agents. In vitro studies evaluating the ability of memantine to inhibit or induce various CYP isoenzymes indicate that memantine is not expected to affect the pharmacokinetics of drugs metabolized by CYP 1A2, 2A6, 2C9, 2D6, 2E1 or 3A4/5. Similarly, in view of the metabolic fate of memantine, drugs that are substrates for, or inducers or inhibitors of, CYP isoenzymes are not expected to interact with memantine [13]. In addition, a study in 52 healthy volunteers found no evidence of pharmacokinetic or pharmacodynamic interactions between memantine and a fixed-dose combination of dextromethorphan/quinidine at steady state [20].
In theory, there could be interactions between memantine and other drugs eliminated by tubular secretion that use the same renal cationic system (e.g. hydrochlorothiazide and triamterene), although clinically significant interactions have not been observed in limited pharmacokinetic drug interaction studies conducted to date [13]. As noted in Sect. 3.2, the clearance of memantine is reduced under alkaline urine conditions (e.g. UTIs), potentially leading to an accumulation of the drug if urine pH is increased by drugs such as carbonic anhydrase inhibitors or sodium bicarbonate [13].
4 Therapeutic Efficacy
4.1 Phase III Trial
The efficacy of memantine ER 28 mg once daily was demonstrated in a randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease who were stabilized and continued to receive their current cholinesterase inhibitor therapy [21]. The phase III, multinational trial included 677 patients with a diagnosis of dementia of the Alzheimer’s type and a score of 3–14 on the Mini-Mental State Examination (MMSE) at the time of screening who were randomized to receive once-daily memantine ER (n = 342) or placebo (n = 335) for 24 weeks. At the time of randomization (i.e. baseline), the MMSE score range was 3–17. Memantine ER was initiated at a dosage of 7 mg once daily and gradually titrated up to a target dosage of 28 mg once daily over the first 4 weeks. Patients were required to tolerate a minimum dosage of 21 mg once daily by week 8 in order to continue in the study. Baseline patient characteristics were well matched between memantine ER and placebo groups, including mean age (76.2 vs. 76.8 years), the proportion of women (71.6 vs. 72.5 %), the proportion of Hispanics (68.3 vs. 69.6 %), mean MMSE score (10.9 vs. 10.6), and the proportion of patients receiving ongoing therapy with oral formulations of donepezil (69.2 vs. 68.1 %), galantamine (21.1 vs. 20.3 %) or rivastigmine (9.4 vs. 12.2 %).
The co-primary endpoints of the trial were the baseline to endpoint score change on the Severe Impairment Battery (SIB), to assess treatment effects on cognition, and the endpoint score on the Clinician’s Interview-Based Impression of Change Plus Caregiver Input (CIBIC-Plus), to assess treatment effects on global clinical status [21]. Secondary outcomes included measures of behaviour, daily functioning and semantic processing ability. Most outcome measures were assessed in terms of the change in score from baseline to week 24 in the intent-to-treat (ITT) population, using the last observation carried forward (LOCF) approach. Assessment scales were administered at regular intervals during the 24-week study period.
As shown in Table 1, memantine ER was superior to placebo on the SIB and CIBIC-Plus (co-primary endpoints), thereby demonstrating its efficacy in this patient population [21]. Memantine ER also achieved significantly greater improvements on the Neuropsychiatric Inventory (NPI), which assesses behaviour, and the verbal fluency test (VFT), which assesses semantic processing ability. The between-group difference did not achieve statistical significance for the baseline to endpoint change in score on the 19-item Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL19), which is used to assess function (Table 1).
4.2 Post Hoc Analyses
A number of post hoc analyses have been conducted using data from the 24-week phase III trial, such as evaluations of treatment response by disease severity and detailed analyses of effect of treatment on cognition, behaviour, function, and language and communication (all reported as abstracts/posters). Most of the exploratory analyses used a mixed-model with repeated measures (MMRM) approach and included observed cases [22–29], which was approximately equal to the number of patients who completed the phase III trial (n = 545), although some evaluated the ITT population with LOCF [25–30] (typically as a type of sensitivity analysis for observed-case results).
