Abstract
Lacosamide is an antiepileptic drug (AED) that has linear pharmacokinetics, predictable blood concentrations, and few drug interactions, setting it apart from other AEDs that require vigorous therapeutic drug monitoring (TDM) such as phenytoin and carbamazepine. However, there have been reports of altered lacosamide pharmacokinetics in some populations. The purpose of this review is to determine whether lacosamide pharmacokinetics are altered in certain patient populations, suggesting the need for TDM. A literature search of Medline, Scopus, Embase, and Cochrane trials was conducted on January 3, 2019 (and then updated on September 2, 2019) to search for articles relevant to the TDM or pharmacokinetics of lacosamide. A total of 56 relevant articles were found and included in this review. Dose of lacosamide is linearly correlated with plasma concentrations and efficacy. However, currently there is no well-established reference range. Overall, the recommended reference ranges varied from 2.2 to 20 mg/L. Lacosamide has very few clinically relevant drug–drug interactions; however, there seems to be a significant drug interaction between lacosamide and enzyme-inducer AEDs. Based on available literature, it appears that lacosamide pharmacokinetics may be altered in severe renal dysfunction, in patients on dialysis and with extremes of age. More evidence is currently needed on lacosamide pharmacokinetics in pregnancy and critical illness. While it is not practical to utilize TDM for all patients, TDM may be useful in patients taking enzyme-inducer AEDs, in patients with decreased renal function or on dialysis, and older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dose of lacosamide is linearly correlated with plasma concentrations and efficacy. However, currently there is no well-established reference range. |
Lacosamide TDM may be useful in patients taking enzyme-inducer antiepileptic drugs, in patients with decreased renal function or on dialysis, and older adults. |
Further studies are needed to determine the need for lacosamide TDM during pregnancy, in pediatrics, and in critically ill patients. |
1 Introduction
Lacosamide is an antiepileptic drug (AED) indicated in many countries as adjunctive therapy in adults for focal seizures [1], although indication of lacosamide is expanded in some countries for use as monotherapy and in pediatrics [2]. The mechanism of action of lacosamide is unique compared to other AEDs in that it enhances the slow inactivation of sodium channels, thereby inhibiting excessive firing of neurons [3]. Lacosamide has a more favorable side-effect profile compared to some of the older AEDs, with the most common side-effects being dizziness, headache, and nausea [4]; however, it is also known to prolong the PR interval [4, 5].
The pharmacokinetic profile of lacosamide has been well established. It has 100% oral bioavailability and reaches peak plasma concentration in 1–4 h [6]. Protein binding is minimal (< 15%) [7], and volume of distribution is about 0.6 L/kg. Elimination is 60% hepatic by CYP3A4, 2C9 and 2C19, and 40% renal [6]. The inactive metabolite formed, O-desmethyl-lacosamide, is also eliminated renally. The average half-life is predicted to be about 13 h in adults [6]. Because of its linear pharmacokinetics, predictable serum concentrations, and minimal drug interactions, current guidelines recommend that therapeutic drug monitoring (TDM) of lacosamide is not necessary. However, there have been reports of altered lacosamide pharmacokinetics in certain populations such as in older adults or those with impaired renal function [7]. Altered serum lacosamide disposition may result in serum concentrations that are too low to maintain efficacy, or elevated with the increased potential to cause adverse effects or toxicity, suggesting the potential need for TDM. Factors that may affect lacosamide pharmacokinetics include renal function, pregnancy status, age, and critical illness.
The aim of this review was to summarize the current evidence pertaining to lacosamide pharmacokinetics and to determine whether lacosamide pharmacokinetics are altered in certain patient populations, suggesting the need for TDM. While other reviews have discussed lacosamide TDM in a broader context [8], this review is the first to examine the potential utility of lacosamide TDM in special populations.
2 Literature Search
Medline, Scopus, Embase, and Cochrane trials databases were searched using the keywords (lacosamide OR vimpat OR copinar) AND (monitor* OR tdm OR pharmacokinetic* OR pk OR “drug administration schedul*” OR “concentration controlled”). Keywords were selected and refined with the assistance of a librarian. The search was repeated to capture any new publications. An abstract and title screening was performed independently by both authors. A full text review was performed to further refine search results. Books, reviews, animal studies, and editorials were excluded. Conference abstracts were included if they were deemed relevant and contained lacosamide blood concentrations. All primary studies that included lacosamide blood concentrations were included. Data extracted from studies included study design, population studied, number of participants, age range, proportion of male participants, lacosamide dosage range, study aim, main results and suggested reference range if included.
3 Results of Literature Search
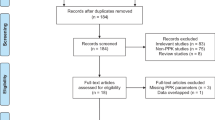
The keyword search was completed on January 3, 2019 and then updated on September 2, 2019 (Fig. 1). Medline, Scopus, Embase, and Cochrane trials produced 215, 340, 712, and 72 results, respectively, for a total of 1,339 records. Two additional records were identified through scanning the reference lists of the reviewed articles. After duplicate removal, 765 records remained. Then, 578 were excluded in the abstract screening, primarily due to irrelevance, leaving 187 for the full text review. Of those, 131 were excluded, primarily due to irrelevance, except for 2 excluded due to inability to translate to English. Therefore, a total of 56 articles were included in the discussion of this paper (12 of which were conference abstracts), including 14 randomized controlled trials (RCTs), 9 secondary analyses, 25 observational studies (10 prospective, 15 retrospective), 1 case series, and 7 case reports. Based on the GRADE working group, the quality of evidence of the included articles ranges from low to very low [9]. Table 1 depicts a summary of the included studies.
