Abstract
During pregnancy, the pharmacokinetics of an antiepileptic drug is altered because of changes in the clearance capacity and volume of distribution. These changes may have consequences for the frequency of seizures during pregnancy and fetal exposure to antiepileptic drugs. In 2009, a review was published providing guidance for the dosing and therapeutic drug monitoring of antiepileptic drugs during pregnancy. Since that review, new drugs have been licensed and new information about existing drugs has been published. With this review, we aim to provide an updated narrative overview of changes in the pharmacokinetics of antiepileptic drugs in women during pregnancy. In addition, we aim to formulate advice for dose modification and therapeutic drug monitoring of antiepileptic drugs. We searched PubMed and the available literature on the pharmacokinetic changes of antiepileptic drugs and seizure frequency during pregnancy published between January 2007 and September 2018. During pregnancy, an increase in clearance and a decrease in the concentrations of lamotrigine, levetiracetam, oxcarbazepine’s active metabolite licarbazepine, topiramate, and zonisamide were observed. Carbamazepine clearance remains unchanged during pregnancy. There is inadequate or no evidence for changes in the clearance or concentrations of clobazam and its active metabolite N-desmethylclobazam, gabapentin, lacosamide, perampanel, and valproate. Postpartum elimination rates of lamotrigine, levetiracetam, and licarbazepine resumed to pre-pregnancy values within the first few weeks after pregnancy. We advise monitoring of antiepileptic drug trough concentrations twice before pregnancy. This is the reference concentration. We also advise to consider dose adjustments guided by therapeutic drug monitoring during pregnancy if the antiepileptic drug concentration decreases 15–25% from the pre-pregnancy reference concentration, in the presence of risk factors for convulsions. If the antiepileptic drug concentration changes more than 25% compared with the reference concentration, dose adjustment is advised. Monitoring of levetiracetam, licarbazepine, lamotrigine, and topiramate is recommended during and after pregnancy. Monitoring of clobazam, N-desmethylclobazam, gabapentin, lacosamide, perampanel, and zonisamide during and after pregnancy should be considered. Because of the risk of teratogenic effects, valproate should be avoided during pregnancy. If that is impossible, monitoring of both total and unbound valproate is recommended. More research is needed on the large number of unclear pregnancy-related effects on the pharmacokinetics of antiepileptic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pharmacokinetics of antiepileptic drugs (AEDs) may change during pregnancy and this may result in a change of effect. |
It is advised to determine the AED concentration twice before conception for every woman with epilepsy to obtain a pre-pregnancy reference concentration (RC) for targeted therapeutic drug monitoring during and after pregnancy. |
Dose adjustment during pregnancy should be considered in combination with risk factors if the AED concentration deviates 15–25% from the RC and should always be considered if the AED concentration deviates more than 25% from the RC. |
1 Introduction
Treatment of epilepsy during pregnancy faces multiple challenges. Clinicians have to find a balance between the risk of increased seizure frequency for both the mother and child on the one hand and fetal exposure to antiepileptic drugs (AEDs) on the other hand. Most pregnant women with epilepsy (WWE) will deliver a healthy child, but some women experience an increase in seizure frequency during pregnancy, which can be harmful especially to the fetus. Fetal loss has been reported as a result of prolonged seizures, and poorer congenital development has been associated with frequent tonic-clonic seizures during pregnancy [1, 2]. Therefore, clinicians pursue a stable AED concentration that is effective enough to avoid seizures. However, maintaining a stable AED concentration during and after pregnancy is difficult owing to the alteration of AED pharmacokinetics including increased volume of distribution, elevated renal clearance, and induction of hepatic metabolism. [3,4,5]. Therefore, it is important to determine the reference concentration (RC) for every AED before pregnancy, which provides guidance for clinicians for the personalized management of individual WWE during and after pregnancy [6].
Many studies on fetal exposure, adverse drug reactions, and Major Congenital malformations associated with AED use during pregnancy have been published in the past decade [1, 7,8,9,10,11,12,13]. However, much less is known about the pharmacokinetics of specific AEDs during pregnancy compared with pre-pregnancy. In addition, little is known about the influence of the changed AED pharmacokinetics on seizure frequency in pregnant WWE. Moreover, guidelines on therapeutic drug monitoring (TDM) and dose adjustments during pregnancy are unclear and differ between countries. In 2009, Harden et al. [3] published on behalf of the American Academy of Neurology (AAN) and the American Epilepsy Society (AES) a practice parameter update of management issues in pregnant WWE. Since that review, new information on included AEDs has become available and new, not included AEDs are increasingly being prescribed. This narrative review aims to provide an updated overview of changes in the pharmacokinetics of AEDs and advice on TDM of 11 different AEDs before, during, and after pregnancy.
