Abstract
The objective of this meta-analysis was to evaluate the effects of coenzyme Q10 (CoQ10) for the treatment of Parkinson’s disease (PD) patients in order to arrive at qualitative and quantitative conclusions about the efficacy of CoQ10. Databases searched included PubMed, Google scholar, CNKI, Wan-Fang, and the Cochrane Library from inception to March 2016. We only included sham-controlled, randomized clinical trials of CoQ10 intervention for motor dysfunction in patients with PD. Relevant measures were extracted independently by two investigators. Weighted mean differences (WMD) were calculated with random-effects models. Eight studies with a total of 899 patients were included. Random-effects analysis revealed a pooled WMD of 1.02, indicating no significant difference when CoQ10 treatment compared with placebo in terms of UPDRS part 3 (p = 0.54). Meanwhile, the effect size of UPDRS part 1, UPDRS part 2, and total UPDRS scores were similar in CoQ10 group with in placebo group (p > 0.05). Moreover, we found CoQ10 was well tolerated compared with placebo group. Subgroup analysis showed that the effect size of CoQ10 in monocentric studies was larger than in multicenter studies. Using the GRADE criteria, we characterized the quality of evidence presented in this meta-analysis as moderate to high level. The current meta-analysis provided evidence that CoQ10 was safe and well tolerated in participants with PD and no superior to placebo in terms of motor symptoms. According to these results, we cannot recommend CoQ10 for the routine treatment of PD right now.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by a progressive loss of dopaminergic neurons within the substantia nigra pars compacta resulting in the loss of motor function, in nonmotor symptoms, and in cognitive decline [1]. It affects approximately 6 million people worldwide, and its prevalence is predicted to more than double by 2030 [2]. Although the pathogenesis of PD is not fully understood at present, the finding that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can cause parkinsonism through the inhibition of complex I in the mitochondrial electron transporter chain generated the idea that a disorder of the mitochondrial respiratory chain is involved in PD [3]. Moreover, previous studies had shown a 30–40% reduction of complex I activity in postmortem substantia nigra and in platelets from patients with PD [4].
Preclinical and clinical investigators are energetically seeking drugs that may delay or slow the progression of PD. Moreover, the discovery of several PD genes whose function is linked to mitochondrial function or oxidative stress, although not proof, strongly supports a pivotal role for mitochondrial involvement in PD-associated neurodegeneration disease [5]. Coenzyme Q10 (CoQ10) is the electron acceptor for complexes I and II, leads to decreased free radical generation and supposed a potent antioxidant [6]. What is more, CoQ10 has neuroprotective effects in multiple in vitro and animal models of neuronal toxicity. Oral supplementation with CoQ10 can reduce the loss of dopamine and dopaminergic axons in the striatum in 1-year-old mice treated with MPTP-induced mouse model of PD [7]. Experiments on monkeys also showed that 10 days of oral supplementation with CoQ10 prior to treatment with MPTP obviously alleviated the loss of nigral dopaminergic neurons [8].
Meanwhile, a recent clinical study showed that 300 mg/day of coenzyme CoQ10 could significantly improve PD symptoms compared with placebo, as judged by total Unified Parkinson’s Disease Rating Scale (UPDRS) scores and that CoQ10 was safe and well tolerated [9]. However, the Parkinson Study Group QE3 Investigators including 600 participants were randomly assigned to receive placebo, 1200 mg/day of CoQ10, or 2400 mg/day of CoQ10. The results indicated that CoQ10 was safe and well tolerated in this population, but showed no evidence of clinical benefit [10]. It was unclear whether CoQ10 could produce superior effects, as randomized controlled trials (RCTs) that have explored the relative efficacies of this compound and placebo have shown inconsistent results. One aforementioned meta-analysis published in 2011 concluded that CoQ10 therapy with 1200 mg/day for 16 months was well tolerated. The improvements in UPDRS part III and Schwab and England were positive. In terms of total and other subscores of UPDRS, the effects of CoQ10 seemed to be less clear [11]. But this conclusion was obtained based on the four RCTs. The statistical power was relatively low. Negida reported that CoQ10 supplementation did not slow functional decline nor provided any symptomatic benefit for patients with PD with only five RCTs [12]. Recently, several high-quality RCTs on CoQ10 in PD had been published [9, 10]. Accumulating studies investigating the effectiveness of CoQ10 have yielded mixed results. Thus, it is critical to integrate and arrange these findings to comprehensively evaluate the efficacy and safety of treatment with CoQ10 in PD patients in order to guide and normalize its use in clinical treatment.
