Abstract
Purpose: The objectives of these analyses were to (1) develop a semimechanistic-physiologic population pharmacokinetic/pharmacodynamic (PK/PD) model to describe neutropenic response to pemetrexed and to (2) identify influential covariates with respect to pharmacodynamic response. Patients and methods: Data from 279 patients who received 1,136 treatment cycles without folic acid or vitamin B12 supplementation during participation in one of eight phase II cancer trials were available for analysis. Starting doses were 500 or 600 mg pemetrexed per m2 body surface area (BSA), administered as 10-min intravenous infusions every 21 days (1 cycle). The primary analyses included 105 patients (279 cycles) for which selected covariates—including vitamin deficiency marker data (i.e., homocysteine, cystathionine, methylmalonic acid, and methylcitrate [I, II, and total] plasma concentrations)—were available. Classical statistical multivariate regression analyses and a semimechanistic-physiologic population PK/PD model were used to evaluate neutropenic response to single-agent pemetrexed administration. Results: The timecourse of neutropenia following single-agent pemetrexed administration was adequately described by a semimechanistic-physiologic model. Population estimates for system-based model parameters (i.e., baseline neutrophil count, mean transit time, and the feedback parameter), which mathematically represent current understanding of the process and physiology of hematopoiesis, were consistent with previously reported values. The population PK/PD model included homocysteine, cystathionine, albumin, total protein, and BSA as covariates relative to neutropenic response. Conclusion: These results support the programmatic decision to introduce folic acid and vitamin B12 supplementation during pemetrexed clinical development as a means of normalizing patient homocysteine levels, thereby managing the risk of severe neutropenia secondary to pemetrexed administration. The current results also suggest that the addition of vitamin B6 supplementation to normalize patient cystathionine levels may further decrease the incidence of grade 4 neutropenia following pemetrexed administration. The results also suggest the use of folic acid as a means of lessening hematologic toxicity following administration of cytotoxic agents other than antifolates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pemetrexed (Alimta, Eli Lilly and Company, Indianapolis, USA) is a promising novel anticancer agent that inhibits multiple enzymes involved in purine and pyrimidine formation. Its mechanism of action and clinical activity is due to inhibition of thymidylate synthase (TS) [19, 48], dihydrofolate reductase (DHFR) and glycinamide ribonucleotide formyltransferase (GARFT) [48] as well as the formation and long-term retention of intracellular polyglutamates, which likely produces a more sustained antitumor effect [33]. Pemetrexed has demonstrated clinical activity in a broad spectrum of tumor types, including mesothelioma, nonsmall cell lung, bladder, head and neck, breast, cervical, colorectal, pancreatic, and gastric cancers [1, 37, 44, 41, 38, 39, 49, 17, 14, 32, 5]. In combination with cisplatin, pemetrexed is approved for the first-line treatment of malignant pleural mesothelioma [53]. As a single agent, it is approved for second-line treatment of nonsmall cell lung cancer [21].
The principle toxicity of pemetrexed following single-agent administration was common toxicity criteria (CTC) grade 3 or 4 neutropenia [20]. Thrombocytopenia was also observed, but it was less frequent and less severe than neutropenia. Relationships between systemic drug exposure (area under the curve [AUC] and maximum plasma concentration [C max]) and nadir absolute neutrophil counts (NANCs) were evaluated previously in a phase I dose-escalation study where pemetrexed was administered between 50 and 700 mg/m2 as a 10-min intravenous infusion every 21 days in 37 patients with solid tumors. Results indicated that NANCs decreased as systemic drug exposure increased [40]. The severity of neutropenia was more accurately predicted according to overall systemic exposure (AUC) rather than a timepoint, at or near the end of, infusion (C max). Hence, a clinically meaningful association was identified between systemic drug exposure and NANCs.
As a class, antifolates have been associated with severe and unpredictable myelosuppression in some patients. Lometrexol is an antifolate that was terminated in its development by Lilly because of severe toxicity. Preclinical and clinical investigations showed that folic acid could modulate lometrexol toxicity and that the maximum tolerated dose could be substantially increased [23, 18, 21]; however, researchers were unable to determine the specific mechanism responsible for the reduction in toxicity [54, 34]. Based on the lometrexol findings, when myelosuppression was identified as a primary pemetrexed toxicity, efforts were undertaken to determine whether functional folate status could be a predictor of pemetrexed toxicity. Thus, a number of vitamin deficiency markers were measured during pemetrexed phase II clinical development. The panel included folic acid and/or vitamin B12 deficiency markers (homocysteine [HCY], methylmalonic acid [MMA], and methylcitrate I and II [MCI and MCII]) and a vitamin B6 deficiency marker (cystathionine [CYS]). Thus, vitamin deficiency markers have been evaluated as potential predictors of pemetrexed toxicity using multivariate stepwise regression methods [34] and potential predictors of neutropenic response based on the population pharmacokinetic/pharmacodynamic (PK/PD) analyses reported in this paper. The current paper describes population PK/PD analyses based on data from more than 200 patients from eight phase II studies of single-agent pemetrexed involving a wide range of advanced stage tumors (see Table 1 [Studies included in the index dataset] in Latz et al. [24]). The analyses utilize a semiphysiologic PK/PD model recently introduced by Friberg et al. [16] to describe myelosuppression following administration of a cytotoxic agent. The model provides a simplified mathematical framework from current conceptual knowledge of physiology to quantitatively mimic the underlying physiologic process of hematopoiesis [30]. The use of a semimechanistic-physiologic model allows a complete characterization of the entire absolute neutrophil count (ANC)-time profile from a sparse sampling scheme.