2 Literature Search
This narrative review includes six AEDs most frequently prescribed for the treatment of epilepsy during pregnancy in European countries: carbamazepine (CBZ), levetiracetam (LEV), lamotrigine (LTG), oxcarbazepine (OXC), topiramate (TPM), and valproate (VPA) [8] and five other AEDs: clobazam (CLB), gabapentin (GBP), lacosamide (LCM), perampanel (PER), and zonisamide (ZNS), which are currently increasingly prescribed in men and women with uncontrolled epilepsy. Seven of these AEDs are discussed by Harden et al. [3]. A literature search was performed to determine whether new publications concerning pharmacokinetics and changes in seizure frequencies during pregnancy of these seven AEDs had been published since the AAN/AES publication and whether TDM might be of any benefit. The remaining four AEDs (CLB, LCM, PER, and ZNS) that are used less frequently, but are expected to be increasingly prescribed in Europe in pregnant women, have been added to obtain an up-to-date overview.
The following strategies were endorsed to find the relevant literature. First, a systematic PubMed search using the MeSH terms ‘drug name’, ‘pregnancy’, and ‘drug monitoring’ per drug was employed. Second, the MeSH terms ‘pregnancy’, ‘epilepsy’, ‘anticonvulsants’, and ‘drug monitoring’ were used. In addition, studies that referred to Harden et al. [3] were selected. The practice parameter update of the AAN and AES formed the starting point for this updated review [3]. Because Harden et al. [3] included studies until October 2007, any literature published between January 2007 and September 2018 was screened for the seven drugs that Harden et al. [3] reviewed. All available relevant literature about the remaining four drugs and relevant articles for background information was included despite the year of publication. Articles were excluded on title and abstract, whereby articles that were not written in English and reviews were excluded from this study. Monotherapy studies were preferred above polytherapy studies because monotherapy studies provide better insight into drug-specific influences during pregnancy. Polytherapies were only included on the premise that the available monotherapy evidence was insufficient and drug–drug interactions were not expected. These polytherapy data are marked (a) in Tables 1 and 2. Reference lists of obtained records were screened to obtain additional studies not identified through database searching. Uptodate.com [14] provided further drug information on the pharmacokinetics of AEDs in the non-pregnant state. A total of 508 records were identified using our search strategy. After removing the duplicates, 373 unique records were screened on title, abstract, and year of publication, which resulted in 37 potentially relevant full-text articles. After reading the full text, eight full-text articles were excluded and 15 additional studies were included from cross-references, which resulted in 44 included studies (Fig. 1).
Tables 1 and 2 provide an overview of the pharmacokinetic changes of AEDs, seizure frequency, and recommendations on TDM before, during, and after pregnancy based on the included studies. When Harden et al. [3]. were unable to present pharmacokinetic changes or recommendations, new information and recommendations were formulated if possible and displayed in italics.
3 Impact of Pregnancy on Antiepileptic Drugs
3.1 Carbamazepine
One study compared seizure frequency during pregnancy with pre-pregnancy [15]. In 22% of the pregnancies, patients experienced an increase in seizures and in 33% of the pregnancies, patients experienced a decrease in seizures during pregnancy compared with pre-pregnancy. In 44% of the patients, the seizure frequency remained stable [15]. Other reports of seizure control during pregnancy agree that most pregnant WWE do not have increased seizure frequency during the trimesters of pregnancy if CBZ is used for seizure control [16, 17]. As a consequence, dose adjustments are not often performed during pregnancy [15, 17]. Clearance of CBZ or CBZ-epoxide is not significantly changed during pregnancy [15, 16]. Unbound CBZ concentrations are increased throughout pregnancy and the total and unbound CBZ-epoxide concentrations slightly decreased during the first trimester and normalized afterwards [15]. A relationship between seizure frequency and changed concentrations of total or unbound CBZ concentrations could not be demonstrated [15,16,17]. Data on postpartum pharmacokinetics are missing.
3.2 Clobazam
One study reported that patients experienced seizures in 72% of the pregnancies with CLB use (most polytherapy) [18]. Data of seizure frequency during pregnancy compared with pre-pregnancy seizure frequency are lacking. Pregnant WWE treated with CLB had more than twice the propensity to have seizures when compared with pregnant WWE who were using LTG, VPA, LEV, or TPM monotherapy [18]. Clobazam was often co-administered with other AEDs in the treatment of epilepsy for better seizure control [19,20,21]. None of the studies have published dose adjustments or changed pharmacokinetics of CLB and its active metabolite N-desmethylclobazam during and after pregnancy.
3.3 Lamotrigine
Use of an oral contraceptive (OC) before conception in combination with LTG treatment is associated with a reduced LTG concentration by approximately 50% [22, 23]. Several studies observed worsening of seizure control and increasing dose and/or addition of another AED (16–100%) more often among LTG pregnancies than in other AED pregnancies [16, 24,25,26,27]. In 19–38% of the pregnancies, the patients experienced an increase in seizures during pregnancy [16, 26, 28] and in 10–33% of the pregnancies, a decrease in seizures during pregnancy [16, 17, 26, 28] compared with pre-pregnancy. In 28–71% of the pregnancies, the seizure frequency remained stable [16, 18, 26, 28]. The clearance of total LTG strongly increased during pregnancy and the total LTG concentration significantly decreased [16, 25, 26, 29, 30]. Postpartum, the elimination rate returned to normal within the first few weeks [25, 30,31,32]. Studies suggest that the decline in total LTG plasma concentration is partly caused by a pregnancy-related enhanced hepatic glucuronidation and partly by an increased renal clearance [25, 29, 30].