4 Therapeutic Drug Monitoring
Several AEDs such as valproic acid, carbamazepine, and phenytoin require plasma concentration monitoring as a way to ensure efficacy and avoid toxicity. Although the TDM of lacosamide is not currently routinely recommended, a number of studies have evaluated how lacosamide plasma concentration correlates with dose and clinical efficacy. Achieving a therapeutic plasma concentration is vital in seizure prevention. Almost all studies included have reported that lacosamide plasma concentrations are linearly related to the dose. Additionally, dose and plasma concentration are proportionally related to clinical efficacy [10]. For example, Ben-Menachem et al. reported increased efficacy of lacosamide in terms of percentage seizure reduction with increasing dose from 200 to 400 to 600 mg/day [11]. However, there was not a significant difference between 400 mg/day and 600 mg/day for focal seizures. In this study, when looking at secondarily generalized tonic–clonic seizures, lacosamide 600 mg/day achieved a significantly greater percentage seizure reduction than 400 mg/day (93.0% vs 59.4%, respectively). The same study also found increased adverse effects with lacosamide 600 mg/day. Similarly, a post hoc analysis looking at exposure of lacosamide and efficacy found an inverse relationship between area under the curve (AUC) and seizure probability over time [12], which is consistent with the correlation between dose and efficacy seen in other studies [10]. On the other hand, one conference abstract of a retrospective observational study looking at the efficacy of intravenous (IV) initiation of lacosamide did not find a relationship between mg/kg dose and plasma concentration [13], but this was likely due to the small sample size and the fact that investigators did not collect times of plasma samples.
Because lacosamide TDM is not routinely practiced, a well-established reference range has not been agreed upon. A few observational studies have suggested reference ranges for lacosamide plasma concentrations, with slight variation between them. Overall, the recommended reference ranges varied from 2.2 to 20 mg/L, and there does not appear to be a consensus on the most acceptable reference range. To illustrate, Payto et al. suggested a reference range of 2.2–19.8 mg/L after analyzing random serum samples at lacosamide doses of 200 and 400 mg/day and finding lacosamide concentrations ranging from 2.2 to 9.8 mg/L and 3.1 to 19.8 mg/L, respectively [14]. Perrenoud et al. recommended a similar but narrower reference range of 10–20 mg/L [15]. In this study they also looked at the efficacy of loading doses of lacosamide, where they found that a loading dose of at least 9 mg/kg was correlated with plasma concentrations within this reference range; however, it was not correlated with clinical response. Furthermore, Svendsen et al. recommended a range of 2.5–10 mg/L because they found that 60 out of 65 patients with good clinical efficacy of lacosamide had plasma concentrations in this range [16]. A limitation with this study was that they did not report the plasma concentrations of patients who were not experiencing efficacy from lacosamide, so we cannot be sure whether those patients also had lacosamide plasma concentrations in this range. On the other hand, one retrospective observational study attempted to define a safety range for lacosamide plasma concentrations in which adverse events do not occur [17]; however, they were unable to find a correlation. They found no significant difference in plasma concentration between those who experienced adverse effects (such as dizziness, visual disturbances, fatigue, nausea, vomiting, headache) and those who did not: 6.7 ± 4.3 mg/L versus 5.12 ± 2.2 mg/L (p = 0.27), respectively.
While lacosamide is traditionally dosed twice daily, one study examined the concentrations of lacosamide at various times per day to describe lacosamide concentration fluctuations [18]. The mean peak drug concentration (Cmax) was about 10 mg/L, with a range from 4.0 to 18.3 mg/L. Thirteen patients received lacosamide twice daily, but in the four who received lacosamide three times daily, there were fewer fluctuations in serum concentrations as well as fewer adverse drug reactions. The authors concluded that dosing lacosamide three times daily may improve tolerability.
There are three studies that examined alternative methods of determining lacosamide concentrations, such as through saliva [19,20,21]. Saliva is a less invasive way of measuring lacosamide concentration, so it would potentially have utility in patients who require a concentration drawn but do not like needles. All three studies found a linear correlation between lacosamide concentrations in the plasma and saliva at various doses. However, in two of the studies, the mean lacosamide concentration in the saliva was consistently slightly greater than the mean lacosamide concentration in the plasma [19, 21]. The authors concluded that saliva may still be an effective way to measure lacosamide concentrations because it exhibits dose proportionality.
Two observational studies compared lacosamide plasma concentrations to cerebrospinal fluid (CSF) concentrations [22, 23]. It is relevant to determine the amount of lacosamide that crosses the blood–brain barrier and enters the central nervous system (CNS) because the CNS is where it exerts its mechanism of action to prevent seizures. Both studies found that both mean plasma and mean CSF concentrations were dose proportional, and there was a linear relationship between plasma and CSF concentrations. However, Michelhaugh et al. only saw this positive correlation in patients on lacosamide as ongoing treatment, and not in lacosamide-naive patients [23]. In patients on lacosamide therapy as maintenance, the dose proportionality proves that lacosamide serum concentration at steady state is an appropriate surrogate for CSF concentration.
Because of the multiple studies that have demonstrated a linear relationship between lacosamide dose and plasma concentrations, it is clear that TDM may be a practical approach to ensure plasma concentrations are within a reference range, once established, in certain populations. However, there is some disagreement on dose–response and adverse reaction proportionality. Several RCTs found increased adverse reactions with increasing dosages of lacosamide [10], but a smaller observational study failed to find a correlation between lacosamide plasma concentration and adverse effects [17]. The latter study was composed of a small sample size of 70 participants, so they may not have been adequately powered to detect a correlation, which may explain the difference in results compared with the RCTs. Overall, more research is needed to develop a well-established reference range for lacosamide TDM, as there is currently some variability in suggested reference ranges. Despite the present limitations in the use of TDM for lacosamide, including lack of a defined reference range, there is likely a place for it in clinical practice in specific populations with altered pharmacokinetics, which will be explored further in this discussion.