The current analyses build upon the work of Friberg et al. [16] by including covariate effects to explain between-patient variability. These analyses were used to identify predictors of neutropenic response to pemetrexed, providing clinically important information for inclusion in product labeling.
Methods
Summary of clinical studies and data assembly
Patients were initially assigned to receive pemetrexed 500 or 600 mg/m2 via a 10-min intravenous infusion every 21 days (1 cycle). Patients enrolled in the studies did not receive folic acid or vitamin B12 supplementation. All participants gave written informed consent, and studies were conducted in accordance with the ethical principles of the most recent version of the Declaration of Helsinki. The PK/PD analysis was performed using a two-stage approach. Empirical Bayesian estimates of clearance (CL), central volume of distribution (V 1), intercompartmental clearance (Q), and peripheral volume of distribution (V 2) resulting from the PK analyses [24] were incorporated into the dataset used for PD analyses to generate predicted plasma concentration-time profiles for the PD model. Additional details about study design and the population PK analyses, the results of which are integrated into the PK/PD analyses presented herein, are found in the previous paper [24].
Blood samples for determination of platelet counts, differential white blood cell counts, and hemoglobin levels were collected at the start of each new cycle (prior to receiving pemetrexed) and weekly thereafter. Blood chemistry evaluations were performed on samples collected at the start of each new cycle and 1 week after receiving pemetrexed. Vitamin deficiency marker (HCY, CYS, MMA, MCI, and MCII) concentrations were measured on samples collected at the start of each new cycle. Clinical parameters (e.g., weight, blood pressure, and pulse) were measured at specific visits at the investigator’s site. Other information (e.g., date of birth and habits) was based on patient self-report. Additional patient parameters (e.g., body surface area [BSA] and estimated creatinine clearance) were derived using standard formulae [45, 13]. Neither granulocyte nor granulocyte monocyte colony-stimulating factors were allowed in these trials.
Hematology, blood chemistry, and vitamin deficiency marker determinations were carried out at central laboratories with common methodology and quality control. Hematology and blood chemistry measurements were performed by Covance Central Laboratory Services (CCLS) Inc., Indianapolis; Geneva, Switzerland; or Sydney, Australia. Vitamin deficiency markers were assayed at University of Colorado Health Sciences Center, Denver, Colorado [4].
ANC data were combined with dosing information, patient demographics (e.g., age and gender) and characteristics (e.g., BSA and weight), clinical laboratory test results (e.g., blood chemistry and vitamin deficiency markers), and PK parameter estimates (CL, V 1, Q, and V 2) to produce the dataset used for population PK/PD analysis. When available, the PK/PD dataset incorporated individual empirical Bayesian estimates of PK parameters; otherwise population estimates based on the population PK model [24], incorporating renal function and BSA, were utilized for patients with missing plasma concentration-time data.
Data from 279 patients who received 1,136 cycles were available for analysis. Vitamin deficiency marker concentrations deemed essential to the analyses [34] were available from 105 patients (279 cycles). Therefore, the subset of data from patients with vitamin deficiency marker results comprised the primary analysis dataset that was used for covariate identification and model development.
Data analysis
Classical statistical multivariate regression analyses of NANCs identified from the observed data were performed using PROC MIXED in Statistical Application Software Version 6.12 [43]. The relationship between pemetrexed exposure and the observed NANC was investigated using a linear statistical model. The analysis of variance (ANOVA) model included fixed covariate effects for lnAUC and cycle and patient identifier as a random effect. The relationship between several other potential covariates (e.g., baseline ANC, pemetrexed AUC, BSA, gender, and smoking status) and the NANC was investigated using similar linear models with these covariates as fixed-effects and patient as a random effect. Statistical testing was performed at the 5% significance level. The time to nadir (T Nadir) and time of recovery to CTC grade 0 toxicity (T Rec,0) were also investigated using similar models.
Since multivariate regression analysis takes into account only the specific timepoints and ANC analysed, which are clustered around 7, 14, and 21-day collections based on protocol design, it does not characterize the entire profile and therefore does not enable an overall understanding of the timecourse of neutropenic response. Therefore, population PK/PD modeling using a semimechanistic-physiologic PK/PD was undertaken to further characterize the timecourse of neutropenia and to identify and understand how covariates affect neutropenic response following pemetrexed administration. All PK/PD analyses were performed with the nonlinear mixed effect modeling (NONMEM, version V) computer program with PREDPP (version V) [8, 47, 51]. Model selection was based on both a change in the minimum value of the objective function for nested models and a visual inspection of the goodness-of-fit plots.
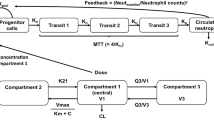
A seven-compartment semimechanistic-physiologic PK/PD model with two PK compartments [24] and five PD compartments [16] was used to characterize the timecourse of ANC following single-agent pemetrexed administration. The PD portion of the model was constructed to mimic physiologic processes and consists of a stem/progenitor cell compartment, three maturation compartments, and the circulation compartment, which represents the circulating neutrophils (Fig. 1).
