Abstract
Background
Pemetrexed is used for the treatment for non-small cell lung cancer and mesothelioma. Patients with renal impairment are withheld treatment with this drug as it is unknown what dose is well tolerated in this population.
Objective
The purpose of our study was to investigate the pharmacokinetics (PK) of pemetrexed in patients with renal impairment.
Methods
A population PK analysis of pemetrexed was performed using non-linear mixed-effects modelling with phase I data obtained from the manufacturer. Additionally, the impact of renal function on pemetrexed PK was assessed with a simulation study using the developed PK model and a previously developed PK model lacking the phase I data.
Results
The dataset included 548 paired observations of 47 patients, with a wide range of estimated glomerular filtration rates (eGFR; 14.4–145.6 mL/min). Pemetrexed PK were best described by a three-compartment model with eGFR (calculated using the Chronic Kidney Disease–Epidemiology Collaboration [CKD-EPI] formula) as a linear covariate on renal pemetrexed clearance. Using the developed model, we found that renal clearance accounts for up to 84% (95% confidence interval 69–98%) of total pemetrexed clearance, whereas the manufacturer previously reported a 50% contribution of renal clearance.
Conclusion
Renal function is more important for the clearance of pemetrexed than previously thought and this should be taken into account in patients with renal impairment. Furthermore, a third compartment may contribute to prolonged exposure to pemetrexed during drug washout.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Understanding the pharmacokinetics of pemetrexed is essential to enable treatment in patients with impaired renal function. |
This population pharmacokinetic analysis of pemetrexed included patients with renal impairment. |
The contribution of renal function to systemic pemetrexed clearance is higher than previously thought. |
Renal impairment can lead to prolonged and possibly toxic exposure to pemetrexed. |
1 Introduction
Pemetrexed is an antifolate drug used for the chemotherapeutic treatment of non-small cell lung cancer (NSCLC), mesothelioma and thymoma [1,2,3]. A single intravenous dose of 500 mg/m2 is administered every 21 days. Although pemetrexed is excreted in the urine [4], dosing recommendations do not include adjustment for renal function. Due to fatal toxicities in a study of pemetrexed in patients with renal impairment, pemetrexed is currently contraindicated when the estimated creatinine clearance (CRCL) is < 45 mL/min [5].
Approximately 25% of the lung cancer population has a CRCL <60 mL/min [6]. Since it is unclear what the well tolerated pemetrexed dose is for patients with impaired renal function, a large group is withheld effective treatment. Understanding the relationship between dose, renal function, pharmacokinetics (PK), toxicity and treatment outcome is essential to enable treatment in patients with impaired renal function and to prevent toxicity in patients who are already treated with pemetrexed.
Existing data on the effect of renal dysfunction on pemetrexed PK are conflicting. In phase I studies, the manufacturer showed that 70–90% of the pemetrexed dose is excreted in the urine as unchanged drug within 24 h after administration, through both tubular secretion and glomerular filtration [4, 5]. However, a large population PK study by the manufacturer, published by Latz et al. in 2006, in which the PK data of 10 phase II trials were pooled for analysis, showed that renal elimination contributed only approximately 50% to the clearance of pemetrexed [7]. Notably, this study and other more recent pemetrexed PK studies excluded patients with moderate to severe renal dysfunction (CRCL <45 mL/min) [8, 9]. Thus, extrapolation of these studies to patients with impaired renal function can be questioned. Therefore, the purpose of our study was to investigate the PK of pemetrexed in patients with renal impairment.
2 Methods
2.1 Data
Rich anonymised PK data collected during the renal impairment study by the manufacturer and as described by Mita et al. [5], were obtained from the manufacturer through the Clinical Study Data Request (CSDR) platform [10]. The following patient demographics were collected for each individual: sex, age, ethnicity, weight, height and serum creatinine. Furthermore, data on pemetrexed dose, infusion rate, sampling times and pemetrexed plasma concentrations were extracted from the dataset. Patients included in the study were not allowed to use aspirin or other non-steroidal anti-inflammatory agents from 2 days before (5 days for longer-acting agents) until 2 days after pemetrexed treatment due to a possible PK interaction.