Materials and methods
Search strategy
To identify studies for inclusion in this meta-analysis, relevant international databases (PubMed, Google scholar, and Cochrane Central Register of Controlled Trials) and two Chinese databases (CNKI and Wan-Fang database) were searched from inception up to March 2016, with different combinations of the following keywords: (CoQ10 or coenzyme Q10 or ubidecarenone or ubiquinone Q10 or Bio-Quinone Q10 or 2,3-dimethoxy-5-methyl-6-decaprenylbenzoquinone or ubisemiquinone radical or ubisemiquinone) AND (Parkinson or PD or Lewy body parkinson or primary parkinsonism or paralysis Agitans). In order to avoid omitting relevant clinical trials, we scanned conference summaries and reference lists of articles identified in the initial searches and contacted authors to obtain additional information for relevant trials.
Inclusion and exclusion criteria
Only those meeting the following criteria were selected for subsequent analysis:
-
1.
We included RCTs that compared CoQ10 to placebo.
-
2.
Subjects were required to have a clinical diagnosis of idiopathic PD, of either sex and with early and midstage PD according to the Hoehn and Yahr stage.
-
3.
CoQ10 alone or in combination with other treatments compared with placebo alone or in combination with same treatments.
-
4.
Effect size was assessed by the motor section of UPDRS (UPDRS part III) as the primary outcome measures; UPDRS subscores as the secondary outcomes.
Data extraction
Two reviewers independently checked all potentially suitable studies by the aforementioned inclusion criteria to perform data extraction. Any disagreements were resolved by discussion. Extracted data included first author, publication year, country, sample size, sample characteristics, study design, intervention characteristics of the trial groups (CoQ10 regimen), timing of outcome measurements (short-term < 12 months; or long-term ≥ 12 months), outcome indexs, changes in UPDRS score from baseline to final visit and adverse events. Results were summarized in a standard summary data sheet. If outcomes were presented from the studies at different time points, we extracted data from the latest time point. For data that could not be directly obtained, good faith efforts were applied to obtain the missing data by dispatching e-mails to the author and researching other studies citing the trial in question.
Risk of bias
The risk of bias of individual RCTs was assessed independently using the Cochrane Handbook for Systematic Reviews of Interventions [13]. The Cochrane tool classifies studies as having low, high, or unclear risk of bias in the following domains: sequence generation, allocation concealment, blinding, missing data, selective reporting, and other biases. Meanwhile, we used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to assess the quality of evidence for core comparisons [14, 15].
Statistical analysis
We considered the main outcome measures as continuous data and then used weighted mean difference (WMD) to express the size of the CoQ10 effect on motor symptoms measured with the UPDRS part III and other UPDRS subscores. A random-effects model was used to calculate pooled effect sizes because it took into account the fact that the true treatment effects had likely varied between the included RCTs and test whether the mean effect size was significantly different from zero. Heterogeneity of effects was evaluated using the Q statistic; the I 2 index was used to estimate the percentage of variation across studies due to heterogeneity rather than chance. Meanwhile, we used a funnel plot to assess the presence of publication bias. When possible, stratified analyses were conducted to examine differences by dosage (≥ 600mg/d vs < 600mg/d).
Results
Study characteristics
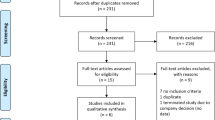
Our initial search of all databases retrieved 456 studies, yet many of these were identified as duplicates. Ultimately, eight eligible studies that met the inclusion criteria were evaluated in this meta-analysis [9, 10, 16–21] (Fig. 1). The basic characteristics of selected studies were listed in Table 1. Eight eligible studies that included 899 participants were included in this review and were conducted in US (3/8, 37.5%), Germany (2/8, 25.0%), China (2/8, 25.0%), and Japan (1/8, 12.5%), respectively, of whom, 453 were randomized to CoQ10 groups (62.03% males), and 446 were randomized to placebo groups (63.4% males). The mean age of those included studies was relatively young at about 63.29 years (CoQ10 63.21 years; placebo 63.37 years). The number of participants randomized into the eight studies included in this meta-analysis ranged from 28 to 399 participants. Among them, four studies were multicenter; the remaining four studies were monocentric. The dosage of CoQ10 was range from 300 to 2400 mg/day. Meanwhile, UPDRS part 3 as the primary outcome was observed in seven studies, UPDRS part 1 and UPDRS part 2 were observed in three and four studies, respectively. Finally, total UPDRS and adverse events were reported in five and three studies, separately. Table 2 showed changes from baseline to each end visit in the primary and secondary outcome measures, including UPDRS part 3, UPDRS part 1, UPDRS part 2, and total UPDRS.