The differential equations governing the semimechanistic-physiologic model are illustrated in Fig. 2. The PK portion of the pemetrexed PK/PD model was parameterized in terms of CL, V 1, Q, and V 2. Parameters for the PD portion of the model included baseline absolute neutrophil count (BAS), mean transit time (MTT), a drug stimulus parameter (DS), and a feedback parameter (FP). MTT represented the average time for a committed stem cell to pass through the three maturation compartments in the bone marrow prior to being released into the circulation; the relationship between MTT and the intercomparmental transfer rate constant (k) is MTT=(n+1)/k where n is the number of maturation compartments. DS was a linear proportionality constant relating drug concentration in the central compartment to the cytotoxic effect of pemetrexed and its intracellular polyglutamates. FP quantified the strength of the feedback action from the colony-stimulating factors that regulated the physiologic process.
Differential equations governing the semimechanistic-physiologic PK/PD model. X 1 drug amount in the central compartment; X 2 drug amount in the peripheral compartment; k 12, k 21, and k 10 microconstants of the pharmacokinetic model; stem the stem/progenitor cell pool size; k prol the stem/progenitor cell proliferation rate constant; DS a linear proportionality constant (dose stimulus) relating drug concentration in the central compartment to the cytotoxic effect of pemetrexed and its intracellular polyglutamates on the proliferation rate constant; V 1 central volume of distribution; BAS the baseline neutrophil counts prior to drug administration; Circ the neutrophil counts in circulation; k the maturation rate constant; FP feedback parameter that quantifies the strength of the feedback action from the colony-stimulating factors that regulate the physiologic process. M 1, M 2, M 3 maturation compartments
The differential equation governing the stem cell compartment (Fig. 2, Eq. 3) is based upon a mass balance over the compartment. The rate of change of stem/progenitor cells in the stem cell pool is equal to the difference between the proliferation rate and the degradation (elimination) rate. The proliferation rate is comprised of three components: proliferation rate under steady state conditions (k prol• Stem), drug effect (1-DS • X 1/V 1), and feedback effect (BAS/Circ) [16]. Figure 3 illustrates the constraints necessary to satisfy steady-state conditions (time, t=0; [prepemetrexed administration]) and their effect upon the rate constants in the model.
Between-patient variability models were examined assuming log-normal distribution of individual BAS, MTT, DS, and FP values, as is typical for physiologic parameters. Additive, proportional, and combined additive and proportional residual error structures were evaluated. The first-order (FO) method of Sheiner and Beal [6, 7], which is based on first-order Taylor series expansion around 0, was utilized as the computational method in NONMEM.
Covariate identification was performed to explain sources of variability with respect to the PD model parameters and to examine their clinical predictive value and potential dosing implications [25]. Patient-specific factors, which could affect either the system-based parameters (BAS, MTT, and/or FP) or the drug effect-related parameter (DS), were evaluated as potential covariates using both linear and nonlinear models. Patient factors considered as potential covariates with respect to each of the PD model parameters included the following continuous variables: age, albumin (ALB), BSA, body weight, creatinine clearance (estimated by Cockcroft-Gault formula using age, weight, and serum creatinine), total bilirubin, total protein (TPR), HCY, CYS, MMA, MCI, MCII, and total methylcitrate. Categorical variables included gender, ethnic origin, alcohol use, smoking status, and treatment cycle. Since overall neutropenic response is a function of all model parameters, each potential covariate was tested for relationships with BAS, MTT, DS, and FP using both linear and nonlinear models (i.e., linear additive, proportional, and power). For linear and proportional models, potential covariates were normalized by their population median; for example, linear models were coded P=Θ1+Θ2•COV where P was the individual’s estimate of the parameter (e.g., BAS, MTT, DS, and FP), Θ1 represented the typical value of the parameter, Θ2 represented the effect of the covariate, and COV was the ratio of the individual’s covariate value to the population median value.
Potentially significant covariates were identified as those factors that, when added to the base model individually, resulted in a decrease in the objective function of 3.84 points or more (P≤0.05 based on χ2 distribution with 1 degree of freedom). Potential covariates were added to the model sequentially, based on the change in objective function for the individual covariate; those covariates that reduced the objective function by the greatest amount were added to the model first. Potential covariates that did not result in a decrease in the objective function of 3.84 points or more on sequential addition to the model were removed from the analysis. Once a full model was established, the process was then reversed, with each potential covariate being removed individually from the full model. Covariates retained in the final model were those associated with a significant increase (≥10.8 points for 1 degree of freedom, P<0.001) in the minimum value of the NONMEM objective function when removed from the full model.
Similar to the pemetrexed population PK model evaluation [24], objective function mapping [46, 29] and leverage analysis [29, 15] were performed to evaluate the robustness of the base and final population PD models. An assessment of predictive ability [31] confirmed that the base model identified for the primary analysis dataset adequately characterized the portion of data that was excluded from the full model development due to lack of vitamin deficiency marker results.
Results
Demographics and patient characteristics
A total of 3,462 ANCs from 279 patients ranging in age from 26–79 years at study entry was available. Patient characteristics (demographics, habits, range, and mean values of weight, BSA, Cockcroft-Gault determination of renal function, ALB, TPR, and vitamin deficiency markers) are provided in Table 1 and were similar for the two datasets; thus, the primary analysis dataset was representative of the complete dataset.
Observed neutrophil and dosing data
Neutrophil data were pooled from eight phase II studies. Data for 1,136 cycles, from 279 patients, were available for PK/PD analysis. The clinical trials were conducted using BSA-based dosing, which yielded a range of doses from 74.9 mg/m2 (126 mg) to 639 mg/m2 (1,362 mg) with a median dose of 596 mg/m2 (1,038 mg). PK/PD analyses were based upon the absolute dose administered. Most patients (>65%) received four or fewer cycles. Most of the ANCs included in the PK/PD analyses (>85%) were from cycles 1 through 6. The timing of ANC determinations were clustered around 7, 14, and 21-day collections, consistent with the protocols for each study as illustrated in Fig. 4.