4.2.1 Response by Disease Severity
The addition of memantine ER to oral cholinesterase inhibitors was associated with statistically significant improvements in cognition, behaviour and global status compared with the addition of placebo (i.e. cholinesterase inhibitor monotherapy) across a wide range of disease severity, as assessed by MMSE scores at baseline [22]. The analysis also showed significant between-group differences in function and verbal fluency favouring memantine ER over placebo, albeit over narrower ranges of baseline MMSE scores. Least-squares mean baseline to endpoint changes were estimated for the memantine ER and placebo groups at each baseline MMSE score for all efficacy outcomes, except CIBIC-Plus, for which the model estimated endpoint scores because CIBIC-Plus measures change from baseline. Significant (p < 0.05) improvements with memantine ER compared with placebo at week 24 were demonstrated on the cognitive (SIB), behaviour (NPI) and global clinical status (CIBIC-Plus) measures across baseline MMSE ranges of 6–13, 7–14 and 5–13, respectively. Memantine ER was also associated with significant (p < 0.05) improvements on daily function (assessed by ADCS-ADL19) in patients with more severe disease (MMSE 4–9) and on verbal fluency in those with moderate disease (MMSE 10–14) [22]. A post hoc analysis of behavioural effects by disease severity has also been reported (see Sect. 4.2.5)
4.2.2 Responder Analysis and Response Across Multiple Outcomes
Findings of a responder analysis significantly favoured memantine ER over placebo (i.e. combination therapy over cholinesterase monotherapy) for some outcomes and response levels [30]. Response levels were defined on the basis of baseline to endpoint score changes attained by the 10th, 25th, 50th, 75th and 90th percentile of placebo recipients. Memantine ER was significantly superior to placebo in terms of the proportion of patients who achieved a response on the SIB at the 90th percentile level (18.7 vs. 10.4 % had improvement ≥12 points; p = 0.003), on the NPI at the 90th percentile level (16.0 vs. 9.7 % had improvement ≥17 points; p = 0.018) and the 75th percentile level (34.9 vs. 25.9 % had improvement ≥8 points; p = 0.016), and on the CIBIC-Plus at the 75th percentile level (35.1 vs. 26.8 % had endpoint score ≤3; p = 0.023).
Results of an analysis evaluating responses across multiple outcomes, including measures of cognition (SIB), function (ADCS-ADL19), behaviour (NPI) and global status (CIBIC-Plus), suggest that memantine ER may provide beneficial effects on multiple clinical domains simultaneously, most notably when cognition and function are improved and when relatively high levels of improvement are observed [23]. For each outcome, the analysis included two levels of response [stabilization (e.g. change from baseline to endpoint of ≥0 on SIB) or improvement (e.g. change from baseline to endpoint of ≥3 on SIB)], and there were 11 possible combinations for responses on multiple (two, three or four) efficacy outcomes. For the response level indicating a clinically notable improvement, memantine ER was superior to placebo for the two-measure combination of ADCS-ADL19/NPI (21.3 vs. 15.8 %; p = 0.042) and for the three-measure combinations of ADCS-ADL19/NPI/SIB (15.4 vs. 9.6 %; p = 0.027) and ADCS-ADL19/SIB/CIBIC-Plus (12.4 vs. 7.4 %; p = 0.030).
4.2.3 Cumulative Efficacy Over Time
To provide more complete information on treatment effects over the entire course of the trial, rather than focusing only on baseline to endpoint changes in clinical domain scores, the mean area under the curve (AUC) for changes from baseline in SIB, CIBIC-Plus, NPI, VFT and ADCS-ADL19 were determined for each treatment group [24]. Results showed that memantine ER was associated with cumulative benefits across multiple clinical domains. When comparing the two treatment groups for mean AUC values from baseline to week 24 for each of these measures, memantine ER was associated with statistically significant benefits of 88 % (p = 0.014) for SIB, 133 % (p = 0.019) for CIBIC-Plus, 109 % (p < 0.001) for NPI and 139 % for VFT (p = 0.014); results were not statistically significant for ADCS-ADL19.
4.2.4 Effect on Cognition
In one post hoc analysis focusing on cognition, memantine ER was associated with statistically significant (p < 0.05) improvements for six of nine individual SIB domains (memory, language, attention, praxis, orientation and construction, but not social interaction, visuospatial ability and orienting to name), as well as all three higher-order cognition subscales of Memory, Language and Praxis (aggregated combinations of domains), compared with placebo [25].