3.4 Levetiracetam
Some studies report a high rate of seizure occurrence and dose increases and/or addition of another AED among LEV pregnancies with inter-individual variability [16, 21, 33,34,35,36]. A relatively large retrospective study reported that 47% of the pregnant WWE taking LEV monotherapy experienced more seizures during pregnancy compared with 1 year before pregnancy [16]. More data on seizure frequency compared with pre-pregnancy seizure frequency are still lacking. A more recent study emphasizes the potential benefit of TDM during LEV pregnancies due to the magnitude of LEV clearance between LEV pregnancies [4]. In addition, pharmacokinetic changes of LEV were observed during and after pregnancy [16, 21, 33,34,35,36]. The total LEV concentration decreased during pregnancy and showed a maximal decline in the third trimester (48–60%) [21, 33,34,35,36,37], owing to an increase of total LEV clearance during pregnancy [4, 16, 35, 36] that returned to normal in the first few weeks after delivery [21, 33, 34]. In addition, there is some evidence for better seizure control when LEV extended release is administered more frequently than once or twice daily [35, 36].
3.5 Oxcarbazepine
In 64–100% of the OXC pregnancies, seizure frequency increased during pregnancy compared with pre-pregnancy seizure frequency [27, 38]. Consequently, dose adjustments were performed in 86–100% of the pregnancies [27, 38]. The total plasma concentration of OXC’s active metabolite LIC decreased gradually throughout pregnancy [38], owing to an increased clearance probably by enhanced hepatic metabolism [25, 27, 30, 32]. It was therefore suggested that seizure deterioration could be caused by the changed LIC plasma concentration [27, 38]. The postpartum elimination returned to normal within the first 4–8 weeks after delivery [38].
3.6 Topiramate
Seizure frequency increased in 47% of the TPM pregnancies (including polytherapy) during the second and third trimesters according to one study [39]. In 27% of those pregnancies, seizure frequency also increased in the first trimester [39]. In the remaining pregnancies, patients were either seizure free before and during pregnancy or the seizure frequency remained stable during pregnancy [39]. As a consequence, high rates of dose increase or the addition of another AED were observed during the second and third trimesters [39]. Topiramate serum concentrations decreased throughout pregnancy and showed a maximum decline during the second and third trimesters [19, 39, 40]. One study presumed an increased clearance of TPM as a result of an increase in renal blood flow and a decrease in tubular reabsorption [19]. Results on the distribution and metabolism of TPM during pregnancy were conflicting between the different studies and evidence on postpartum elimination was lacking.
3.7 Valproate
Seizures occurred in 25–40% of the VPA pregnancies (including polytherapy) according to the reviewed studies [17, 41]. One study reports an increased seizure occurrence on VPA monotherapy during the second and third trimesters compared with the first trimester [17]. Comparison of seizure frequencies before pregnancy and during pregnancy has not been described yet. Dose adjustments were not often performed during VPA pregnancies, only in 7% [17]. Furthermore, unbound VPA fraction was found to be highly variable [41] and the effect of pregnancy on VPA clearance was not studied.
3.8 Zonisamide
An increase in seizures was reported in 33% of the ZNS pregnancies, which especially occurred in the second and third trimesters [42]. In addition, breakthrough seizures occurred in 40% of the pregnancies (including polytherapy) in WWE who were seizure free in the pre-pregnancy year [42] and dose adjustments were frequently performed during pregnancy [42]. More data on seizure frequency compared with pre-pregnancy seizure frequency are lacking. Several studies report an increased ZNS clearance and a decreased ZNS plasma concentration during pregnancy [16, 42, 43]. The decline of ZNS concentration is probably associated with the observed increase of seizure frequency [42]. Evidence for changed clearance after pregnancy was lacking.
3.9 Gabapentin, Lacosamide, and Perampanel
To our knowledge, there are no data on the pharmacokinetics, seizure frequency, or dose adjustments of GBP, LCM, and PER during and after pregnancy.
4 Clinical Implications
Although the evaluation of the safety of AED use during pregnancy has received much scientific attention over the last few years [1, 7, 8, 12], knowledge about the pharmacokinetics and its influence on seizure frequency is still scarce. An important reason may be the reluctance to study new drugs in pregnant women because of their unknown risks for teratogenic effects. Nevertheless, new information about the pharmacokinetics of AEDs during pregnancy based on prospective studies and case reports in pregnant WWE has become available, leading to this update of the practice parameter update of Harden et al. [3] of the AAN and AES.
4.1 Pregnancy-Related Changes
A general recommendation for AED use during pregnancy is to prescribe AEDs at the lowest effective dose. In addition, monotherapy is preferred above polytherapy and TDM-guided dosing is recommended for some AEDs. However, drug-specific recommendations can only be made if the drug-specific pharmacokinetic changes during pregnancy are clear and when these changes are associated with an increased risk of seizures. Increased plasma volume may lead to an increased volume of distribution, which potentially reduces the AED concentration [5, 44]. It is suggested that circulating pregnancy hormones could compete with highly protein-bound AEDs for protein binding. Furthermore, the serum albumin level may also be reduced as a result of the increased plasma volume. These pregnancy-related changes could increase the unbound (pharmacologically active) fraction and affect the clearance of (especially highly) protein-bound AEDs. Pregnancy-related increased renal blood flow and glomerular filtration rate may reduce the serum concentrations of AEDs eliminated through the kidneys [44]. In addition, activities of some cytochrome P450 and uridine diphosphate glucuronosyltransferase isoenzymes are induced during pregnancy, which may lead to increased metabolism of AEDs predominantly metabolized by these isoenzymes. We have searched for evidence in the literature for drug-specific pharmacokinetic changes during pregnancy, which is presented in Table 1 and described per AED.