Empirical analysis of observed neutrophil data
Table 2 summarizes the results of the multivariate regression analyses for the association between AUC and each of three clinically relevant features of the ANC versus time profile (NANC, T Nadir, or T Rec,0). Lower NANC, longer T Nadir, and longer T Rec,0 were all associated with higher overall systemic exposure. Additionally, lower NANCs were associated with lower BSA (P=0.03), smokers (P=0.01), and females (P=0.04). Vitamin deficiency markers did not correlate with NANC, T Nadir, or T Rec,0 using multivariate regression analyses.
Population PK/PD base model identification
As systemic drug exposure was found to be the most influential factor that described the degree of neutropenic response to pemetrexed administration, a semimechanistic-physiologic population PK/PD model that incorporated drug exposure was used to further explore the overall timecourse of neutropenia and to characterize sources of variability in the PD response. The base structural PK/PD model (Table 3) included proportional between-patient variability (ηs) for BAS, MTT, DS, and FP and a proportional residual error term (ε). Covariance between structural model parameters was not included in the statistical model since the goodness-of-fit plots did not indicate an improvement in the model’s predictive ability.
A comparison of goodness-of-fit plots (Fig. 5) confirmed that the base model identified for primary analysis adequately characterized the data excluded from full-model development due to lack of vitamin deficiency marker data. The mean prediction errors (MPEs) (data included in primary analysis=−0.380; data excluded from primary analysis=0.051) indicated that the base-model performance was at least as good for the subset of data included in the primary analysis as for the data excluded. These results supported the predictive ability of the final base model and the use of only the subset of ANCs with vitamin deficiency marker data available for covariate identification and model development.
Final population PK/PD model
In the final population PK/PD model (Table 4), the magnitude of the estimates of between-patient variability and residual error is consistent with those reported previously [16]. The estimate of residual variability is consistent with known physiologic intraindividual variation in ANCs [28]. The addition of CYS, HCY, ALB, TPR, and BSA onto the basic PK/PD model decreased between-patient variability in BAS from 32.9 to 30.3%, in MTT from 10.4 to 9.85%, and in FP from 38.7 to 27.6%, while between-patient variability increased in DS from 42.3 to 45.6%. Proportional residual variability also decreased from 37.5 to 35.6%. Goodness-of-fit for the final population model is represented graphically (Fig. 6) by the agreement between predicted (IPRED) and observed (DV) ANCs as well as by weighted residual (IWRES) values. Representative examples of individual patient data, together with predicted ANC-time profiles, are provided in Fig. 7.
Population-predicted ANC-time profiles (i.e., PRED versus time) following a 500-mg/m2 dose for patient characteristics represented in the analysis dataset (Fig. 8, gray-shaded area of the graph) depict between-patient variability in ANC response that is explained by patient factors included in the final population PK/PD model.
Final population PK/PD model: timecourse of predicted absolute neutrophil counts (PRED) following 500 mg/m2 pemetrexed. Lines: Solid black curve the overall “typical” patient in the analysis dataset (i.e., median values for each of the covariates contained in the final model); gray shading predictions based on the population PK/PD model for each of the patients in the analysis dataset, assuming a 500 mg/m2 dose; dashed horizontal lines hematologic toxicity grades (grade 1 <2, grade 2 <1.5, grade 3 <1, grade 4 <0.5)
Final population PK/PD model evaluation
Objective function mapping and leverage analysis supported the validity of the model to describe neutropenic response to pemetrexed in this patient population. Objective function mapping confirmed the absence of local minima and showed the parameters to be well estimated. Leverage analyses also demonstrated that each structural parameter included in the model was well estimated, with no significant differences observed when subsets of patient data were systematically removed from the index dataset. These results indicate that no subset of the patient population had an undue influence on the model estimates.
Covariate relationships
Patient-specific factors that affect either the system-based parameters (BAS, MTT, FP) or the drug effect-related parameter (DS) in this semimechanistic-physiologic model lead to changes in the ANC-time profile and were evaluated in these analyses. The final PK/PD model included relationships between HCY and BAS, CYS and BAS, CYS and DS, ALB and MTT, TPR and DS, and BSA and DS. The effects of individual covariates on the structural parameters of the model and on the timecourse of neutropenia were examined by varying each covariate individually (Fig. 9) and are summarized in Table 5. CYS was identified as a covariate with respect to both BAS and DS. The effects counteract each other relative to the nadir; an increase in plasma CYS corresponds to an increase in BAS, resulting in a higher NANC, and an increase in DS, resulting in a lower NANC. Thus, since NANC decreases and T Nadir increases with increased CYS, the effect of CYS on DS provides a more pronounced impact on the nadir than BAS does.
Relationships between covariates and structural parameters (left panel) and effect of covariates on timecourse of neutropenic response to pemetrexed (right panel). Left panel: Open circles individual empirical Bayesian estimates of model parameters. Right panel: Effect of varying covariate on the timecourse of ANCs following pemetrexed administration. Heavy solid curves the “typical” patient in the analysis dataset (median values for each of the covariates in the final model). Dashed curves the effect of varying the covariate indicated. Gray horizontal lines hematologic toxicity grades (grade 1<2, grade 2<1.5, grade 3<1, grade 4<0.5)
Inclusion of cycle as a covariate with respect to BAS, MTT, DS, or FP did not reveal any systematic effects on these model parameters. The lack of cycle effect with respect to DS indicated that the direct effect of the drug did not change over time with ongoing pemetrexed treatment. The remaining patient factors examined as potential covariates (e.g., gender, patient age, smoking status, alcohol consumption, ethnic origin) failed to meet the statistical criteria for inclusion in the final model. Therefore, there is no evidence that these factors influence the magnitude and timecourse of neutropenia following pemetrexed administration.