4.1 Drug–Drug Interactions
One factor that may make TDM desirable in clinical practice is the presence of unpredictable or significant drug interactions. Drug interactions may occur at any stage of the drug’s passage through the body, including absorption, distribution, metabolism, and excretion. Several older AEDs have many clinically important drug interactions because they are liver microsomal enzyme inducers or inhibitors, resulting in low or high concentrations, respectively, of the interacting medication. Although lacosamide does not act as a clinically significant enzyme inducer or inhibitor, it is metabolized by CYP 3A4, 2C9, and 2C19, so there is a potential for significant drug–drug interactions [1, 6].
As AEDs are often used in combination when patients do not attain seizure freedom with monotherapy, there have been multiple studies assessing for interactions between lacosamide and other AEDs. Two studies in healthy volunteers looked at combining lacosamide with valproic acid [24] and carbamazepine [25], which influence liver microsomal enzymes by inhibition and induction, respectively. The authors in both studies found that neither the pharmacokinetics of lacosamide were affected by either AED, nor were the pharmacokinetics of valproic acid or carbamazepine influenced by lacosamide coadministration. Similarly, other trials have demonstrated that the pharmacokinetics of lacosamide generally do not seem to be affected by coadministration with other AEDs [4, 10, 22, 25, 26]. On the other hand, in a retrospective observational study with 973 patients with epilepsy, May et al. found that coadministration with an enzyme-inducer AED resulted in a 30–40% decrease in lacosamide trough plasma concentrations, with a larger effect when more than one enzyme-inducer AED was coadministered with lacosamide [28]. However, there was no significant effect of enzyme-inhibitor AEDs on lacosamide trough concentrations, which is consistent with the other reported studies. Likewise, four other studies found a decreased concentration–dose ratio with lacosamide and concomitant enzyme-inducer AEDs compared with lacosamide monotherapy [15, 28,29,30]. In addition to the pharmacokinetic interactions, pharmacodynamic interactions between lacosamide and other sodium-channel blocking AEDs such as carbamazepine, lamotrigine, and phenytoin, have been reported. Novy et al. described such interaction in a case series of seven patients. Lacosamide doses were standard and plasma concentrations ranged from 1.3 to 12 mg/L [32]. The authors of this study concluded that the patients described, although having lacosamide concentrations within or slightly below the suggested reference range, had increased CNS adverse effects. A study by Hillenbrand et al. had similar findings, in which patients on lacosamide with a concomitant sodium channel blocker AED had a significantly increased incidence of dizziness [17]. Taken together, this suggests the possibility of a pharmacodynamic interaction between lacosamide and other sodium channel blocking AEDs, resulting in an increase in adverse effects, so should be used together cautiously.
Lacosamide has also been studied in combination with several other commonly used medications to assess for drug–drug interactions. Administration of lacosamide with oral contraceptives, omeprazole, or digoxin have been found not to result in any clinically significant drug interaction or concentration changes [33,34,35]. Notably, all of these studies were conducted in healthy volunteers, which may not necessarily be generalizable to patients on medications for epilepsy.
Overall, lacosamide has few drug–drug interactions and typically exhibits predictable pharmacokinetics. Digoxin, oral contraceptives, omeprazole, and enzyme-inhibiting AEDs have all shown to have no significant effect on efficacy or pharmacokinetics of lacosamide when coadministered with lacosamide. However, there is some disagreement on whether enzyme-inducing AEDs impact the pharmacokinetics of lacosamide, with several studies finding no effect, and other studies finding a significant effect. Because several studies did find a decrease in lacosamide plasma concentrations when given concomitantly with enzyme-inducing AEDs, TDM may be of benefit to monitor this and ensure lacosamide concentrations do not drop drastically when an interacting AED is added on.
4.2 Drug Formulations
An advantage that lacosamide has over other AEDs is that it is available in an IV formulation, which is important for emergent treatment of seizures, such as in status epilepticus, or in situations where oral administration is not feasible for a patient. Because lacosamide has near 100% bioavailability, the doses of lacosamide in IV versus oral administration are similar [6]. Multiple studies have evaluated the safety, efficacy, and pharmacokinetics of IV lacosamide compared with oral lacosamide, and have shown that IV lacosamide is equally efficacious and tolerable [6, 35,36,37,38]. Bioequivalence of IV and oral lacosamide was demonstrated in a study in healthy volunteers by Cawello et al. [37]. As in oral lacosamide, IV lacosamide administration resulted in a linear relationship between dose and plasma concentration; maximum mean concentration immediately following IV administration was approximately 6 mg/L for 200 mg of lacosamide, 9 mg/L for 300 mg of lacosamide, and 12 mg/L for 400 mg of lacosamide [38]. This shows that a single IV loading dose of lacosamide results in similar plasma concentrations to equivalent oral daily doses at steady state, which was also demonstrated through simulations by Cawello and Andreas [40].