Risk of bias
The quality score of studies were ranged from two to six out of a total six points, of which one study got two points; one study got three points; two studies got four points; three studies got five points; and one study got six points (Table 3). All studies described the method of random sequences generation. Six studies gave information that allowed the assessment of whether an adequate concealment of allocation procedure was used. Only two studies reported the blinding of participants and six studies described intention-to-treat analyses (ITT) in this review. Four studies existed other sources of bias. Overall, all of the included studies were deemed to have a low to moderate risk of bias.
Meta-analysis
For UPDRS part 3, there were 809 participants included in the analysis, we pooled the whole data to process and found no significant difference when CoQ10 treatment compared with placebo (n = 809, WMD 1.02, 95% CI −2.21 to 4.24, p = 0.54 > 0.05, Fig. 2). Meanwhile, there was obvious heterogeneity for the analysis of UPDRS part 3 between studies (Tau2 = 17.36, Chi2 = 1246.91, p < 0.00001, I 2 = 100%, Fig. 2). After sequentially excluding each study, the results of UPDRS part 3 and heterogeneity were consistent (I 2 range from 76 to 100%; WMD 0–1.46). Data on the mental-rated UPDRS part 1 score were available from three studies with 580 participants included. We used a random effects model as well as the three independent studies that showed severe heterogeneity in the consistency of the trial results (Tau2 = 0.08, Chi2 = 6.88, p = 0.03, I 2 = 71%, Fig. 3a). Meanwhile, we found no significant difference between CoQ10 and placebo (WMD 0.02, 95% CI −0.36 to 0.40, p = 0.93 > 0.05, Fig. 3a). Moreover, there were four studies that compared CoQ10 with placebo which were included in the efficacy analysis; the effect size of UPDRS part 2 score is similar in CoQ10 group than in placebo group (n = 613, WMD −0.10, 95% CI −1.09 to 0.89, p = 0.84 > 0.05; Heterogeneity test: Tau2 = 0.71, Chi2 = 13.19, p = 0.004, I 2 = 77%, Fig. 3b). Finally, five included studies measured the changes in total UPDRS score, with CoQ10 from 300 to 2400 mg/day. However, there were no differences between coenzyme Q10 at any dose and placebo (n = 641, WMD 0.78, 95% CI −0.84 to 2.40, p = 0.34 > 0.05, Fig. 3c). There was moderate heterogeneity between studies (Tau2 = 1.63, Chi2 = 9.52, p = 0.05, I 2 = 58%, Fig. 3c). Since all the analysis showed moderate to severe heterogeneity, we should interpret results with caution. Because of the small number of the studies, we combined the four outcomes to test publication bias. The funnel plot was almost symmetric for the effects of CoQ10 on UPDRS score, which did not suggest any obvious publication bias (Fig. 4). In the subgroup analysis for the outcome measure according to UPDRS part 3, we also found no significant statistical difference between high doses CoQ10 (≥ 600 mg) compared with placebo (WMD 2.27, 95% CI: −3.43 to 7.97, P = 0.44 > 0.05, Fig. 5). Undoubtedly, low doses CoQ10 (< 600 mg) is not effective in PD compared with placebo (WMD −0.37, 95% CI: −0.83 to 0.09, P = 0.11 > 0.05, Fig. 5).
Adverse events
Adverse events that occurred in the three studies are summarized in Table 4. There was no significant difference of adverse events between the CoQ10 and placebo (p > 0.05, Table 4). All in all, we found CoQ10 was well tolerated compared with placebo group. The most commonly occurring adverse events across the three studies were pain (Back pain or Joint pain; 9.6 vs 11.0%, p = 0.70), infection (mainly upper respiratory tract infection and Urinary tract infection; 7.6 vs 7.9%, p = 0.98), anxiety (7.9 vs 7.2%, p = 0.80), headache(6.6 vs 7.6%, p = 0.69), depression (4.1 vs 5.8%, p = 0.31), nausea (6.9 vs 5.8%, p = 0.53), diarrhea (5.2 vs 4.5%, p = 0.61), nasopharyngitis (4.1 vs 4.5%, p = 0.94), dizziness (4.5 vs 4.5%, p = 0.93), insomnia (6.2 vs 4.1%, p = 0.45), tremor (5.8 vs 3.8%, p = 0.33), and constipation (4.5 vs 3.1%, p = 0.49) and so on.
Quality of the evidence
Using the GRADE criteria, we characterized the quality of evidence presented in this meta-analysis as moderate to high level. Meanwhile, GRADE judgments for all core comparisons of the primary and secondary outcome can be found in Table 5.