Discussion
The semimechanistic-physiologic PK/PD model used in these analyses was developed in order to provide an understanding of the timecourse of ANC following single-agent pemetrexed administration and to identify patient-specific factors that influence neutropenic response to pemetrexed. The analyses utilized a semimechanistic-physiologic PK/PD model recently introduced by Friberg et al. [16] to describe myelosuppression following pemetrexed administration. Typical values (i.e., central tendencies) for the model parameters were BAS 5.19×109/l, MTT 107 h, DS 0.229, and FP 0.192.
Parameters in physiologically based PD models are either system-based (physiologically based) or drug effect-related [13]. In the model used in these analyses, BAS, MTT, and FP are all system-based parameters and provide an adapted mathematical representation of current understanding of the underlying physiology. In contrast, DS is a drug effect-related parameter; it relates the concentration of pemetrexed in plasma to the effect of the drug at the site of action (stem cell). Changes in any of the four parameters, whether the parameter is system-based or drug effect-related, alter the ANC-time profile. Therefore, patient-specific factors affecting any of the four PD parameters led to alterations in the ANC-time profile and were evaluated in these analyses. The current work built upon the earlier work of Friberg et al. [16] by including covariate effects to explain between-patient variability.
Since the system parameters characterize the physiology of hematopoesis in cancer patients, they should be relatively consistent between similar populations. Estimates for BAS, MTT, and FP from our analyses were consistent with those reported by Friberg et al. [16], which confirmed the adequacy of the model to describe the current data. In general, estimates of between-patient variability were also similar for the two analyses, although between-patient variability in MTT was slightly lower than that in Friberg’s analyses. This is likely due to the inclusion of between-patient variability in FP in the current model, which picks up some of the between-patient variability in MTT. It is noteworthy that between-patient variability in DS was similar to that reported for other agents (docetaxel, paclitaxel, etoposide, DMDC, CPT-11, and vinflunine) that were studied by Friberg et al. Since pemetrexed PK variability is relatively low, this means that pemetrexed dose-ANC variability is not higher, and possibly may be lower, than those agents previously evaluated with the same model.
One of the primary toxicities associated with single-agent pemetrexed administration is neutropenia [20]. Elevations in vitamin deficiency marker concentrations of HCY, MMA, and CYS have been linked to deficiencies in functional folate status as well as to deficiencies in vitamins B12 and B6 [4, 50]. Earlier work by Niyikiza et al. [34] showed that the incidence of CTC grade 4 neutropenia in patients receiving pemetrexed correlated with a patient’s functional folate status, as characterized by the pretreatment total HCY concentration. Results of Niyikiza et al. were used as a guide in the selection of covariates evaluated during model development.
In our evaluations, an initial empirical analysis identified AUC, BSA, smoking, and gender as potential predictors of response (observed NANC; T Nadir; and T Rec,0) but, interestingly, did not identify vitamin deficiency markers (e.g., HCY, CYS, MMA) as predictors of response. A semimechanistic-physiologic population PK/PD model comprised of a PK component (thereby incorporating AUC) was then used to further explore and characterize the impact of patient factors on the overall timecourse of neutropenia following pemetrexed administration and included covariate relationships between CYS and BAS, CYS and DS, HCY and BAS, ALB and MTT, TPR and DS, and BSA and DS. Disparities in covariate identification between the multivariate regression analysis and the PK/PD model are likely related to methodology. In the regression analyses, NANCs are directly identified from the observed data; covariate relationships are then identified based on these NANCs that likely do not occur at the time of the actual nadir due to protocol design (ANCs taken at 7, 14, and 21 days). In the population PK/PD model, the overall timecourse of neutropenic response is described based on all ANC-time data contained in the analysis dataset. Covariate relationships are then identified, which describe how ANCs deviate from the central tendency of the ANC-time profile for patients with the characteristic being evaluated, regardless of the timing of the ANC. Thus, regression analyses provide information only about the discrete timepoints identified as nadirs and provide an opportunity for chance findings, whereas the population PK/PD-modeling approach provides information about the overall ANC-time profile.
Since vitamin deficiency marker data (HCY, CYS, MMA, MCI, MCII, and total methylcitrate) were available for only 25% of the cycles, population PK/PD model development was completed on the subset of the ANC data for which vitamin metabolite data were available. Consistent with the empirical approach used by Niyikiza et al. [34], pretreatment vitamin deficiency marker status was shown to influence neutropenic response following single-agent pemetrexed administration in these analyses. HCY, a marker for folate, vitamin B12 [47], and vitamin B6 deficiency [50], was identified as a covariate relative to BAS. CYS, a marker for vitamin B6 deficiency [50], which did not correlate with severe neutropenia in the Niyikiza evaluation, was shown to be a significant covariate both with respect to the system-based parameter, BAS, and the drug effect-related parameter, DS.