In addition to IV formulation of lacosamide, other formulations of lacosamide have been used in studies, including an oral syrup and a subcutaneous injection. Cawello et al. compared the bioavailability and pharmacokinetics of lacosamide in an oral tablet versus oral syrup in a randomized, open-label crossover trial [21]. The tablet and syrup demonstrated bioequivalence, with similar mean time for the drug to reach maximum drug concentration (tmax), Cmax, and AUCs. In a case report by Remi et al., the authors described a palliative male patient who lost the ability to swallow, and a subcutaneous injection was preferred to an IV injection [41]. This patient was previously on lacosamide 400 mg/day and was switched to off-label subcutaneous lacosamide at a dose of 200 mg undiluted IV solution for two doses. No seizures were experienced during the time of the subcutaneous injections, indicating apparent efficacy of lacosamide. Serum lacosamide concentration immediately prior to the subcutaneous injection was 3.56 mg/L, peak lacosamide concentration (50 min after subcutaneous dose) was 5.44 mg/L, and trough concentration was 3.67 mg/L.
One advantage of lacosamide is the availability of an IV formulation, which has been found to be bioequivalent to the tablet. Oral syrup and subcutaneous formulation may also be of benefit when choosing among antiepileptic drugs.
4.3 Pregnancy
It is known that physiological changes due to pregnancy result in altered pharmacokinetics of drugs including increased renal clearance, altered hepatic metabolism, and increased volume of distribution, among other changes [42]. Lacosamide pharmacokinetics are susceptible to these physiological changes during pregnancy since it undergoes both hepatic metabolism and renal excretion. There are limited studies that have assessed the use of lacosamide in pregnancy, and currently the drug monograph relies on animal studies to provide a recommendation [1]. In particular, there is very limited evidence on the effect of pregnancy status on lacosamide plasma concentrations. Only one study analyzing lacosamide plasma concentrations in pregnancy was identified [43]. Ylikotila et al. describe a case report of a woman with focal secondarily generalized seizures who started lacosamide in her eighth week of pregnancy. The child born at 36 weeks had no malformations. Measured lacosamide concentrations in the mother and cord blood immediately after delivery were 3.8 ± 1.1 mg/L and 3.9 ± 1.2 mg/L, respectively, demonstrating that lacosamide must freely cross the placenta. Based on lacosamide concentrations drawn from breast milk 5 days postpartum and the child 8 days postpartum, they estimated a relative infant dose (RID) of 1.8%. An RID of < 10% is generally considered to have minimal clinical impact on infants [44].
Currently, more evidence is needed on whether lacosamide kinetics are altered in pregnancy. Because lacosamide dosage requirements may change during pregnancy, and it is vital for the mother to remain seizure free in pregnancy to avoid harm to the fetus, TDM may be of benefit to monitor lacosamide plasma concentrations.
4.4 Renal Impairment
Renal function is an important consideration for many medications, as many require dose adjustments with decreasing renal function, and some may be contraindicated below a certain concentration of renal function. Medications that are renally eliminated, such as lacosamide, may build up in the body with declining renal function, and have the potential to cause toxicity. One way to ensure a drug is not building up to a toxic concentration is through use of TDM. Cawello et al. demonstrated that as renal function declines, plasma concentration of lacosamide increases due to a decrease in renal clearance [7]. To illustrate, clearance of lacosamide was 2.13 L/h in healthy volunteers, 1.74 L/h in moderate renal impairment, and 1.34 L/h in severe renal impairment. A decrease in maximum daily dose of lacosamide to 250–300 mg/day (compared with the typical 400 mg/day) was recommended in adults with severe renal impairment and end-stage renal disease, but not mild or moderate renal impairment.
Renal replacement therapy has the potential to significantly remove lacosamide due to the characteristics of lacosamide including small molecular weight, low protein binding [7], 40% renal elimination [6], and small volume of distribution. Two case reports have examined lacosamide use in continuous venovenous hemofiltration (CVVH) in critically ill patients [45, 46]. In both cases, Cmax at steady state was around 7 mg/L, but in the case study by Wieruszewski et al., they found that the minimum drug concentration (Cmin) fell below the reference range [46]. Although the authors recommended dosing of lacosamide as in normal kidney function, they suggest that use of TDM may be warranted to ensure concentrations within a suggested reference range of 5–12 mg/L. On the other hand, Cawello et al. found that in patients on hemodialysis, systemic exposure of lacosamide was significantly decreased, so they may require dose supplementation of up to 50% to maintain concentrations within a reference range [7].
Due to the limited data on the use of lacosamide in patients with renal dysfunction or on renal replacement therapy, and small sample sizes of available studies, more evidence is required to form a clear recommendation regarding pharmacokinetic changes of lacosamide in renal dysfunction and renal replacement therapy. Because current data suggest that lacosamide plasma concentrations may increase in severe renal impairment and systemic exposure is decreased with hemodialysis, TDM may be useful to monitor lacosamide plasma concentrations of such patients.
4.5 Older Adults
A variety of factors change as people age, including body composition and presence of comorbidities, with both affecting the pharmacokinetics of medications. Older adults generally have higher relative lipid content and lower total body water, which affect drug distribution, and may have decreased renal and hepatic clearance [47]. There are several studies investigating the pharmacokinetic changes of lacosamide in an older population, and generally they have found that age in itself does not have a significant effect on lacosamide plasma concentrations [29, 47,48,49,50]. To illustrate, in a post hoc analysis including both healthy subjects and patients with epilepsy, Schafer et al. found that although younger subjects had lower Cmax at steady state (4.8 mg/L vs 6.2 mg/L), AUC, and higher clearance than older subjects, there was no significant difference when body weight or volume of distribution was adjusted for [49]. However, several of the studies that found no effect of age on lacosamide pharmacokinetics were fairly small, so they may not have been powered to show a difference. In contrast, a large retrospective analysis by May et al. found that adults aged > 60 years had 20% higher lacosamide serum trough concentrations than those aged < 60 years, suggesting the need for dose reduction in older adults [28].