2.2 Population Pharmacokinetic Modelling
A population PK analysis was performed using the non-linear mixed-effect modelling software package NONMEM V7.4 (Icon plc, Dublin, Ireland). The following proxies for renal function were tested as continuous covariates for pemetrexed clearance: estimated CRCL (calculated using the Cockcroft–Gault formula [11]) and estimated glomerular filtration rates (eGFR; calculated using the Modification of Diet in Renal Disease [MDRD] [12] and Chronic Kidney Disease–Epidemiology Collaboration [CKD-EPI] [13] formulae). MDRD and CKD-EPI were used as absolute values, and thus uncorrected for body surface area (BSA). When including renal function as a covariate for clearance, we estimated both the non-renal contribution to clearance (CLNR) and the renal clearance (CLR). The renal function estimate (CRCL or eGFR) that resulted in the best model fit (decrease in objective function value [OFV]) and largest decrease in interindividual variability (IIV) was retained in the final model. Model selection and diagnostics were performed in line with best practice [14]. A detailed description of the PK analysis can be found in the electronic supplementary material.
2.3 Assessment of the Impact of Renal Function on Pemetrexed Pharmacokinetics
After model development, we compared the manufacturer’s model (published by Latz et al. [7]) and the model developed herein, on several aspects. First, we assessed the difference in exposure, using the target area under the concentration-time curve from the start of infusion until infinity (AUC). In a virtual study, a cohort of 1000 patients was simulated with NONMEM V7.4 using Monte Carlo simulations. Age, sex, height and weight were extracted from the National Health and Nutrition Examination Survey (NHANES) database [15], and serum creatinine was randomly drawn from a normal distribution based on a median (male 110 µmol/L, female 95 µmol/L) with 25% variability (based on clinical data). These variables were used to calculate CRCL. Dosing was based on BSA according to the drug label (500 mg/m2). Subsequently, pemetrexed exposure (AUC) was simulated for these individuals using the manufacturer’s PK model and the model developed herein. This was performed for a population with a CRCL ≥45 mL/min and a population with a CRCL < 45 mL/min. To compare exposure between these groups, the geometric means of the AUCs with the coefficient of variation were calculated.
Second, the disposition of pemetrexed was investigated visually. We simulated one PK curve up to 96 h after administration, with both models using a systemic pemetrexed clearance of 3 L/h for a typical individual with impaired renal function (age 40 years, height 180 cm, weight 70 kg, BSA 1.85 m2). A 3 L/h clearance was chosen as it represents a typical individual with decreased pemetrexed clearance, for example due to renal impairment.
3 Results
3.1 Dataset Characteristics
The final dataset consisted of 47 patients with a total of 548 paired observations of time and plasma concentrations over a time window of 0–72 h after administration. Table 1 shows the baseline characteristics of the population. Approximately three-quarters of the population were male and the median age was 62 years (range 25–79), with a wide range in eGFR (14.4–145.6 mL/min, calculated using the CKD-EPI).
3.2 Population Pharmacokinetic Model
The PK data were best described by a three-compartment model. Inclusion of renal function as a covariate for clearance of pemetrexed resulted in significant improvement of the model (p < 0.0001). Inclusion of the eGFR calculated using the CKD-EPI formula [13] explained approximately 45% of the IIV in clearance (reduction from 38.7 to 21.0% IIV in clearance). Of the three tested renal function formulae, CKD-EPI best explained the observed IIV in clearance. Typical population values for CLR and CLNR (with 95% confidence intervals) were 3.42 L/h (2.80–3.99) and 0.66 L/h (0.24–1.13). For central volume of distribution (V1) and peripheral volume of distribution (V2 and V3), typical values were 6.70 L (5.93–7.53), 8.01 L (7.20–8.95) and 1.23 L (1.02–1.55), respectively. The detailed results of the base model and covariate models are described in the electronic supplementary material.