Another analysis showed that the benefits of memantine ER on cognition (assessed using the SIB instrument) were observed as early as week 8, and memantine ER was associated with sustained cognitive improvement at week 24 [26]. At weeks 12 (p < 0.05), 18 (p < 0.01) and 24 (p < 0.001), the mean improvement from baseline SIB score was significantly greater among memantine ER than placebo recipients. The analysis also considered five SIB response levels (improvement of ≥0, ≥5, ≥10, ≥15 and ≥20 points), and the proportion of patients in each treatment group who achieved and maintained a response was compared. Significantly more memantine ER than placebo recipients maintained SIB responses of ≥5 (26.1 vs. 17.0 %; p = 0.014) or ≥10 (14.6 vs. 7.6 %; p = 0.012) points from week 8 to week 24. Likewise, significantly more memantine ER than placebo recipients maintained 12-week SIB responses of ≥5 (28.5 vs. 19.9 %; p = 0.025), ≥10 (16.3 vs. 8.2 %; p = 0.005) or ≥15 (9.5 vs. 4.1 %; p = 0.015) points through to week 24 [26]. In both studies, findings were generally similar whether observed cases or the LOCF approach were used [25, 26].
4.2.5 Effect on Behaviour
Post hoc analyses focusing on behavioural benefits of memantine ER (assessed by NPI) include an evaluation of effects across a range of disease severity [27] and an assessment of sustained effects [28]. In the former, patients were divided into subgroups of disease severity according to baseline MMSE scores (overall range assessed was 4–14) [27]. Mean baseline NPI scores indicated a mild degree of behavioural impairment in both treatment groups (see Table 1). Across subgroups, memantine ER was associated with placebo-corrected improvements of 2.7–3.1 points in total NPI scores, and between-group differences achieved statistical significance for baseline MMSE scores of 7 (p < 0.05), 8, (p < 0.05), 9 (p < 0.01), 10 (p < 0.01), 11 (p < 0.001), 12 (p < 0.01), 13 (p < 0.01) and 14 (p < 0.05).
The other analysis demonstrated sustained behavioural improvement with memantine ER, as assessed by changes from baseline in NPI scores at various time points [28]. Statistically significant between-group differences favouring memantine ER over placebo in NPI score improvements were observed at weeks 12 (p = 0.05), 18 (p = 0.01) and 24 (p < 0.01). In the respective treatment groups, 49.3 and 45.4 % of patients had an improvement of ≥3 points from baseline to week 12. For patients with NPI scores at all three follow-up visits, 39.5 % of memantine ER recipients compared with 28.9 % of placebo recipients maintained this level of response at weeks 12, 18 and 24 (p < 0.05). Findings were similar in a sensitivity analysis using the LOCF approach.
4.2.6 Effect on Language and Communication
Patients who received memantine ER performed significantly better on some language-related items from the SIB than those who received placebo in the phase III trial [29]. In this post hoc analysis, the efficacy of memantine ER on language abilities was assessed using a compilation of relevant items from the SIB to form three subscales (Naming, Reading/Writing and Comprehension/Repetition/Discourse) and assessing the changes in scores from baseline to week 24. Its efficacy in terms of functional communication was determined from baseline to endpoint changes in a composite functional communication score, which was derived from a compilation of three items from the ADCS-ADL19 and 15 items from the Caregiver Perceived Burden Questionnaire. Although there was not a statistically significant between-group difference on the Naming subscale of the SIB, memantine ER was associated with statistically significant improvements on the Reading/Writing subscale (p = 0.002) and the Comprehension/Repetition/Discourse subscale (p = 0.048). There was no statistically significant between-group difference for the change in functional communication score. These findings using observed cases were similar in sensitivity analyses (using the LOCF and MMRM approaches).
4.2.7 Effect on Functional Abilities
Results of another post hoc analysis indicate that memantine ER may be associated with improvements in activities of daily living (ADL) related to higher-level processing [31]. The analysis compared treatment groups for baseline to endpoint changes in scores for individual items and four factor-derived subscales from the ADCS-ADL19 instrument. Memantine ER demonstrated a statistically significant advantage over placebo for three of the nineteen individual items in the observed-case analysis: eating (p = 0.027), clearing the table (p = 0.015) and finding belongings (p = 0.030). The LOCF analysis did not confirm these findings, although statistical superiority (p < 0.05) of memantine ER was shown for eating, clearing a table and conversing when the MMRM approach was used. In the ADL factor analysis, memantine ER was associated with a statistically significant advantage (p = 0.014) on the subscale for higher level functions, which included using a telephone, conversing, clearing a table, finding belongings, obtaining a beverage and disposing of litter. Sensitivity analyses using the LOCF and MMRM approaches confirmed this finding.