Although currently the majority of studies have not found any impact of age on lacosamide concentrations and thus recommend standard dosing, one study suggests that older adults may require lower dosing to prevent accumulation of lacosamide. It may be beneficial to monitor lacosamide plasma concentrations in older adults periodically to ensure stable concentrations and prevent toxicity.
4.6 Pediatrics
Children have substantially different physiological characteristics from adults, and these characteristics change throughout the life of a child. For example, stomach pH varies, which alters absorption of medications; total body water is higher than in adults, which alters volume of distribution; and drug metabolism varies throughout pediatric age groups [52]. Because of these changes, drug pharmacokinetics in pediatrics is often much different from that in adults. Lacosamide is currently approved as an antiepileptic in children aged ≥ 4 years in the European Union [2], but is not approved for pediatric use in Canada [1]. The current literature on the pharmacokinetics of lacosamide in pediatric populations is limited. A recent retrospective observational study examined the effect of age, dose, sex, and duration of treatment on lacosamide pharmacokinetics in children [53]. The only significant factor on lacosamide concentrations (normalized by dose) was age; children aged between 13 and 17 years had significantly higher concentration–dose ratios than children aged between 6 and 12 years and children aged < 6 years. Another study investigated the utility of TDM of lacosamide in children and found that lacosamide was more effective when the plasma concentration was > 10 mg/L [54].
Four studies examined the use of lacosamide with concomitant AEDs and the effect on pharmacokinetics in pediatrics [28, 53, 55, 56]. Martin et al. described lacosamide use with concomitant AEDs, and found that AUC was increased by 14% with coadministration of valproate, and decreased by 17% with coadministration of enzyme-inducer AEDs [55]. However, the authors acknowledge that this change is small, and children likely have similar exposure as in adults. Similarly, Burns et al. found no effect of enzyme-inducer AEDs on lacosamide pharmacokinetics in children, although their sample size was small (n = 3) [53]. In contrast, in a secondary analysis, Winkler et al. examined lacosamide use in pediatrics, and found that lacosamide clearance increased by about 53% when administered with a concomitant enzyme-inducer AED [56]. May et al. found a similar reduction of AUC of 46% when children were given concomitant enzyme-inducer AEDs compared with monotherapy [28].
In a secondary analysis and simulation of 2 previous trials in children, Winkler et al. compared the pharmacokinetics of IV and oral lacosamide in children with epilepsy with that in healthy adults [57]. They demonstrated that the AUC in pediatrics of oral and IV lacosamide was comparable, so the weight-based dosing used for oral lacosamide can likely be applied to IV lacosamide.
Currently, lacosamide pharmacokinetics in children compared with adults is not well elucidated. There is some disagreement on the extent of the impact of enzyme-inducer AEDs in pediatrics, as seen in adults. A simulation found that lacosamide exposure was similar with IV and oral administration, so can be dosed similarly. There is currently a lack of definitive evidence regarding TDM of lacosamide in pediatrics, so at this point it is unknown whether TDM would be useful in this population. Further studies are needed.
4.7 Race
The pharmacokinetics of lacosamide has been compared among various races in multiple studies, including Caucasian, African-American, Korean, Chinese, and Japanese. Two conference abstracts of retrospective observational studies described lacosamide pharmacokinetics in African-Americans compared with Caucasians [51, 58]. Both studies concluded that lacosamide serum concentrations seem to be lower in African-American people than in Caucasian people. For example, in their small study of 19 participants, Basha et al. found that serum concentrations were comparable between the two groups at a dose of 200 mg/day; however, at a dose of 400 mg/day, mean lacosamide concentration of African-American participants was 5.94 mg/L, and Caucasian participants was 9.00 mg/L [58]. While this difference was not statistically significant, it may suggest that African-American people require higher doses of lacosamide to maintain a plasma concentration similar to that of Caucasian people.
A randomized controlled trial by Hong et al. investigated the safety and efficacy of lacosamide in an Asian population, including Chinese and Japanese people, which was a population that had not been previously studied [59]. While this study did not directly compare Asian to Caucasian people, they found similar mean plasma concentrations and dose proportionality of lacosamide to what has typically been seen in studies of Caucasian populations; about 5 mg/L at a dose of 200 mg/day and 10 mg/L at a dose of 400 mg/day. Another study found a significant interaction between enzyme-inducer AEDs and lacosamide in Japanese patients, which is similar to that seen in previous studies in Caucasians [60]. In contrast, Kim et al. compared lacosamide pharmacokinetics in Korean and Caucasian people and found a relatively increased AUC of lacosamide in Korean people [61]. However, this was likely due to a lower mean body weight and height in the Korean participants compared with Caucasian people.
Overall, the pharmacokinetics of lacosamide are likely comparable between Asian and Caucasian populations, especially when difference in mean body weight is accounted for. However, based on the limited studies available, lacosamide administration to African-American people may result in lower plasma concentrations compared with Caucasian people. Because of this, TDM may be necessary in African-American people to ensure plasma concentrations within a reference range and increase the likelihood of seizure prevention. Regardless, further studies are needed.