3.3 Effect of Renal Function on Pemetrexed Pharmacokinetics
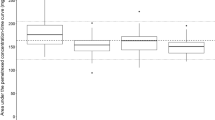
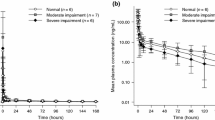
The box and whiskers plot in Fig. 1 depicts the predicted exposure according to both models (manufacturer’s model and the present model), for two separate groups: CRCL <45 mL/min and CRCL ≥ 45 mL/min. In their study, Latz et al. concluded that renal elimination contributed to the clearance of pemetrexed by approximately 50%. This is reflected in Fig. 1, where it can be observed that exposure seems to be in the same order of magnitude regardless of renal function, with moderate variability (white bars). With our model, developed using the data of patients with a wide range of renal function, a major impact of renal function on pemetrexed exposure can be observed from both the increased variability in AUC as well as the increased exposure in the renal impairment group. We predict that pemetrexed exposure in patients with renal impairment is approximately 1.7-fold higher than previously postulated by the manufacturer (see Table 2). Figure 2 shows two simulated PK curves, one for each model, using the same systemic clearance of pemetrexed of 3 L/h. For the present model, the impact of the presence of a third compartment on the concentration-time curve can be observed, resulting in prolonged higher exposure at approximately 48 h after drug administration and onwards.
Box and whiskers plot for simulation exposure using both the present model and the manufacturer’s model, separated for two categories of renal function (CRCL of <45 and ≥45 mL/min, respectively). The box represents the 25th–75th percentiles with geometric mean, and the whiskers indicate the 2.5th–97.5th percentile. The dots represent the outliers. CRCL creatinine clearance
Two concentration-time curves of pemetrexed using both models with the same systemic pemetrexed clearance of 3 L/h, to demonstrate the difference in distribution compartments. The black line represents the curve of the typical individual simulated using the manufacturer’s model, and shows two compartments, while the light-grey line represents the present model, identifying the third compartment >24 h. AUC area under the concentration-time curve
4 Discussion
This thorough PK analysis of pemetrexed in a population that included patients with impaired renal function, led to two major findings. First, we found that renal function is more important for pemetrexed clearance than the 50% contribution previously described by the manufacturer [16]. The manufacturer’s analysis did not include patients with impaired renal function (CRCL < 45 mL/min). We showed, in a representative population, that CLR accounts for up to 84% of total pemetrexed clearance, which is in line with the manufacturer’s early mass balance studies showing that 70–90% of the administered dose could be recovered in urine [4]. Thus, impaired renal function has a more pronounced impact on pemetrexed exposure than previously thought. Our second important finding relates to the disposition of pemetrexed. To date, it has been found that pemetrexed distributes over two compartments [7,8,9]. The data used in this analysis included sampling up to 72 h after administration and this revealed the presence of a third compartment. A third compartment can suggest the presence of extravascular fluid or a difference in redistributing tissues. It is unknown what holds true for pemetrexed, but as this is a hydrophilic drug, extravascular distribution is plausible. However, Dickgreber et al. showed no effect of third space fluid on the PK and toxicity of pemetrexed [17]. This implicates that during drug washout, there can be prolonged exposure to higher concentrations of pemetrexed than previously thought. The driving mechanism for pemetrexed toxicity is the subject of discussion. Mita et al. showed no correlation between renal function (and thus exposure) and non-haematological toxicities [5]. With regard to haematotoxicity, it is hypothesized that neutropenia is associated with the total exposure (AUC) [18, 19]. Based on this, it has been suggested that the dose should be adjusted to reach