4.2 Determine the Reference Concentration Before Pregnancy
According to the reviewed studies, it is generally recommended to determine the trough RC of AEDs before conception when the WWE are on stable seizure control on the lowest effective AED dose and to maintain this AED concentration throughout pregnancy by adjusting the dose. However, determining of the RC before conception is not always performed in practice (especially when a pregnancy is unplanned). Hence, we emphasize the importance of determining the individual RC during stable seizure control for every AED before conception. We recommend to measure trough concentrations (for total and/or unbound concentration, see Table 2) two times before conception to take intra-individual differences into account. We also recommend measuring total and unbound AEDs trough concentrations when the AED is highly protein bound (≥ 90%) and to measure only total serum trough concentrations when the AED is not highly protein bound (< 90%).
Furthermore, it is important to know that plasma concentrations of some AEDs can be influenced by discontinuing estrogen-containing OCs [22, 23]. Use of these OCs in combination with LTG is associated with a reduction in the LTG concentration by approximately 50% [22, 23]. Estrogen-induced hepatic glucuronidation has been implicated in the decline of LTG concentrations [22, 23]. Hence, when WWE have been using OCs before planning pregnancy, the LTG concentration will rise strongly after discontinuing the OCs [45]. Based on this knowledge, we recommend to measure the LTG concentration twice and determine the RC on continuous use of the OC. Thereafter, we advise to reduce the LTG dose by 50% at the same time of discontinuing the OC to maintain the RC. Within 1 week to 1 month after discontinuing the OC, we advise monitoring a third trough LTG concentration to determine if the LTG concentration is comparable with the previous RC. Repeated monitoring and adjustment of the LTG dose could be necessary to titrate the WWE on stable seizure control before conception. Other AEDs with a major elimination pathway through glucuronidation, such as OXC’s metabolite LIC (50–60%) and VPA (30–50%) [14], are also likely to show a decrease in concentration because of the influence of OCs, which should be taken into account by measuring the RC. However, for LIC and VPA, this effect is of less importance compared with LTG, which is almost fully eliminated through glucuronidation [46]. No evidence has been reported yet for a possible influence of discontinuing the OC on the pharmacokinetics of other AEDs. Nevertheless, there is a possibility that changing hormone levels may have some influence on the AED concentration. Because we assume the WWE will be on stable seizure control 1 month after discontinuation of the OC, we advise to determine the RC 1 month after discontinuing the OC and before conception. The importance of determining the RC and the different strategies for determining the RC when the WWE used OCs underlines the need for intensive pre-pregnant guidance of the WWE by clinicians.
4.3 Monitoring and Dose Adjustment During Pregnancy
For WWE receiving AED treatment without routine TDM advice during pregnancy, clinicians should decide when TDM could be of value in treating WWE receiving AED treatment during pregnancy. Therefore, clinical features should be taken into account. Therapeutic drug monitoring of those AEDs could be helpful when clinical features deteriorate. Nevertheless, the EMPiRE study from Thangaratinam et al. [47] claims that there is no evidence to suggest that TDM-guided dosing of AEDs in pregnancy improves seizure control compared with dosing guided by clinical effects. One inclusion criterion in this study was ‘viable pregnancy of < 24 weeks gestation’, which means that they could not compare AED concentrations during pregnancy with pre-pregnancy concentrations for every included patient. As a result, the often sharp fall of LTG concentration and possibly other AEDs in the beginning of pregnancy could have been missed, which misleads the conclusion. In our view and using this knowledge, TDM can be very helpful in providing clinicians guidance to avoid seizures during pregnancy.
After deciding TDM could be beneficial for the improvement of the individual AED therapy, trough AED concentrations should be compared with the pre-pregnancy RCs. Based on the results, clinicians have to decide if a dose adjustment is required. Many reviewed studies recommend a dose adjustment during pregnancy. However, clear advice when to perform a dose adjustment is lacking.
Therefore, we have formulated a guide on when to perform dose adjustments of AEDs during pregnancy, which can be considered by clinicians when WWE are pregnant. This guidance is based on two sources of drug variation allowed in practice: the variation between analytical results and the batch-to-batch variation in drug content. The variation between analytical results of drug concentrations in blood allowed by the US Food and Drug Administration [48] and the European Medicines Agency [49] amounts to 15%. Following that variation, we assume that deviations in AED concentrations less than 15% from the RC could be the result of the analysis and do not necessarily require dose adjustments. The actual amount of the active ingredient of a drug dispensed may vary from 90 to 110% of the amount declared on the label [50], which results in a maximum batch-to-batch variation of 20%. The total accepted variation of those variations together will be the maximum: √(202 + 152) = 25%. Following this calculation, deviations in AEDs through concentrations between 15 and 25% from the RC are more likely to cause seizures or side effects. Therefore, we suggest to consider a dose adjustment in this range taking risk factors into account. For example, polytherapy of AEDs, type of seizures and, most important, seizure occurrence in the year prior to pregnancy are such risk factors [3, 16, 18, 24]. Seizure occurrence in the pre-pregnancy month is even associated with a 15 times higher risk of developing seizures during pregnancy [18]. In the case of WWE being seizure free during the year prior to pregnancy, we suggest that a dose adjustment is not necessary. Last, we advise a dose adjustment when the drug concentration decreases more than 25% from the RC to pursue the RC and avoid seizures. However, the risk on fetal exposure should always be taken into account.