4.8 Critical Illness
Critical illness involves multisystem dysfunction (including respiratory, neurological, and cardiovascular problems), is life-threatening for patients and requires admission to an intensive care unit (ICU) for management. There are many considerations involved regarding the pharmacokinetics of medications in these patients, including medications specific to their illness causing drug interactions, changes in blood flow from the periphery to core organs leading to altered bioavailability, altered fluid status and inflammation resulting in altered distribution, and changes in drug metabolism due to the potential effect on liver microsomal enzymes and blood flow to the liver [62]. Additionally, renal excretion can change due to impaired renal function, requirement for dialysis, or augmented renal clearance [63]. Because pharmacokinetic studies are typically carried out in healthy volunteers, it can be difficult to extrapolate the information gathered from those studies to critically ill patients with many physiological changes. While there are retrospective studies that have demonstrated safety of lacosamide use in the ICU [64, 65], there is very limited evidence regarding potential pharmacokinetic changes of lacosamide in critically ill patients. A retrospective observational study of patients with refractory status epilepticus in the ICU described the efficacy of a weight-based IV loading dose of lacosamide [50]. The most efficacious loading dose in attaining a concentration in their suggested reference range of 15–20 mg/L was 10–12 mg/kg, although there was significant heterogeneity in plasma concentration based on loading dose. There are two limitations of this study, including the fact that the clearance and half life of lacosamide were not reported, making it difficult to determine if augmented renal clearance was occurring in the study population. Additionally, the relationship between dose and efficacy was not stated, so we cannot be sure that attaining lacosamide concentration in the reference range correlated with efficacy of the medication. More evidence is needed in the critically ill population to elucidate pharmacokinetic changes of lacosamide.
4.9 Other
There are several other factors that may have an effect on the pharmacokinetics of lacosamide, including sex and presence of intellectual disabilities. Additionally, two case reports of lacosamide toxicity have been described.
The majority of studies did not find a difference in lacosamide plasma concentrations between sexes [5, 15, 28, 29, 48, 54]. Several of the studies at first found higher mean plasma concentrations of lacosamide in women; however, when the lower mean body weight of women was adjusted for, there was no significant difference.
One conference abstract of a retrospective observational study described comparable efficacy of lacosamide in people with and without intellectual disabilities [66]. While the authors did not report lacosamide serum concentrations to compare the two groups, they found that the serum concentration in both groups was 22 mmol/L. The concentration–dose ratio was similar between groups, at 0.07 in those with an intellectual disability and 0.06 in those without, so presumably the mean serum concentrations in each group was also similar. Likely, there are no major differences in the pharmacokinetics of lacosamide in people with intellectual disabilities compared with people without.
There have been three case reports associated with lacosamide toxicity. In a case report by Berei et al., a 70-year-old female experienced life-threatening ventricular tachycardia after administration of lacosamide 200 mg IV loading dose, then 300 mg IV every 12 h [67]. However, in this case study, lacosamide concentration at 2 days after starting lacosamide were within the suggested reference range at 12.4 mg/L. Using the Naranjo Probability Scale, the authors concluded that the relationship between lacosamide and ventricular tachycardia was ‘probable’. In another case report, a 26-year-old male experienced lacosamide toxicity after ingestion of 6,000 mg of lacosamide in a suicide attempt [68]. Lacosamide concentration at 10 h post-ingestion was well above the reference range at 44.5 mg/L. This overdose resulted in a paradoxical tonic–clonic seizure. Notably, there was no change in this patient’s renal function as a result of lacosamide toxicity. Another case report of lacosamide toxicity, including mental status changes and abnormal ECG, demonstrated the success of utilizing hemodialysis to clear lacosamide and bring the patient back to baseline [69].
4.10 Limitations
There are several limitations of this review. The majority of studies included are observational in nature, thus are subject to bias due to lack of randomization and blinding. Additionally, due to the relatively small sample size of many of the included studies there is the possibility that they were inadequately powered to detect significant differences. Another limitation is that many of the pharmacokinetic studies were conducted in healthy volunteers, which may not be entirely generalizable to a population with epilepsy.
4.11 Practical Aspects of Lacosamide TDM
If a clinician does choose to utilize TDM for lacosamide, there are several considerations. First, it is important to measure plasma concentrations at steady state, which is based on the half-life of the drug. It generally takes 3–5 half lives for a drug to reach a steady state. Based on its half-life of about 13 h in patients with normal renal and hepatic function, lacosamide is likely at steady state in 39–65 h (~ 2–3 days). With regard to sampling time, measuring a trough concentration (i.e., immediately before the next dose) is most consistent with the literature on TDM of lacosamide and will facilitate comparison of results overcoming concentration fluctuations [18]. Although it can be measured in some laboratories, lacosamide assay is not widely available. However, there are multiple methods that have been utilized in the literature, including LC–MS/MS [70, 71], GC–MS [72] and SPE-MS/MS [73]. Another consideration is the cost–benefit relationship of utilizing TDM; however, there is not currently any information on a cost–benefit relationship of lacosamide TDM. Further studies are needed.
5 Conclusion
Although TDM is not routinely performed to assess plasma concentrations of lacosamide, it may be a good way to ensure efficacy of seizure prevention and prevent toxicity and adverse reactions. If TDM is utilized, it is important to consider the plasma concentration together with the clinical context when making therapeutic decisions. While it may not be practical to utilize TDM for all patients, especially because lacosamide has predictable linear kinetics, there are some situations when TDM may be useful. The current literature shows that populations where TDM may be beneficial are in patients taking enzyme-inducer AEDs because of a potential drug interaction, in patients with decreased renal function or on dialysis and older adults. However, more evidence is needed in these situations, as well as in pediatrics, pregnancy, and in critical illness, to elaborate on potential pharmacokinetic variability of lacosamide due to physiological changes that occur during these times. It is also imperative to establish a reference range for lacosamide that is accepted worldwide.
References
UCB Pharma. Vimpat Product Monograph (Canada). 2018.