a target based on renal function, instead of BSA [7, 19, 20]. AUC-based dosing in a 21-day cycle is also routinely applied for carboplatin, where CRCL and the desired AUC are used to calculate the patient’s individual dose [21]. It is unknown whether this hypothesis holds true for pemetrexed, as it is known that for other antifolate drugs, such as methotrexate, haematological toxicity is threshold-driven [22]. For example, in an early phase I trial, it was shown that prolonged exposure to low concentrations of pemetrexed from daily administration resulted in severe neutropenia as a dose-limiting toxicity, with a maximum tolerated dose of only 4 mg/m2 for 5 consecutive days without supplementation of vitamin B12 or folic acid [23]. The predominant role of time above the threshold concentration in determining toxicity is supported by the observation that the maximum tolerated dose of pemetrexed administered in a 21-day cycle, also without vitamin supplementation, was markedly higher (600 mg/m2) [4]. Threshold-driven toxicity will be an issue, particularly in renal impairment, as clearance becomes so low that the pemetrexed plasma concentration exceeds the toxicity threshold for a prolonged period. This would explain why previous studies with pemetrexed in patients with renal impairment [5] were not successful. Currently, the PK determinant for the efficacy of pemetrexed is a topic of discussion. Dose adjustment to reach an AUC target will probably entail toxicity concerns when there is low systemic clearance, for example due to renal dysfunction. To allow safe and effective treatment in renal impairment, innovative interventions are needed to overcome toxicity. For example, rescue therapy with folinic acid, as widely applied with pemetrexed’s structural analogue methotrexate [24], may be a feasible option.
A limitation of this study is that the number of patients with severe renal impairment (eGFR <30 mL/min) was limited due to the toxicity concerns that arose during the conduction of the phase I study. Nonetheless, as it stands, these data are the only currently available data to elucidate the clinical PK of pemetrexed in patients with impaired renal function.
5 Conclusion
Overall, we found that the contribution of renal function was greater than previously thought and that a third compartment may contribute to prolonged exposure during drug washout. Since both factors may contribute to pemetrexed toxicity, they should be accounted for when developing dosing strategies for pemetrexed in patients with renal impairment.
The present PK model can be used to further unravel the PK–toxicity relationship of pemetrexed. In parallel, we must think of innovative strategies to overcome the haematological toxicity of pemetrexed in patients with impaired renal function, such as rescue therapy with folinic acid.
References
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S, Committee EG. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–237.
Baas P, Fennell D, Kerr KM, Van Schil PE, Haas RL, Peters S, Committee EG. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v31–9.
Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v40–55.
Rinaldi DA, Kuhn JG, Burris HA, Dorr FA, Rodriguez G, Eckhardt SG, et al. A phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol. 1999;44(5):372–80.
Mita AC, Sweeney CJ, Baker SD, Goetz A, Hammond LA, Patnaik A, et al. Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol. 2006;24(4):552–62.
Launay-Vacher V, Etessami R, Janus N, Spano JP, Ray-Coquard I, Oudard S, Gligorov J, Pourrat X, Beuzeboc P, Deray G, Morere JF, Renal Insufficiency Anticancer Medications Study G. Lung cancer and renal insufficiency: prevalence and anticancer drug issues. Lung. 2009;187(1):69–74.
Latz JE, Chaudhary A, Ghosh A, Johnson RD. Population pharmacokinetic analysis of ten phase II clinical trials of pemetrexed in cancer patients. Cancer Chemother Pharmacol. 2006;57(4):401–11.
Visser S, Koolen SLW, de Bruijn P, Belderbos HNA, Cornelissen R, Mathijssen RHJ, et al. Pemetrexed exposure predicts toxicity in advanced non-small-cell lung cancer: A PROSPECTIVE cohort study. Eur J Cancer. 2019;121:64–73.