4.3 Subgroup Analysis in Donepezil-Treated Patients
In the phase III trial, as in clinical practice, donepezil was the most commonly used cholinesterase inhibitor, and prospectively defined assessments of treatment efficacy were conducted in the subgroup of patients with moderate to severe Alzheimer’s disease who received ongoing therapy with a stable dosage regimen of donepezil [32]. The prospective analyses were conducted in the ITT population with LOCF and included 456 donepezil-treated patients who were randomized to receive memantine ER (n = 232) or placebo (n = 224). Findings were similar to those of the overall population in that baseline to endpoint changes in scores significantly favoured memantine ER on the SIB (p = 0.001), NPI (p = 0.009) and VFT (p < 0.001), while no significant between-group difference was observed on the ADCS-ADL19; however, in this subgroup, in contrast to the overall population, the between-group difference in CIBIC-Plus score was not statistically significant. A post hoc observed-cases analysis using the MMRM approach showed a statistical advantage for memantine ER over placebo at all visits for scores on the SIB, NPI, VFT and CIBIC-Plus, but not on the ADCS-ADL19, and these results were supported by a post hoc AUC analysis [32].
4.4 Pooled Analyses
Various pooled analyses of clinical trials with memantine, including the phase III trial with memantine ER (Sect. 4.1) and ≥1 trial with memantine IR, have been conducted to compare the effects of memantine (IR or ER) plus cholinesterase inhibitors with monotherapy or placebo for 6 months in patients with moderate to severe Alzheimer’s disease (all presented as posters). Among the main findings of these analyses is that memantine plus cholinesterase inhibitor therapy stabilized or improved low or moderate levels of agitation/aggression (assessed by NPI) compared with cholinesterase inhibitor monotherapy or placebo [33]. Also, the addition of memantine to cholinesterase inhibitor therapy provided significant improvements across a range of neuropsychiatric symptoms (assessed by NPI) [34], and the addition of memantine to ongoing donepezil therapy was associated with a significant advantage over donepezil monotherapy for several items on the ADCS-ADL19, suggesting improvements in both basic and instrumental activities of daily living [35].
5 Tolerability
Memantine ER was generally well tolerated in the 24-week, randomized, double-blind, placebo-controlled, phase III trial discussed in Sect. 4.1 [21]. The safety population included 341 patients in the memantine ER group and 335 patients in the placebo group who received at least one dose of study medication. Adverse events were solicited from patients and caregivers using non-leading questions at all study visits, including any contact up to 30 days after study completion. The mean daily dosage of memantine ER was 27.0 mg and 92.1 % of patients received the target/maximum dosage of 28 mg once daily. There were no statistical comparisons between treatment groups for safety and tolerability data.
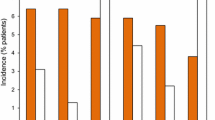
Treatment-emergent adverse events were reported in 62.8 % of patients in the memantine ER group and 63.9 % of those in the placebo group [21]. Figure 1 includes all adverse events reported by ≥2.0 % of memantine ER recipients that were numerically more common in the active treatment group than in the placebo group. The most common adverse events were headache, diarrhoea and dizziness. In addition, dizziness, depression, increased bodyweight, constipation, somnolence, back pain and abdominal pain were reported with at least twice the frequency in the memantine ER group compared with the placebo group. The only laboratory abnormality that occurred in at least twice as many memantine ER than placebo recipients was low haemoglobin (2.4 vs. 1.1 %). In the respective groups, 9.9 and 6.3 % of patients withdrew from the study because of adverse events. In the memantine ER group, the most common adverse event leading to discontinuation of therapy was dizziness (1.5 % of patients).