Other expert opinions on adjusting AED doses during pregnancy are mostly based on the results of LTG pregnancies. Their approach is to adjust the AED dose when the AED concentration falls below 65% of the pre-pregnancy baseline concentration because it has been demonstrated that the seizure frequency of WWE on LTG pregnancies increased when this occurred [4, 26]. However, it has not been demonstrated that this also applies to other AEDs than LTG.
We agree that we have formulated a stricter regime of when to perform a dose adjustment of AEDs to protect the WWE and the unborn from seizures during pregnancy. However, our approach with the different deviation categories of 0–15%, 15–25%, and > 25% of the RC is similar to the Danish policy of pursuing the RC during pregnancy [51]. Future prospective research could use our systematic approach of when to perform TDM of AEDs during pregnancy. This allows future studies to acquire pharmacokinetic and pharmacotherapeutic knowledge in a structural manner.
4.4 Monitoring and Dose Adjustments after Delivery
The pharmacokinetics of AEDs will return to the pre-pregnancy situation after delivery. Therefore, dose adjustments performed during pregnancy could lead to an overdose and side effects after pregnancy. As the pharmacokinetics and clearance of AED can rapidly return to normal after birth, we have to consider a dose adjustment after delivery. Major changes in dose during pregnancy will request an almost immediate start of tapering the dose after delivery towards pre-pregnancy concentrations. Smaller adjustments in dose during pregnancy require minor and less frequent interventions. Therefore, we advise monitoring trough AED concentrations and gradually adjusting the dose over 0–21 days after delivery until normalization, when the AED dose was adjusted during pregnancy. Post-partum drug monitoring is not required when the AED dose has not been changed during pregnancy. It is helpful to instruct the WWE that specific toxic symptoms of the used AED, such as diplopia, dizziness, and ataxia, can occur if the dose is not adjusted rapidly enough. In addition, we advise not to pursue pre-pregnancy values but concentrations that are slightly higher to provide the WWE with more seizure protection after delivery as the women may experience more stress and sleep deprivation.
4.5 Therapeutic Drug Monitoring of Specific Antiepileptic Drugs
Because of minimal changes in CBZ concentrations and clearances during pregnancy that appeared not to be related with increased seizure frequency, TDM of CBZ was suggested as unnecessary during pregnancy. However, the sample sizes of the reviewed studies are small, which necessitates future larger studies for confirmation of this suggestion. Based on the literature, TDM of CBZ currently seems not useful or profitable for WWE, as concentrations cannot be related to seizure occurrence. Therefore, we suggest to determine only the total trough RC of CBZ and CBZ-epoxide before pregnancy for comparison purposes. A change of CBZ treatment should depend on clinical signs, seizure risks, and previous doses until more evidence on CBZ is obtained in larger studies.
The reviewed studies of LTG and LEV pregnancies conclude that seizure deterioration is a consequence of a decline in concentrations of LTG and LEV, respectively. Although many of the reviewed LEV studies included patients receiving AED polytherapy, it is expected that the pharmacokinetics of LEV does not change as a result of polytherapy because LEV has a low protein binding and few drug–drug interactions. Hence, we can use the pharmacokinetics of LEV during polytherapy as a reference to discuss the changed LEV concentrations. Therefore, seizure occurrence of WWE treated with LTG or LEV during pregnancy might be controlled by routine monitoring of the total LTG or LEV concentrations and dose adjustments. Hence, we advise for these pregnancies to measure the total serum RC of LTG and LEV twice before conception, monthly during pregnancy, and after delivery until stable seizure control is obtained with gradual dose adjustments over 0–21 days. Dose adjustments of LTG and LEV could be necessary during and after pregnancy depending on the clinical features, seizure risk factors, adverse drug reactions, and previous dose. When the WWE are not on stable seizure control with twice-daily LEV immediate-release tablets, it can be considered to divide the daily dose over three of four times a day or change to slow-release tablets if available. Because of the short half-life of LEV, especially during pregnancy, slow-release LEV tablets have preference, if available.
Seizure deterioration during OXC pregnancy has been associated with decreased total LIC trough concentrations [38], which reinforces TDM before, during, and after pregnancy. However, one patient experienced an increase in the seizure frequency of more than 50% despite a 6% increase in LIC plasma concentration, which suggests other factors than plasma concentrations may contribute to seizure deterioration [38]. Support for this finding is limited and more research on OXC use during pregnancy is needed. Considering this, we advise a TDM regime with necessary dose adjustments before, during, and after pregnancy comparable to LTG and LEV pregnancies.