Notably, elevated HCY concentrations, which are associated with folate deficiency, were shown to lower BAS. A decrease in BAS shifts the entire ANC-time profile downward (Fig. 9). Since BAS is a system-based parameter and, therefore, drug independent, this result implies that the findings of Niyikiza and colleagues might be generalized to other anticancer agents; that is, elevated vitamin deficiency markers may correlate with increased incidence and severity of neutropenia for any cytotoxic drug. This implies that vitamin supplementation theoretically may be beneficial for patients receiving any cytotoxic drug, not just antifolates. In pemetrexed clinical trials, patients receiving cisplatin with folate and vitamin B12 supplementation appeared to experience less toxicity than those who did not receive supplementation [3]. Branda et al. [9, 10, 11] have provided preclinical and clinical evidence suggesting that folate supplementation may ameliorate chemotherapy-induced neutropenia for cytotoxic agents other than antifolates, including cyclophosphamide, 5-FU, and doxorubicin. If confirmed, the use of folic acid as a general means of lessening hematologic toxicity following administration of cytotoxic agents would join a growing body of literature implicating the importance of folic acid for a diverse group of diseases and situations [12]. Whether or not folate and vitamin B12 supplementation can reduce severe neutropenia and/or other toxicities associated with cytotoxic agents other than antifolates is beyond the scope of the current work and requires further investigation.
An increase in CYS concentrations was shown to be associated with an increase in BAS and DS. The effects counteract each other relative to the nadir; an increase in BAS results in a higher NANC, and an increase in DS results in a lower NANC. Thus, since NANC decreases and T Nadir increases with increased CYS (Fig. 9), the effect of CYS on DS provides a more pronounced impact on the nadir than BAS does and suggests that an association between increased CYS and lower NANC might be pemetrexed-specific. Since increased CYS would be expected to be associated with decreased white blood cell count [4], it is unclear why an increase in CYS corresponded to an increase in BAS in these analyses.
Therefore, these PK/PD analyses indicate that patients with folate, vitamin B12, and vitamin B6 deficiencies have a greater probability of experiencing severe neutropenia, although potentially by different mechanisms. Although elevated CYS is associated with vitamin B6 deficiency, it has also been shown to occur collaterally with HCY [4, 50] by a mechanism that is independent of vitamin B6 deficiency. Based on the study by Niyikiza et al. [34] and an established order of effectiveness for normalizing plasma HCY by vitamin supplementation (highest with folic acid, followed by vitamin B12, and lowest with vitamin B6 [22]), folic acid and vitamin B12 supplementation was implemented to normalize patients’ plasma HCY levels in pemetrexed clinical trials. This clinical intervention was shown to quickly normalize patient HCY, maintain normalized HCY throughout therapy, and dramatically decrease the incidence of drug-related deaths and severe nonhematologic and hematologic toxicities, including neutropenia; however, despite this intervention, a 9% incidence of grade 4 neutropenia still occurred in pemetrexed patients supplemented with folic acid and vitamin B12 [35]. The identification of CYS as a covariate in these PK/PD analyses suggests that normalization of patient CYS might offer an additional means of further decreasing the incidence of grade 4 neutropenia and warrants further investigation.
Similar to the findings of the Uppsala group, with respect to application of the semimechanistic-physiologic model following single-agent paclitaxel administration [26], a decrease in MTT with decreasing ALB levels was also observed in these analyses. Maas speculated that this observation was not likely because of a direct action of ALB on cell maturation rate. Rather, they indicated that this phenomenon might be caused by the influence of interleukin-6 as it increased the rate of cell maturation [52] as well as acted as an inhibitor of ALB production [27, 55, 36].
Finally, TPR and BSA were both identified as covariates with respect to DS. Decreases in both covariates were associated with an increase in the dose-strength parameter, which translates into increased toxicity. Since pemetrexed is 81% protein bound, a decrease in TPR would result in an increase in unbound fraction and could potentially result in an increased cytotoxic effect. This also suggests the possibility that free pemetrexed in plasma, rather than total, might further enhance the model’s capability to describe the neutropenic response; however, one would expect the use of free drug in plasma to be more critical for drugs that are highly protein bound. The specific reason for the correlation between BSA and DS is not understood.
The patients included in this analysis received pemetrexed without vitamin supplementation. Therefore, the findings presented here are applicable only to single-agent pemetrexed administration without vitamin co-therapy. Further analyses are necessary to characterize neutropenic response following doublet therapy (e.g., pemetrexed+cisplatin) and will require some modification to the model [42]. Additional analyses, incorporating data from patients who have received vitamin co-therapy, are necessary to further characterize the impact of vitamin co-therapy in ameliorating pemetrexed hematologic toxicity.
Further, it should be noted that pemetrexed is polyglutamated intracellularly, and the polyglutamates are the cytotoxic species; however, the current PD model is based on concentrations of pemetrexed in plasma. Thus, although the current model describes the timecourse of neutropenia in cancer patients and is physiologically plausible based on existing knowledge of the hematopoitic system, the intracellular polyglutamates could serve as the basis for alternative PK/PD models.
In summary, a semimechanistic-physiologic model has adequately described the timecourse of ANC following single-agent pemetrexed administration. Patient-specific factors that explained variability in each of 4 model parameters were identified, and the impact of these covariates on the timecourse of neutropenia examined. The results from these analyses support the programmatic decision to introduce folic acid and vitamin B12 supplementation during pemetrexed clinical development as a means of normalizing patient HCY, thereby managing the risk of severe neutropenia secondary to pemetrexed administration. Interestingly, the data also suggest that adding vitamin B6 to supplementation as a means of normalizing patient CYS may further decrease the incidence of grade 4 neutropenia following pemetrexed administration.