Treatment-emergent adverse events in the phase III trial in patients with moderate to severe Alzheimer’s disease receiving ongoing cholinesterase inhibitor therapy who were randomized to memantine extended-release (ER) (target dosage 28 mg once daily) or placebo for 24 weeks [21]
Adverse events reported with memantine ER in the phase III study in patients with moderate to severe Alzheimer’s disease [21] are generally similar to those reported with memantine IR in randomized, double-blind, placebo-controlled trials in patients with dementia, in which dizziness, headache, confusion and constipation were the most common adverse events (incidence ≥5 % and higher than placebo) [6].
6 Dosage and Administration
Memantine ER (7, 14, 21 and 28 mg) capsules are administered orally once daily and may be taken with or without food [13]. The recommended starting dosage is 7 mg once daily and the dosage should be increased in increments of 7 mg to the recommended maintenance dosage of 28 mg once daily, which is also the recommended maximum dosage of memantine ER. The minimum interval between dosage increases is 1 week and the dosage should only be increased if the previous regimen was well tolerated. Memantine ER capsules should be swallowed whole or they may be opened and the contents sprinkled on applesauce to facilitate swallowing. To transition patients from memantine IR 10 mg twice daily, memantine ER 28 mg once daily should be started the day following the last dose of memantine IR 10 mg. Dosage adjustment is not required in patients with mild or moderate renal impairment. For patients with severe renal impairment (CLCR 5–29 mL/min), the recommended maintenance (and maximum) dosage is 14 mg once daily. Dosage adjustment is not needed in patients with mild or moderate hepatic impairment; memantine ER has not been evaluated in patients with severe hepatic impairment. Local prescribing information should be consulted for additional information on precautions, contraindications, drug interactions and use in special patient populations.
7 Place of Memantine Extended Release in the Management of Alzheimer’s Disease
Memantine has been used in the management of Alzheimer’s disease for more than a decade and is a well-established treatment option. Memantine ER capsules (maintenance/maximum dosage 28 mg once daily) were approved in the USA in 2010 and became available more recently. The purported advantages of memantine ER over the older formulation include a more convenient dosage regimen and lower pill burden that may improve adherence to therapy; also, memantine ER capsules may be opened and the contents sprinkled on applesauce for patients who have difficulty swallowing. Memantine ER provides a higher total daily dosage than the recommended memantine IR regimen, although possible clinical benefits of the higher dosage regimen have not been assessed in a comparative trial. Nevertheless, pharmacokinetic data indicate greater drug exposure with memantine ER 28 mg once daily compared with memantine IR 10 mg twice daily (Sect. 3), and the slow release of drug from the ER formulation may have contributed to the relatively low frequency of adverse events reported in the phase III trial with memantine ER 28 mg once daily (Sect. 5) [21]. The authors of the phase III study noted that the frequency of adverse events with memantine ER in their trial was generally lower than that reported in previous studies with memantine IR in patients with moderate to severe Alzheimer’s disease, although this observation would need to be tested in a study directly comparing these formulations [21].
Pharmacological treatment options for Alzheimer’s disease are currently limited to cholinesterase inhibitors and memantine. These drugs can provide symptomatic improvement in Alzheimer’s disease, but they do not provide disease-modifying effects [1, 36]. Among the cholinesterase inhibitors available in the USA, donepezil and rivastigmine transdermal system are approved for all stages of Alzheimer’s disease, whereas oral rivastigmine, galantamine and tacrine (which is rarely used because of associated hepatotoxicity) are approved for mild to moderate Alzheimer’s disease, and a high-dose formulation of donepezil is approved for moderate to severe disease [37, 38]. Memantine ER and IR are approved for moderate to severe Alzheimer’s disease, either alone or in combination with a cholinesterase inhibitor [6, 13]. Also of note is that a fixed-dose combination of memantine ER plus donepezil (Namzaric™) recently received US FDA approval [39]. In general, the selection of drug therapy for patients with Alzheimer’s disease should be based on individualized assessment and take into consideration tolerability, ease of administration and cost, as the effectiveness of these drugs varies from person to person and there is no strong evidence favouring one agent over another in terms of efficacy [1, 36]. However, short-term trials in patients with moderate to severe Alzheimer’s disease generally indicate that combination therapy with memantine plus cholinesterase inhibitors provides greater benefit than cholinesterase inhibitors alone [40], as was demonstrated in the phase III trial with memantine ER (Sect. 4) [21].