According to a recent study TPM (one of the newer AEDs), clearance is significantly increased during the second and third trimesters of pregnancy resulting in lower drug concentrations [4]. This is presumably caused by a pregnancy-related increased renal blood flow and decreased tubular reabsorption because TPM is excreted unchanged by the kidneys [19]. To our knowledge, the study of Voinescu et al. [4] is the first study to confirm the relationship between the decreased TPM concentrations and increased seizure frequency [4]. Based on this study, we recommend a TDM regime of before, during, and after pregnancy comparable with LTG, LEV, and OXC pregnancies.
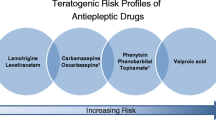
There is strong evidence that VPA use during pregnancy is associated with an increased risk on congenital malformations. Therefore, the International League Against Epilepsy, European Academy of Neurology and the European Medicines Agency [52] have recently recommended that VPA should preferably be avoided during pregnancy [53]. However, several studies have found that the teratogenic risk of VPA is dose dependent and recommend not to prescribe VPA at doses exceeding 500–600 mg/day in fertile women. Continuing the administration of VPA during pregnancy can only be considered for women who are well controlled on a low dose of VPA 500–600 mg/day with no other alternatives being available [10, 53, 54]. Furthermore, it is suggested that the unbound VPA concentration increases as result of a decrease in serum albumin during pregnancy, when at the same time, the total VPA concentration will decrease [41]. However, WWE and partners must be very clearly informed of the teratogenic risks and possible long-term effects on the child. Therefore, when it is decided to continue VPA during pregnancy, we advise TDM of total and unbound VPA concentrations in the same schedule as LTG, LEV, and OXC pregnancies. In addition, it could be considered to divide the daily dose of sustained-release VPA over three or four times a day to reduce the teratogenic risk as a result of high peak concentrations.
Data on the pharmacokinetics and sufficient data on seizure occurrence during pregnancy are lacking for WWE treated with CLB, GBP, LCM, and PER during pregnancy. This is mainly owing to the lack of teratogenic safety data, which have resulted in the limited use of these AEDs in pregnant women. Therefore, guidelines for performing TDM during pregnancy are lacking for these AEDs. There is some evidence of an increased clearance and decreased serum concentration of ZNS during pregnancy. However, the study included pregnancies on the polytherapy of ZNS with other AEDs, which makes it difficult to rule out the effects of the other drugs on ZNS [32]. Therefore, more research on these AEDs during pregnancy is needed. Hence, we recommend caution when prescribing one of these AEDs during pregnancy and the monitoring of trough concentrations (total concentrations of GBP, LCM, and ZNS and total and unbound concentrations of CLB, dmCLB, and PER due to strong protein binding [55]) twice before conception to determine the RC and guide the dose adjustment of those AEDs during pregnancy when the patient deteriorates or the risk for seizure occurrence has increased.
4.6 Further Research
Although much is already known about the pharmacokinetics of some AEDs during pregnancy, more research on the pharmacokinetic changes of some other AEDs during pregnancy is needed. In particular, data on the pharmacokinetics of CLB, GBP, LCM, PER, and ZNS during pregnancy are not available. Moreover, the relationship between the pharmacokinetic changes of AEDs and changes in seizure frequency should be investigated more precisely and perhaps result in physiology-based population pharmacokinetic models in pregnant women. In addition, for some AEDs, only polytherapy data are available, which confounds the ‘drug-specific’ effect on seizure frequency and the effect of pregnancy on drug pharmacokinetics. This reinforces the need for research on AED monotherapies. Furthermore, factors other than drug plasma concentrations may contribute to seizure deterioration as suggested in one OXC case report [38], which encourages more research on seizure protection during pregnancy.
5 Conclusions
This review has added new insights into the pharmacokinetic changes of AEDs and seizure frequency and provides guidance on the monitoring and management of AEDs during pregnancy. We advise to measure RCs of all AEDs twice before conception. The recommendations on TDM of AEDs during pregnancy can be divided into those that are recommended to routinely measure, which include LEV, LIC, LTG, TPM and VPA if avoiding use during pregnancy is impossible. Monitoring during pregnancy should be considered in CLB, GBP, LCM, PER, and ZNS pregnancies depending on clinical features and seizure-predicting factors. The role of TDM is questionable during CBZ pregnancies. We advise considering dose adjustments of AEDs during pregnancy when the AED concentration decreases between 15 and 25% from the pre-pregnancy RC in combination with risk factors. Seizure occurrence in the pre-pregnancy year is the most important seizure-predicting factor during pregnancy. We advise dose adjustment if the AED concentration decreases more than 25% from the RC, and postpartum monitoring only when the AED dose was changed during pregnancy. Although new information on AED pharmacokinetics during pregnancy has become available, more research on TDM of AED use during pregnancy is still needed.
References
Meador KJ, Baker GA, Browning N, et al. Effects of fetal antiepileptic drug exposure: outcomes at age 4.5 years. Neurology. 2012;78:1207–14.