Due to the complexity of the current model, it is difficult to visualize the effect of each parameter on the ANC-time profile without performing a series of simulations. A subsequent paper [25] will use simulations based on this model to extract clinically relevant features from the ANC-time profile (i.e., NANC, T Nadir, and T Rec,0) that are of interest to the practicing physician and the clinical development team. Simulated outcomes (central tendency and probability distribution) will be used to further characterize the magnitude of the effect of drug exposure and the effect of the significant covariates on the clinically relevant features of the ANC-time profile. Results from these simulations were used to quantitatively evaluate differences between dosing strategies and the impact of folate and vitamin B12 deficiency on neutropenic response to pemetrexed.
References
Adjei AA (2003) Pemetrexed (Alimta): a novel multitargeted antifolate agent. Expert Rev Anticancer Ther 3:145–156
Alati T, Shih C, Pohland RC, Lantz FJ, Grindey GB (1992) Evaluation of the mechanism(s) of inhibition of the toxicity, but not the antitumor activity of lomotrexol (DDATHF) by folic acid. Proc Am Assoc Cancer Res 33:407
Alimta Drug Approval Package, Medical Review. Food and Drug Administration Web site. Available at: http://www.fda.gov/cder/foi/nda/2004/21-462.pdf_Alimta_Medr_P6.pdf. Page 257. Accessed September 20, 2004
Allen RH, Stabler SP, Savage DG, Lindenbaum J (1993) Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J 7:1344–1353
Bajetta E, Celio L, Buzzoni R et al (2003) Phase II study of pemetrexed disodium (Alimta) administered with oral folic acid in patients with advanced gastric cancer. Ann Oncol 14:1543–1548
Beal SL, Sheiner LB (1982) Estimating population kinetics. Crit Rev Biomed Eng 8:195–222
Beal SL, Sheiner LB (1988) Heterskedastic nonlinear regression. Technometrics 30:327–338
Beal SL, Sheiner LB (1992) NONMEM user’s guide NONMEM project group. University of California, San Francisco
Branda RF, Nigels E, Lafayette AR, Hacker M (1998) Nutritional folate status influences the efficacy and toxicity of chemotherapy in rats. Blood 92:2471–2476
Branda RF, Chen Z, Brooks EM, Naud SJ, Trainer TD, McCormack JJ (2002) Diet modulates the toxicity of cancer chemotherapy in rats. J Lab Clin Med 140:358–368
Branda RF, Naud SJ, Brooks EM, Chen Z, Muss H (2004) Effect of vitamin B12, folate, and dietary supplements on breast carcinoma chemotherapy-induced mucositis and neutropenia. Cancer 101:1058–1064
Carmel R, Jacobsen DW (2001) (eds) Homocysteine in health and disease. Cambridge University Press, New York
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Cripps C, Burnell M, Jolivet J et al (1999) Phase II study of first-line LY231514 (multitargeted antifolate) in patients with locally advanced or metastatic colorectal cancer: an NCIC Clinical Trials Group study. Ann Oncol 10:1175–1179
Ette EI (1997) Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 37:486–495
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO (2002) Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 20:4713–4721
Goedhals L, van Wijk AL (1998) MTA (LY231514) in advanced carcinoma of the cervix. Ann Oncol 9(suppl 4):70
Grindey GB, Alati T, Shih C (1991) Reversal of the toxicity but not the antitumor activity of lometrexol by folic acid. Proc Am Assoc Cancer Res 32:324
Grindey GB, Shih C, Barnett CJ et al (1992) LY231514, a novel pyrrolopyrimidine antifolate that inhibits thymidylate synthase (TS). Proc Am Assoc Cancer Res 33:411
Hanauske AR, Chen V, Paoletti P, Niyikiza C (2001) Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist 6:363–373
Hanna N, Shepherd FA, Fossella FV et al (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589–1597
Homocysteine Lowering Trialists’ Collaboration (1998) Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ 316:894–898
Laohavinij S, Wedge SR, Lind MJ et al (1996) A phase I clinical study of the antipurine antifolate lomotrexol (DDATHF) given with oral folic acid. Invest New Drugs 14:325–335
Latz JE, Chaudhary A, Ghosh A, Johnson RD (2006) Population pharmacokinetic analysis of ten phase II clinical trials of pemetrexed in cancer patients. Cancer Chemother Pharmacol 57. DOI 10.1007/s00280-005-0036-1
Latz JE, Rusthoven JJ, Karlsson MO, Ghosh A, Johnson RD (2006) Clinical application of a semimechanistic-physiologic population PK/PD model for neutropenia following pemetrexed therapy. Cancer Chemother Pharmacol 57. DOI 10.1007/s00280-005-0035-2
Maas H (2001) Development of a semi-physiological population PK/PD model of hematological toxicity: evaluation of across-drug consistency. MSc Thesis. Uppsala University. Uppsala, Sweden
Mackiewicz A, Speroff T, Ganapathi MK, Kushner I (1991) Effects of cytokine combinations on acute phase protein production in two human hepatoma cell lines. J Immunol 146:3032–3037
Maes M, Stevens W, Scharpe S et al (1994) Seasonal variation in peripheral blood leukocyte subsets and in serum interleukin-6 and soluble interleukin-2 and −6 receptor concentrations in normal volunteers. Experientia 50:821–829
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm 20:511–528
McKenzie SB (1996) Textbook of hematology. Williams and Wilkins, Baltimore, pp 22–29, pp 58–67