Srinivasan M, Chaturvedula A, Fossler MJ, Patil A, Gota V, Prabhash K. Population pharmacokinetics of pemetrexed in adult non-small cell lung cancer in Indian patients. J Clin Pharmacol. 2019;59(9):1216–24.
Clinical Study Data Request. https://www.clinicalstudydatarequest.com/Posting.aspx?ID=19619&GroupID=SUMMARIES. Accessed 20 Nov 19.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology C. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–72.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Byon W, Smith MK, Chan P, Tortorici MA, Riley S, Dai H, et al. Establishing best practices and guidance in population modeling: an experience with an internal population pharmacokinetic analysis guidance. CPT Pharmacometr Syst Pharmacol. 2013;2:e51.
Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) [Nov 2019]. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed 6 Nov 2019.
Latz JE, Schneck KL, Nakagawa K, Miller MA, Takimoto CH. Population pharmacokinetic/pharmacodynamic analyses of pemetrexed and neutropenia: effect of vitamin supplementation and differences between Japanese and Western patients. Clin Cancer Res. 2009;15(1):346–54.
Dickgreber NJ, Sorensen JB, Paz-Ares LG, Kjestrup Schytte T, Latz JE, Schneck KB, et al. Pemetrexed safety and pharmacokinetics in patients with third-space fluid. Clin Cancer Res. 2010;16(10):2872–80.
Latz JE, Karlsson MO, Rusthoven JJ, Ghosh A, Johnson RD. A semimechanistic-physiologic population pharmacokinetic/pharmacodynamic model for neutropenia following pemetrexed therapy. Cancer Chemother Pharmacol. 2006;57(4):412–26.
Latz JE, Rusthoven JJ, Karlsson MO, Ghosh A, Johnson RD. Clinical application of a semimechanistic-physiologic population PK/PD model for neutropenia following pemetrexed therapy. Cancer Chemother Pharmacol. 2006;57(4):427–35.
de Rouw N, Croes S, Posthuma R, Agterhuis DE, Schoenmaekers JJAO, Derijks HJ, et al. Pharmacokinetically-guided dosing of pemetrexed in a patient with renal impairment and a patient requiring hemodialysis. Lung Cancer. 2019;130(April):156–8.
Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7(11):1748–56.
Chabner BA, Young RC. Threshold methotrexate concentration for in vivo inhibition of DNA synthesis in normal and tumorous target tissues. J Clin Invest. 1973;52(8):1804–11.
McDonald AC, Vasey PA, Adams L, Walling J, Woodworth JR, Abrahams T, et al. A phase I and pharmacokinetic study of LY231514, the multitargeted antifolate. Clin Cancer Res. 1998;4(3):605–10.
Bleyer WA. The clinical pharmacology of methotrexate: new applications of an old drug. Cancer. 1978;41(1):36–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflicts of interests
Nikki de Rouw, Rene J. Boosman, Alwin D.R. Huitema, Luuk B. Hilbrands, Elin M. Svensson, Hieronymus J. Derijks, Michel M. van den Heuvel, David M. Burger, and Rob ter Heine have no conflicts of interest to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Manufacturer’s consent for publication.
Availability of data and material
Not applicable.
Code availability
Supplementary file.
Authors’ contributions
Study concepts: NR, RJB, ADRH, LBH, EMS, HJD, MMH, DMB, RH. Study design: NR, RJB, ADRH, RH. Data acquisition and control: NR, RJB, RH. Data analysis and interpretation and statistical analysis: NR, RH. Manuscript preparations: NR, RH. Manuscript editing and revisions: NR, RJB, ADRH, LBH, EMS, HJD, MMH, DMB, RH.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
de Rouw, N., Boosman, R.J., Huitema, A.D.R. et al. Rethinking the Application of Pemetrexed for Patients with Renal Impairment: A Pharmacokinetic Analysis. Clin Pharmacokinet 60, 649–654 (2021). https://doi.org/10.1007/s40262-020-00972-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00972-1