Discussion
As the second most common neurodegenerative disease after Alzheimer’s disease, PD is a major global economic burden that will be increasing with the ‘aging’ of our society [22]. Previous meta-analysis had concluded that CoQ10 therapy with 1200 mg/day was well tolerated by patients with PD. Meanwhile, there were improvements in activities of daily living (ADL) UPDRS and Schwab and England for coenzyme CoQ10 at 1200 mg/day for 16 months versus placebo. For total and other subscores of UPDRS, the effects of CoQ10 seemed to be less clear [11]. However, in the present meta-analysis, our study further confirmed that there was no significant difference between CoQ10 and placebo on the UPDRS part 3 scores (WMD 1.02, p = 0.54 > 0.05). What is more, our results demonstrated that CoQ10 has the similar efficacy on the UPDRS part 1 (WMD 0.02, p = 0.93 > 0.05), UPDRS part 2 (WMD −0.10, p = 0.84 > 0.05), and total UPDRS (WMD 0.78, p = 0.34 > 0.05) scores when compared with placebo groups. In safety outcomes, three studies evaluated the incidence of adverse events and, of these, CoQ10 was well tolerated and there was no significant difference of adverse events between the CoQ10 and placebo. Finally, using the GRADE system to test, we supposed the quality of evidence of our meta-analysis as moderate to high.
Over the past decade, interest in the roles of nutritional supplements in neurodegenerative disease had intensified. One of these supplements, CoQ10, was an essential cofactor involved in mitochondrial oxidative phosphorylation as well as a potent antioxidant [7]. Strong evidence had now emerged supporting the role of oxidative stress and defective energy metabolism in the pathogenesis of PD [23]. Consequently, there was a robust scientific rationale for testing this compound as a potential neuroprotective therapy. Studies of in vitro and animal models of PD had demonstrated potential neuroprotective effects of CoQ10 [24]. With this data in mind, several clinical trials of CoQ10 have been performed in PD patients. Meanwhile, CoQ10 is widely available in multiple formulations and is very well tolerated with minimum adverse events, making it an attractive potential therapy [9, 10, 18]. However, in the present meta-analysis, neither of the active treatment groups even from high dosages of CoQ10 (>1000 mg/day) showed any benefit in terms of UPDRS score compared with the placebo group. It is possible that mitochondrial oxidative damage may be a consequence of other pathological processes rather than the primary cause of PD as well as the multi-mechanism involved in PD and, therefore, that targeting this pathway would not be expected to provide benefit in PD. In addition, how to achieve optimizing administration remains to be understood due to that different form of CoQ10 demonstrated different blood levels [25]. In addition, major studies included early PD patients in this review, the levels of oxidative stress may not be as high in early stage of PD, and CoQ10 treatment may not show the same benefits as that during advanced PD [26]. This maybe the underlying reason contributes to the discrepancy between this meta and preclinical researches.
This article updates preceding meta-analyses with the inclusion of new RCTs. However, in this meta-analysis, our study does not support the hypothesis that restoring the impaired energy metabolism of the diseased dopaminergic neurons leads to symptomatic benefits in PD. In addition, future research should also attempt to establish a more precise relationship between CoQ10 effect and patients’ clinical and demographic characteristics, such as medication use, stage of disease, side of onset, and dominant motor symptoms. Overall, in view of these results, we cannot recommend CoQ10 for the routine treatment of PD patients right now.
Regarding the limitations of the present meta-analysis, a number of weaknesses of this study should be considered. First, this meta-analysis only included eight studies and, therefore, type-II errors due to chance cannot be entirely ruled out as an alternative explanation for our main findings [27]. Meanwhile, in most analyses a significant level of heterogeneity has been observed, but the low number of studies does not enable meta-regression analyses [28]. Second, we acknowledge that our search strategy which is likely to include studies in English and Chinese database due to the language barrier, nevertheless, takes no account of other languages that may lead to certain degree selective bias [29]. Finally, few participants were younger than 60 years at this meta-analysis. Therefore, we can not offer definite age-specific treatment recommendations according to these results. Nevertheless, our meta-analysis also had possessed some advantages. First, although the number of RCTs on the efficacy of CoQ10 in PD is small, the total sample size is relatively large, confirming the efficacy of the technique in the control of motor signs, as well as all of the included trials were well designed and deemed to have a low risk of bias and provided hopefully evidences. Second, the long follow-up period between initiation and availability of the results provided the comprehensive information.