Mentre F, Ebelin ME (1997) Validation of population pharmacokinetic/pharmacodynamic analyses: review of proposed approaches. In: Balant LP, Aarons L (eds) The population approach: measuring and managing variability in response, concentration and dose. Commission of the European Communities, Brussels, pp 147–160
Miller KD, Picus J, Blanke C et al (2000) Phase II study of the multitargeted antifolate LY231514 (ALIMTA, MTA, pemetrexed disodium) in patients with advanced pancreatic cancer. Ann Oncol 11:101–103
Moran RG (1999) Roles of folylpoly-gamma-glutamate synthetase in therapeutics with tetrahydrofolate antimetabolites: an overview. Semin Oncol 26(2 suppl 6):24–32
Niyikiza C, Baker SD, Seitz DE et al (2002) Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther 1:545–552
Niyikiza C, Hanauske AR, Rusthoven JJ et al (2002) Pemetrexed safety and dosing strategy. Semin Oncol 29(6 Suppl 18):24–29
Odamaki M, Kato A, Kumagai H, Hishida A (2004) Counter-regulatory effects of procalcitonin and indoxyl sulphate on net albumin secretion by cultured rat hepatocytes. Nephrol Dial Transplant 19:797–804
O’Dwyer PJ, Nelson K, Thornton DE (1999) Overview of phase II trials of MTA in solid tumors. Semin Oncol 26:99–104
Paz-Ares L, Tabernero J, Moyano A et al (1999) Significant activity of the multitargeted antifolate MTA (LY231514) in advanced transitional cell carcinoma (TCC) of the bladder: results of a phase II trial. Eur J Cancer 35(suppl 2):S81
Pivot X, Raymond E, Laguerre B et al (2001) Pemetrexed disodium in recurrent locally advanced or metastatic squamous cell carcinoma of the head and neck. Br J Cancer 85:649–655
Rinaldi DA, Kuhn JG, Burris HA et al (1999) A phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol 44:372–380
Rusthoven JJ, Eisenhauer E, Butts C et al (1999) Multitargeted antifolate LY231514 as first-line chemotherapy for patients with advanced non-small cell lung cancer: a phase II study National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 17:1194–1199
Sandström M, Lindman H, Nygren P, Lidbrink E, Bergh J, Karlsson MO (2005) Model describing the relationship between pharmacokinetics and hematologic toxicity of the epirubicin-docetaxel regimen in breast cancer patients. J Clin Oncol 23:413–421
SAS Institute Inc (1997) SAS/STAT software: changes and enhancements through release 6.12. Cary, NC: SAS Institute Inc
Scagliotti GV, Shin DM, Kindler HL et al (2003) Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol 21:1556–1561
Shargel L, Yu ABC (1985) Applied biopharmaceutics, 2nd edn. Appleton-Century-Crofts, Norwalk, pp 310–312
Sheiner LB (1986) Analysis of pharmacokinetic data using parametric models. III. Hypothesis tests and confidence intervals. J Pharmacokinet Biopharm 14:539–555
Sheiner LB, Steimer JL (2000) Pharmacokinetic/pharmacodynamic modeling in drug development. Annu Rev Pharmacol Toxicol 40:67–95
Shih C, Gosset L, Gates S et al (1996) LY231514 and its polyglutamates exhibit potent inhibition against both human dihydrofolate reductase (DHFR) and thymidylate synthase (TS): multiple folate enzyme inhibition. Ann Oncol 7(Suppl 1):85
Spielmann M, Martin M, Namer M, duBois A, Unger C, Dodwell DJ (2001) Activity of pemetrexed (ALIMTA, multitargeted antifolate, LY231514) in metastatic breast cancer patients previously treated with an anthracycline and a taxane: an interim analysis. Clin Breast Cancer 2:47–51
Stabler SP, Lindenbaum J, Savage DG, Allen RH (1993) Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood 81:3404–3413
Sun H, Fadiran EO, Jones CD et al (1999) Population pharmacokinetics. A regulatory perspective. Clin Pharmacokinet 37: 41–58
Suwa T, Hogg JC, English D, Van Eeden SF (2000) Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol 279:H2954–H2960
Vogelzang NJ, Rusthoven JJ, Symanowski J et al (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
Wedge SR, Laohavinij S, Taylor GA, Boddy A, Calvert AH, Newell DR (1995) Clincial pharmacokinetics of the antipurine antifolate (6R)-5,10- dideaza-5,6,7,8-tetrahydrofolic acid (Lomotrexol) administered with an oral folic acid supplement. Clin Cancer Res 1:1479–1486
Yoshioka M, Mori Y, Miyazaki S, Miyamoto T, Yokomizo Y, Nakajima Y (1999) Biological functions of recombinant bovine interleukin 6 expressed in a baculovirus system. Cytokine 11:863–868
Acknowledgements
The authors wish to thank the following individuals for their assistance with the conduct of these analyses and preparation of this manuscript: Dinesh Dealwis, Lena Friberg, and S Thomas Forgue for their insightful scientific comments; Mary Brandes Dugan and Pete Fairfield for their scientific writing and editorial support; and, David B. Radtke for operations and project management support.
Author information
Authors and Affiliations
Corresponding author
Additional information
James J Rusthoven, Robert D Johnson were employees of Eli Lilly and Company, Indianapolis, USA, at the time this work was completed
Rights and permissions
About this article
Cite this article
Latz, J.E., Karlsson, M.O., Rusthoven, J.J. et al. A semimechanistic-physiologic population pharmacokinetic/pharmacodynamic model for neutropenia following pemetrexed therapy. Cancer Chemother Pharmacol 57, 412–426 (2006). https://doi.org/10.1007/s00280-005-0077-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-0077-5