In the large, multinational, phase III trial discussed in Sect. 4, the addition of memantine ER 28 mg once daily to ongoing cholinesterase inhibitor therapy in patients with moderate to severe Alzheimer’s disease was associated with modest but statistically significant improvements on measures of cognition (using the SIB instrument) and global clinical status (CIBIC-Plus), which were the co-primary endpoints of the study [21]. Add-on therapy with memantine ER was also statistically superior to placebo (i.e. cholinesterase monotherapy) on measures of behaviour (NPI) and verbal fluency (VFT). While these results reflect changes in scores from baseline to study endpoint (week 24) for the overall cohort (Sect. 4.1), a post hoc analysis showed treatment effects over the entire course of the trial (Sect. 4.2.3). Several other post hoc analyses provided additional information, such as detailed assessments of treatment effects on cognition, behaviour, function, and language and communication, as well as treatment response by disease severity (Sect. 4.2). These data, as well as a prospectively defined assessment of treatment efficacy in the subgroup of patients receiving donepezil (Sect. 4.3), support and expand upon the findings of the key phase III trial.
In the only cost-effectiveness analysis focusing on the newer formulation, the addition of memantine ER to cholinesterase inhibitor therapy dominated (i.e. was less costly and more effective than) cholinesterase inhibitor monotherapy in patients with moderate to severe Alzheimer’s disease (reported as an abstract/poster) [41]. The analysis modelled costs and outcomes over a 3-year time horizon using data from the 24-week phase III study (Sect. 4.1) and other clinical trial data, and was conducted from the healthcare payer and societal perspectives in the USA. Results were similar from both perspectives and were primarily driven by reductions in the need for institutional care in patients receiving add-on therapy with memantine ER.
Although the phase III trial with memantine ER as add-on therapy to cholinesterase inhibitors was relatively short (≈6 months) in duration [21], the long-term benefits of combination therapy with memantine IR and cholinesterase inhibitors have been shown in several observational studies in patients with probable Alzheimer’s disease [42–45]. For example, in one analysis (n = 382) with a mean follow-up of 30 months, combination therapy was associated with a slowing of cognitive and functional decline compared with cholinesterase monotherapy or no pharmacotherapy [43]. Another study (n = 943) showed that combination therapy extended the time to nursing home admission compared with cholinesterase monotherapy or no pharmacotherapy [44]. In addition, persistent treatment with cholinesterase inhibitors and/or memantine slowed clinical disease progression assessed by multiple cognitive, functional and global outcome measures in another analysis [45].
In conclusion, memantine has been used for more than a decade in the management of patients with moderate to severe Alzheimer’s disease, and memantine ER capsules (maintenance/maximum dosage 28 mg once daily) became available more recently in the USA for this indication. The efficacy and tolerability of memantine ER 28 mg once daily was demonstrated in a large, multicentre, phase III trial. It is unclear whether memantine ER 28 mg once daily, with its more convenient regimen and higher total daily dosage, provides clinical advantages over memantine IR 10 mg twice daily, as it has not been evaluated in a comparative clinical trial.
Data selection sources:
Relevant medical literature (including published and unpublished data) on memantine extended release was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) (searches last updated 15 Apr 2015), bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Memantine, Namenda, ER, extended release
Study selection: Studies in patients with Alzheimer’s disease who received memantine extended release. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. http://www.alz.org/downloads/Facts_Figures_2014.pdf. Accessed 25 Nov 2014.
Alzheimer’s Disease International. The global impact of dementia 2013–2050. http://www.alz.co.uk/research/GlobalImpactDementia2013.pdf. Accessed 25 Nov 2014.
Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369(24):2275–7.
Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6(4):37.
McKeage K. Memantine. CNS Drugs. 2009;23(10):881–97.
Namenda (memantine hydrochloride): US prescribing information. 2013. http://www.frx.com/pi/namenda_pi.pdf. Accessed 15 Apr 2015.
Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–41.
van Dyck CH, Tariot PN, Meyers B, et al. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21(2):136–43.
Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366(10):893–903.
Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–24.
Atri A, Molinuevo JL, Lemming O, et al. Memantine in patients with Alzheimer’s disease receiving donepezil: new analyses of efficacy and safety for combination therapy. Alzheimers Res Ther. 2013;5(1):6.