Teramo K, Hiilesmaa V. Pregnancy and fetal complications in epileptic pregnancies. In: Janz D, Dam M, Bossi L, Helge H, Richens A, Schmidt D, editors. Epilepsy, pregnancy, child. New York: Raven Press; 1982. p. 53–9.
Harden CL, Pennell PB, Koppel BS, et al. Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding. Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142–9.
Voinescu PE, Park S, Chen LQ, et al. Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy. Neurology. 2018;91(13):e1228–36. https://doi.org/10.1212/WNL.0000000000006240.
Tomson T, Landmark CJ, Battino D. Antiepileptic drug treatment in pregnancy: changes in drug disposition and their clinical implications. Epilepsia. 2013;54:405–14.
Patsalos PN, Berry DJ, Bourgeois BF, et al. Antiepileptic drugs: best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49:1239–76.
Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10:609–17.
Tomson T, Battino D, Bonizzoni E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530–8.
Briggs GG, Freeman RK, Towers CV. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. Philadelphia: Lippincott Williams and Wilkins; 2017.
Campbell E, Kennedy F, Russell A. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry. 2014;85:1029–34.
Holmes L, Harvey E, Coull B. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132–8.
Güveli BT, Rosti RO, Güzeltas A, et al. Teratogenicity of antiepileptic drugs. Clin Psychopharmacol Neurosci. 2017;15:19–27.
Meador KJ, Baker GA, Browning N, et al. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011;134:396–404.
uptodate.com. Available from: https://www.uptodate.com/contents/search. Accessed 21 June 2018.
Johnson EL, Stowe ZN, Ritchie JC, et al. Carbamazepine clearance and seizure stability during pregnancy. Epilepsy Behav. 2014;33:49–53.
Reisinger TL, Newman M, Loring DW, et al. Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav. 2013;29:13–8.
Battino D, Tomson T, Bonizzoni E, et al. Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia. 2013;54:1621–7.
Thomas S, Syan U, Devi J. Predictors of seizures during pregnancy in women with epilepsy. Epilepsia. 2012;53:2010–3.
Öhman I, Sabers A, de Flon P, et al. Pharmacokinetics of topiramate during pregnancy. Epilepsy Res. 2009;87:124–9.
Patsalos PN, Gougoulaki M, Sander JW. Perampanel serum concentrations in adults with epilepsy: effect of dose, age, sex and concomitant anti-epileptic drugs. Ther Drug Monit. 2016;38:358–64.
López-Fraile IP, Cid AO, Juste AO, et al. Levetiracetam plasma level monitoring during pregnancy, delivery, and postpartum: clinical and outcome implications. Epilepsy Behav. 2009;15:372–5.
Sabers A, Buchholt J, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151–4.
Sabers A, Ohman I, Christensen J, et al. Oral contraceptives reduce lamotrigine plasma levels. Neurology. 2003;61:570–1.
Vajda F, O’Brien T, Lander C, et al. The efficacy of the newer antiepileptic drugs in controlling seizures in pregnancy. Epilepsia. 2014;55:1229–34.
Öhman I, Beck O, Vitols S. Plasma concentrations of lamotrigine and its 2-N-glucuronide metabolite during pregnancy in women with epilepsy. Epilepsia. 2008;49:1075–80.
Pennell PB, Peng L, Newport DJ, et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology. 2008;70:2130–6.
Wegner I, Edelbroek P, De Haan GJ, et al. Drug monitoring of lamotrigine and oxcarbazepine combination during pregnancy. Epilepsia. 2010;51:2500–2.
Sabers A, Petrenaite V. Seizure frequency in pregnant women treated with lamotrigine monotherapy. Epilepsia. 2009;50:2163–6.
Reimers A, Brodtkorb E. Second-generation antiepileptic drugs and pregnancy: a guide for clinicians. Expert Rev Neurother. 2012;12:707–17.
Polepally AR, Pennell PB, Brundage RC, et al. Model-based lamotrigine clearance changes during pregnancy: clinical implication. Ann Clin Transl Neurol. 2014;1:99–106.
Fotopoulou C, Kretz R, Bauer S, et al. Prospectively assessed changes in lamotrigine-concentration in women with epilepsy during pregnancy, lactation and the neonatal period. Epilepsy Res. 2009;85:60–4.
Tomson T, Battino D. Pharmacokinetics and therapeutic drug monitoring of newer antiepileptic drugs during pregnancy and the puerperium. Clin Pharmacokinet. 2007;46:209–19.
Novy J, Hubschmid M, Michel P, et al. Impending status epilepticus and anxiety in a pregnant woman treated with levetiracetam. Epilepsy Behav. 2008;13:564–6.
Westin A, Reimers A, Helde G, et al. Serum concentration/dose ratio of levetiracetam before, during and after pregnancy. Seizure. 2008;17:192–8.
Garrity LC, Turner M, Standridge SM. Increased levetiracetam clearance associated with a breakthrough seizure in a pregnant patient receiving once/day extended-release levetiracetam. Pharmacotherapy. 2014;34:e128–32.
Cappellari AM, Cattaneo D, Clementi E, et al. Increased levetiracetam clearance and breakthrough seizure in a pregnant patient successfully handled by intensive therapeutic drug monitoring. Ther Drug Monit. 2015;37:285–7.