UCB Pharma. Vimpat Product Monograph (EMA). 2018.
Errington AC, Stöhr T, Heers C, Lees G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol. 2008;73(1):157 LP-169.
Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, et al. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50(3 CC-Epilepsy):443–53.
Kropeit D, Johnson M, Cawello W, et al. Lacosamide cardiac safety: a thorough QT/QTc trial in healthy volunteers. Acta Neurol Scand. 2015;132(5):346–54.
Cawello W, Boekens H, Bonn R. Absorption, disposition, metabolic fate and elimination of the anti-epileptic drug lacosamide in humans: mass balance following intravenous and oral administration. Eur J Drug Metab Pharmacokinet. 2012;37(4):241–8.
Cawello W, Fuhr U, Hering U, Maatouk H, Halabi A. Impact of impaired renal function on the pharmacokinetics of the antiepileptic drug lacosamide. Clin Pharmacokinet. 2013;52(10):897–906.
Patsalos PN, Spencer EP, Berry DJ, et al. Therapeutic drug monitoring of antiepileptic drugs in epilepsy: a 2018 update. Ther Drug Monit. 2018;40(5):526–48.
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94.
Chung S, Ben-Menachem E, Sperling MR, et al. Examining the clinical utility of lacosamide: pooled analyses of three phase IIIII clinical trials. CNS Drugs. 2010;24(12):1041–54.
Ben-Menachem E, Biton V, Jatuzis D, et al. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48(7):1308–17.
Lindauer A, Laveille C, Stockis A. Time-to-seizure modeling of lacosamide used in monotherapy in patients with newly diagnosed epilepsy. Clin Pharmacokinet. 2017;56(11):1403–13.
Morris G, Burgos J, Van Kampen A, Hanson M, Dagman J. Intravenous intiation of lacosamide for status epilepticus and following video EEG monitoring. Epilepsy Curr. 2015; 15(1):157.
Payto D, Foldvary-Schaefer N, So N, Bruton M, Wang S. Establishment of expected ranges for the random serum concentration of lacosamide and desmethyl lacosamide. Clin Chem. 2014;60(10 Suppl. 1):S247–8.
Perrenoud M, Andre P, Alvarez V, et al. Intravenous lacosamide in status epilepticus: correlation between loading dose, serum levels, and clinical response. Epilepsy Res. 2017;135:38–42.
Svendsen T, Brodtkorb E, Baftiu A, et al. Therapeutic drug monitoring of lacosamide in norway: focus on pharmacokinetic variability, efficacy and tolerability. Neurochem Res. 2017;42(7):2077–83.
Hillenbrand B, Wisniewski I, Jürges U, Steinhoff BJ. Add-on lacosamide: a retrospective study on the relationship between serum concentration, dosage, and adverse events. Epilepsy Behav. 2011;22(3):548–51.
Sattler A, Schaefer M, May TW, et al. Fluctuation of lacosamide serum concentrations during the day and occurrence of adverse drug reactions–first clinical experience. Epilepsy Res. 2011;95(3):207–12.
Brandt C, Bien CG, Helmer R, May TW. Assessment of the correlations of lacosamide concentrations in saliva and serum in patients with epilepsy. Epilepsia. 2018;59(4):e34–9.
Greenaway C, Ratnaraj N, Sander JW, Patsalos PN. Saliva and serum lacosamide concentrations in patients with epilepsy. Epilepsia. 2011;52(2):258–63.
Cawello W, Bokens H, Nickel B, et al. Tolerability, pharmacokinetics, and bioequivalence of the tablet and syrup formulations of lacosamide in plasma, saliva, and urine: saliva as a surrogate of pharmacokinetics in the central compartment. Epilepsia. 2013;54(1):81–8.
May TW, Brandt C, Helmer R, et al. Comparison of lacosamide concentrations in cerebrospinal fluid and serum in patients with epilepsy. Epilepsia. 2015;56(7):1134–40.
Michelhaugh SK, Basha M, Rhoney DH, Shah AK, Mittal S. Acute or chronic use of lacosamide does not alter its distribution between serum and cerebrospinal fluid. Epilepsia. 2015;56(11):1732–7.
Cawello W, Bonn R. No pharmacokinetic interaction between lacosamide and valproic acid in healthy volunteers. J Clin Pharmacol. 2012;52(11):1739–48.
Cawello W, Nickel B, Eggert-Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50(4):459–71.
Cawello W. Exposure to lacosamide in blood plasma during adjunctive therapy and monotherapy: pharmacokinetic analysis of data from a conversion to lacosamide monotherapy study. Epilepsia. 2015;56(Suppl. 1):53.
Chung S, Sperling MR, Biton V, et al. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia. 2010;51(6):958–67.
May TW, Helmer R, Bien CG, et al. Influence of dose and antiepileptic comedication on lacosamide serum concentrations in patients with epilepsy of different ages. Ther Drug Monit. 2018;40(5):620–7.
Contin M, Albani F, Riva R, et al. Lacosamide therapeutic monitoring in patients with epilepsy: effect of concomitant antiepileptic drugs. Ther Drug Monit. 2013;35(6):849–52.
Markoula S, Teotonio R, Ratnaraj N, et al. Lacosamide serum concentrations in adult patients with epilepsy: the influence of gender, age, dose, and concomitant antiepileptic drugs. Ther Drug Monit. 2014;36(4):494–8.
Ota M, Demura A, Hashi S, Kinoshita M. Pharmacokinetic drug–drug interaction between lacosamide and other antiepileptic drugs in Japanese adult patients with epilepsy. Epilepsia. 2018;59(Suppl. 3):S272.