Hendrix S, Ellison N, Stanworth S, et al. Evidence for an additive effect of memantine and donepezil: consistent conclusions from DOMINO-AD study and memantine clinical trial program (poster). In: 7th clinical trials conference on Alzheimer’s disease; 2014.
Namenda XR (memantine hydrochloride) extended release capsules: US prescribing information. 2014. http://www.frx.com/pi/namendaxr_pi.pdf. Accessed 15 Apr 2015.
Lyseng-Williamson KA, McKeage K. Once-daily memantine: a guide to its use in moderate to severe Alzheimer’s disease in the EU. Drugs Aging. 2013;30(1):51–8.
Makino KM, Porsteinsson AP. Memantine: a treatment for Alzheimers disease with a new formulation. Aging Health. 2011;7(3):349–62.
Ong WY, Tanaka K, Dawe GS, et al. Slow excitotoxicity in Alzheimer’s disease. J Alzheimers Dis. 2013;35(4):643–68.
Gilling KE, Jatzke C, Parsons CG. Agonist concentration dependency of blocking kinetics but not equilibrium block of N-methyl-d-aspartate receptors by memantine. Neuropharmacology. 2007;53(3):415–20.
Volbracht C, van Beek J, Zhu C, et al. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006;23(10):2611–22.
Parsons CG, Danysz W, Dekundy A, et al. Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res. 2013;24(3):358–69.
Pope LE, Schoedel KA, Bartlett C, et al. A study of potential pharmacokinetic and pharmacodynamic interactions between dextromethorphan/quinidine and memantine in healthy volunteers. Clin Drug Investig. 2012;32(8):e1–15.
Grossberg GT, Manes F, Allegri RF, et al. The safety, tolerability, and efficacy of once-daily memantine (28 mg): a multinational, randomized, double-blind, placebo-controlled trial in patients with moderate-to-severe Alzheimer’s disease taking cholinesterase inhibitors. CNS Drugs. 2013;27(6):469–78.
Tocco M, Hendrix S, Miller M, et al. Clinical benefits of extended-release memantine (28 mg, once daily) as a function of disease severity in people with moderate to severe Alzheimer’s disease: post hoc analysis from a randomized trial (abstract no. P3-271 plus poster). Alzheimers Dement. 2013;9(4 Suppl.):P655.
Graham S, Hendrix S, Miller M, et al. Response across multiple outcome measures in a randomized trial of extended-release memantine (28 mg once daily) in patients with moderate-to-severe Alzheimer’s disease (abstract no. P3-386 plus poster). Alzheimers Dement. 2012;8(4 Suppl.):P591.
Atri A, Tocco M, Hendrix S, et al. Cumulative benefits of extended-release memantine (28 mg, once daily) across clinical domains in patients with moderate to severe alzheimer’s disease: an area under the curve analysis (abstract no. NR 16 plus poster). Am J Geriatr Psychiatry. 2014;22(3, Suppl.):S120–S1.
Tocco M, Hendrix S, Miller M, et al. Effects of extended-release memantine (28 mg, once daily) on cognitive domains in patients with moderate to severe Alzheimer’s disease: post hoc analysis of a randomized trial (abstract no. 26 plus poster). Consult Pharm. 2011;26(10):750.
Tocco M, Hendrix S, Miller ML, et al. Sustained cognitive improvement with extended-release memantine (28 mg, once daily) in moderate to severe Alzheimer’s disease (abstract no. M1116 plus poster). Ann Neurol. 2012;72(Suppl. 16):S42.
Graham SM, Hendrix S, Miller ML, et al. Extended-release memantine (28 mg, once daily) provides behavioral benefits across a wide range of disease severity in patients with moderate to severe Alzheimer’s disease: post hoc analysis from a randomized trial (abstract no. NR 16 plus poster). Am J Geriatr Psychiatry. 2013;21(3):S139.
Cummings J, Hendrix S, Miller M, et al. Extended-release memantine (28 mg, once daily) and sustained behavioral improvement: post hoc responder analysis from a randomized trial in patients with moderate to severe Alzheimer’s disease (abstract no. P04.197 plus poster). Neurology. 2012;78.