Tomson T, Palm R, Källén K, et al. Pharmacokinetics of levetiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia. 2007;48:1111–6.
Petrenaite V, Sabers A, Hansen-Schwartz J. Seizure deterioration in women treated with oxcarbazepine during pregnancy. Epilepsy Res. 2009;84:245–9.
Westin AA, Nakken KO, Johannessen SI, et al. Serum concentration/dose ratio of topiramate during pregnancy. Epilepsia. 2009;50:480–5.
Ornoy A, Zvi N, Arnon J, et al. The outcome of pregnancy following topiramate treatment: a study on 52 pregnancies. Reprod Toxicol. 2008;25:388–9.
Johannessen Landmark C, Huuse Farmen A, Larsen Burns M, et al. Pharmacokinetic variability of valproate during pregnany: implications for the use of therapeutic drug monitoring. Epilepsy Res. 2018;141:31–7.
Reimers A, Helde G, Becser Andersen N, et al. Zonisamide serum concentrations during pregnancy. Epilepsy Res. 2018;144:25–9.
Oles KS, Bell WL. Zonisamide concentrations during pregnancy. Ann Pharmacother. 2008;42:1139–41.
Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008.
Wegner I, Edelbroek P, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388–93.
Herzog AG, Blum AS, Farina EL, et al. Valproate and lamotrigine level variation with menstrual cycle phase and oral contraceptive use. Neurology. 2009;72:911–4.
Thangaratinam S, Marlin N, Newton S, et al. AntiEpileptic drug Monitoring in PREgnancy (EMPiRE): a double-blind randomised trial on effectiveness and acceptability of monitoring strategies. Health Technol Assess. 2018;22:1–152.
FDA, CDER, CVM. Bioanalytical method validation guidance for industry. Silver Spring: Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER) and Center for Veterinary Medicine (CVM); 2018.
EMA. Guideline on bioanalytical method validation. Eur Med Agency Comm Med Prod Hum Use. 2015;44:1–23.
Art. 3 van het Besluit Geneesmiddelenwet. 2018. Available from: http://wetten.overheid.nl/BWBR0021672/2018-01-01#Paragraaf2. Accessed 22 Nov 2019.
Sabers A. Algorithm for lamotrigine dose adjustment before, during, and after pregnancy. Acta Neurol Scand. 2012;126:e1–4.
European Medicines Agency. New measures to avoid valproate exposure in pregnancy endorsed. London: European Medicines Agency (EMA); 2018. p. 1–4.
International League Against Epilepsy (ILAE) and European Academy of Neurology (EAN). Valproate in the treatment of epilepsy in women and girls. Pre-publication summary of recommendations from a joint Task Force of ILAE-Commission on European Affairs and European Academy of Neurology (EAN). 2018. Available from: https://www.ilae.org/files/ilaeGuideline/ValproateCommentILAE-0315.pdf. Accessed 22 Nov 2019.
Hernandez-Diaz S, Smith C, Shen A. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78:1692–9.
Patsalos PN, Zugman M, Lake C, et al. Serum protein binding of 25 antiepileptic drugs in a routine clinical setting: a comparison of free non-protein-bound concentrations. Epilepsia. 2017;58:1234–43.
Kacirova I, Grundmann M, Brozmanova H. Concentrations of carbamazepine and carbamazepine-10,11-epoxide in maternal and umbilical cord blood at birth: influence of co-administration of valproic acid or enzyme-inducing antiepileptic drugs. Epilepsy Res. 2016;122:84–90.
de Leon J, Spina E, Diaz FJ. Clobazam therapeutic drug monitoring: a comprehensive review of the literature with proposals to improve future studies. Ther Drug Monit. 2013;35:30–47.
Burns M, Baftiu A, Opdal M, et al. Therapeutic drug monitoring of clobazam and its metabolite: impact of age and comedication on pharmacokinetic variability. Ther Drug Monit. 2016;38:350–7.
Shorvon S, Perucca E, Engel J Jr. The treatment of epilepsy. 4th ed. Chichester: Wiley; 2016.
Kacirova I, Grundmann M, Brozmanova H. Serum levels of lamotrigine during delivery in mothers and their infants. Epilepsy Res. 2010;91:161–5.
Lyseng-Williamson K, Yang L. Spotlight on topiramate in epilepsy. CNS Drugs. 2008;22:171–4.
Sills G, Brodie M. Pharmacokinetics and drug interactions with zonisamide. Epilepsia. 2007;48:435–41.
Kawada K, Itoh S, Kusaka T, et al. Pharmacokinetics of zonisamide in perinatal period. Brain Dev. 2002;24:95–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
Inge J. Arfman, Elisabeth A. Wammes-van der Heijden, Peter G.J. ter Horst, Danielle A. Lambrechts, Ilse Wegner, and Daan J. Touw have no conflicts of interest that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Arfman, I.J., Wammes-van der Heijden, E.A., ter Horst, P.G.J. et al. Therapeutic Drug Monitoring of Antiepileptic Drugs in Women with Epilepsy Before, During, and After Pregnancy. Clin Pharmacokinet 59, 427–445 (2020). https://doi.org/10.1007/s40262-019-00845-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00845-2