Conclusions
The current meta-analysis provided evidence that CoQ10 was safe and well tolerated in participants with PD and no superior to placebo in terms of motor symptoms. According to these results, we cannot recommend CoQ10 for the routine treatment of PD right now.
References
Klingelhoefer L, Reichmann H (2015) Pathogenesis of Parkinson disease-the gut-brain axis and environmental factors. Nat Rev Neurol 11(11):625–636
Dorsey ER, Constantinescu R, Thompson JP et al (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68(5):384–386
Przedborski S, Tieu K, Perier C et al (2004) MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J Bioenerg Biomembr 36(4):375–379
Schapira AH, Mann VM, Cooper JM et al (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55(6):2142–2145
Burchell VS, Gandhi S, Deas E et al (2010) Targeting mitochondrial dysfunction in neurodegenerative disease: part I. Expert Opin Ther Targets 14(4):369–385
Shults CW (2005) Therapeutic role of coenzyme Q(10) in Parkinson’s disease. Pharmacol Ther 107(1):120–130
Spindler M, Beal MF, Henchcliffe C (2009) Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatric Dis Treat 5:597–610
Horvath TL, Diano S, Leranth C et al (2003) Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson’s disease. Endocrinology 144(7):2757–2760
Yoritaka A, Kawajiri S, Yamamoto Y et al (2015) Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson’s disease. Parkinsonism Relat Disord 21(8):911–916
Beal MF, Oakes D, Shoulson I et al (2003) A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol 71(5):543–552
Liu J, Wang L, Zhan SY et al (2011) Coenzyme Q10 for Parkinson’s disease. Cochrane Database Syst Rev (12):Cd008150. doi:10.1002/14651858
Negida A, Menshawy A, El Ashal G et al (2016) Coenzyme Q10 for patients with Parkinson’s disease: a systematic review and meta-analysis. CNS Neurol Disord: Drug Targets 15(1):45–53
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 343:d5928
Atkins D, Best D, Briss PA et al (2004) Grading quality of evidence and strength of recommendations. BMJ (Clinical research ed) 328(7454):1490
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Jie Z (2014) Clinical effects and safety of coenzyme Q10 in Parkinson disease. China Foreign Med Treat 23:79–80
Wang XY, Yang ZM, Zhang XJ, et al. (2014) Clinical observation of coenzyme Q10 in Parkinson disease. HeBei J TCM 36:151–153
Storch A, Jost WH, Vieregge P et al (2007) Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol 64(7):938–944
NINDS NET-PD Investigators (2007) A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology 68(1):20–28
Muller T, Buttner T, Gholipour AF et al (2003) Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci Lett 341(3):201–204
Shults CW, Oakes D, Kieburtz K et al (2002) Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 59(10):1541–1550
Akushevich I, Kravchenko J, Ukraintseva S et al (2012) Age patterns of incidence of geriatric disease in the U.S. elderly population: medicare-based analysis. J Am Geriatr Soc 60(2):323–327
Choi H, Park HH, Koh SH et al (2012) Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13 K pathway. Neurotoxicology 33(1):85–90
Sikorska M, Lanthier P, Miller H et al (2012) Nanomicellar formulation of coenzyme Q10 (Ubisol-Q10) effectively blocks ongoing neurodegeneration in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model: potential use as an adjuvant treatment in Parkinson’s disease. Neurobiol Aging 35(10):2329–2346
Seet RC, Lim EC, Tan JJ et al (2014) Does high-dose coenzyme Q10 improve oxidative damage and clinical outcomes in Parkinson’s disease? Antioxid Redox Signal 21(2):211–217
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinson’s Dis 3(4):461–491
Thorlund K, Imberger G, Walsh M et al (2011) The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis–a simulation study. PLoS One 6(10):e25491
Dias S, Sutton AJ, Welton NJ et al (2013) Evidence synthesis for decision making 3: heterogeneity–subgroups, meta-regression, bias, and bias-adjustment. Med Decis Mak 33(5):618–640
Guyatt GH, Oxman AD, Montori V et al (2011) GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol 64(12):1277–1282
Acknowledgements
We gratefully acknowledge Prof Jie Chen from Wenzhou Medical University for his help in guiding and revising the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The study was supported by the Projects of National Science Foundation of China (No. 81600977).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Zhen-Guo Zhu and Miao-Xuan Sun have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, ZG., Sun, MX., Zhang, WL. et al. The efficacy and safety of coenzyme Q10 in Parkinson’s disease: a meta-analysis of randomized controlled trials. Neurol Sci 38, 215–224 (2017). https://doi.org/10.1007/s10072-016-2757-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-016-2757-9