Tocco M, Hendrix S, Miller ML, et al. Effects of extended-release memantine (28 mg, once daily) on language and communication abilities in patients with moderate to severe Alzheimer’s disease (abstract no. M1156 plus poster). Ann Neurol. 2012;72(Suppl. 16):S52–3.
Graham SM, Hendrix S, Miller ML, et al. Efficacy of extended-release memantine (28 mg, once daily): post hoc responder analysis from a randomized trial in patients with moderate to severe Alzheimer’s disease (abstract no. 27 plus poster). Consult Pharm. 2011;26(10):750.
Tocco M, Hendrix S, Miller ML, et al. Effects of extended-release memantine (28 mg, once daily) on individual activities of daily living in patients with moderate to severe Alzheimer’s disease: post hoc factor analysis of a randomized trial (abstract no. 20 plus poster). Consult Pharm. 2011;26(10):747.
Grossberg G, Alva G, Hendrix S, et al. Efficacy and tolerability of memantine extended release added to stable donepezil regimen in individuals with moderate to severe Alzheimer’s disease: subset analysis of a randomized clinical trial (abstract no. P1-370 plus poster). Alzheimers Dement. 2014;10(4):P450.
Hendrix S, Ellison N, Pejovic V, et al. Adding memantine to cholinesterase inhibitor treatment stabilizes or improves low to moderate agitation in patients with moderate to severe Alzheimer’s disease: post hoc analysis of five randomized trials (poster no. LB 54). In: Annual meeting of the American Association for Geriatric Psychiatry. 2015.
Hendrix S, Ellison N, Pejovic V, et al. Adding memantine to stable cholinesterase inhibitor therapy in patients with moderate to severe Alzheimer’s disease is associated with improvement in various neuropsychiatric symptoms: a pooled analysis (poster no. LB 55). In: Annual meeting of the American Association for Geriatric Psychiatry. 2015.
Hendrix S, Ellison N, Pejovic V, et al. Effects of add-on memantine on daily functioning in patients with moderate to severe Alzheimer’s disease receiving stable donepezil treatment (poster). In: 12th international conference on Alzheimer’s and Parkinson’s Diseases. 2015.
Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148(5):370–8.
Singh I, Grossberg GT. High-dose donepezil or memantine: next step for Alzheimer’s disease? Current Psychiatry. 2012;11(6):20.
Exelon Patch (rivastigmine transdermal system): US prescribing information. 2015. http://www.pharma.us.novartis.com/product/pi/pdf/exelonpatch.pdf. Accessed 15 Apr 2015.
Actavis and Adamas announce FDA approval of Namzaric™, a fixed-dose combination of memantine extended-release and donepezil hydrochloride. 2014. http://www.prnewswire.com/news-releases/actavis-and-adamas-announce-fda-approval-of-namzaric-a-fixed-dose-combination-of-memantine-extended-release-and-donepezil-hydrochloride-300013883.html. Accessed 12 Jan 2015.
Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3):e000917.
Thibault CSL, Stillman IO, Chen S, et al. Cost-effectiveness of memantine extended release for treatment of moderate-to-severe Alzheimer’s disease in the United States (abstract no. P2-320 plus poster). Alzheimers Dement. 2014;10(4 Suppl.):P596–P7.
Zhu CW, Livote EE, Scarmeas N, et al. Long-term associations between cholinesterase inhibitors and memantine use and health outcomes among patients with Alzheimer’s disease. Alzheimers Dement. 2013;9(6):733–40.
Atri A, Shaughnessy LW, Locascio JJ, et al. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209–21.
Lopez OL, Becker JT, Wahed AS, et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80(6):600–7.
Rountree SD, Chan W, Pavlik VN, et al. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimers Res Ther. 2009;1(2):7.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes based on any comments received were made by the author on the basis of scientific and editorial merit. Greg Plosker is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: A. Atri, Memory Disorders Unit, Massachusetts General Hospital, Boston, MA, USA; S. Gauthier, McGill Center for Studies in Aging, Alzheimer Disease and Related Disorders Unit, Douglas Mental Health University Institute, Montreal, QC, Canada.
Rights and permissions
About this article
Cite this article
Plosker, G.L. Memantine Extended Release (28 mg Once Daily): A Review of Its Use in Alzheimer’s Disease. Drugs 75, 887–897 (2015). https://doi.org/10.1007/s40265-015-0400-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0400-3