Novy J, Patsalos PN, Sander JW, Sisodiya SM. Lacosamide neurotoxicity associated with concomitant use of sodium channel-blocking antiepileptic drugs: a pharmacodynamic interaction? Epilepsy Behav. 2011;20(1):20–3.
Cawello W, Rosenkranz B, Schmid B, Wierich W. Pharmacodynamic and pharmacokinetic evaluation of coadministration of lacosamide and an oral contraceptive (levonorgestrel plus ethinylestradiol) in healthy female volunteers. Epilepsia. 2013;54(3):530–6.
Cawello W, Mueller-Voessing C, Fichtner A, et al. Pharmacokinetics of lacosamide and omeprazole coadministration in healthy volunteers: results from a phase I, randomized, crossover trial. Clin Drug Investig. 2014;34(5):317–25.
Cawello W, Mueller-Voessing C, Andreas J-O. Effect of lacosamide on the steady-state pharmacokinetics of digoxin: results from a phase I, multiple-dose, double-blind, randomised, placebo-controlled, crossover trial. Clin Drug Investig. 2014;34(5):327–34.
Biton V, Rosenfeld WE, Whitesides J, et al. Intravenous lacosamide as replacement for oral lacosamide in patients with partial-onset seizures. Epilepsia. 2008;49(3):418–24.
Cawello W, Bonn R, Boekens H. Bioequivalence of intravenous and oral formulations of the antiepileptic drug lacosamide. Pharmacology. 2012;90(1–2):40–6.
Fountain NB, Krauss G, Isojarvi J, et al. Safety and tolerability of adjunctive lacosamide intravenous loading dose in lacosamide-naive patients with partial-onset seizures. Epilepsia. 2013;54(1):58–65.
Krauss G, Ben-Menachem E, Mameniskiene R, et al. Intravenous lacosamide as short-term replacement for oral lacosamide in partial-onset seizures. Epilepsia. 2010;51(6):951–7.
Schaefer C, Cawello W, Andreas JO. Immediate steady state concentrations in plasma after oral or intravenous lacosamide loading dose. Epilepsy Curr. 2015;15(Suppl. 1):524–5.
Rémi C, Zwanzig V. Subcutaneous use of lacosamide. J Pain Symptom Manag. 2016;51(2):e2–4.
Ward RM, Varner MW. Principles of pharmacokinetics in the pregnant woman and fetus. Clin Perinatol. 2019;46(2):383–98.
Ylikotila P, Ketola RA, Timonen S, et al. Early pregnancy cerebral venous thrombosis and status epilepticus treated with levetiracetam and lacosamide throughout pregnancy. Reprod Toxicol. 2015;57:204–6.
Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343(2):118–26.
Franquiz MJ, Kalaria SN, Armahizer MJ, et al. Lacosamide pharmacokinetics in a critically ill patient receiving continuous venovenous hemofiltration. Pharmacotherapy. 2018;38(2):e17–21.
Wieruszewski PM, Lopez-Ruiz A, Albright RC et al. Lacosamide pharmacokinetics in a critically ill patient during continuous renal replacement therapy. J Pharm Pract. 2018:897190018803765.
Klotz U. The elderly—a challenge for appropriate drug treatment. Eur J Clin Pharmacol. 2008;64(3):225–6.
Cawello W, Schafer C. A system of equations to approximate the pharmacokinetic parameters of lacosamide at steady state from one plasma sample. Epilepsy Res. 2014;108(6):1068–75.
Schaefer C, Cawello W, Waitzinger J, Elshoff J-P. Effect of age and sex on lacosamide pharmacokinetics in healthy adult subjects and adults with focal epilepsy. Clin Drug Investig. 2015;35(4):255–65.
Ramsay RE, Sabharwal V, Khan F, et al. Safety & pK of IV loading dose of lacosamide in the ICU. Epilepsy Behav. 2015;49:340–2.
Mahulikar A, Yarranguntla K, Shah A, Seraji-Bozorgzad N, Basha M. Racial influence on antiepileptic drug levels. Neurol. 2018;90(15):P5.259.
Nahata MC, Taketomo C. Pediatrics. In: DiPiro JT, Talbert RL, Yee GC, editors. Pharmacotherapy: A Pathophysiologic Approach, 10th edition. New York: McGraw-Hill Education; 2017.
Burns ML, Nikanorova M, Baftiu A et al. Pharmacokinetic variability and clinical use of lacosamide in children and adolescents in Denmark and Norway. Ther Drug Monit. 2019;41(3):340–347.
Kronenfeld N, Kohn E, Lezinger M, et al. Lacosamide monitoring in the serum of children with refractory epilepsy. Arch Dis Child. 2019. https://doi.org/10.1136/archdischild-2019-esdppp.54.
Martin P, Stockis A, Jolling K. Population pharmacokinetic modelling and simulation to support dose recommendations for lacosamide in children. Epilepsia. 2018;59(Suppl. 3):S78.
Winkler J, Schoemaker R, Stockis A. Population pharmacokinetics of adjunctive lacosamide in pediatric patients with epilepsy. J Clin Pharmacol. 2018. https://doi.org/10.1002/jcph.1340.
Winkler J, Schoemaker R, Stockis A. Modeling and simulation for the evaluation of dose adaptation rules of intravenous lacosamide in children. Epilepsy Res. 2019;149:13–6.
Bautista RE, Graham C, Mukardamwala S. Do lacosamide serum levels differ in African American and caucasian groups? Epilepsy Curr. 2012;12(1).
Hong Z, Inoue Y, Liao W, et al. Efficacy and safety of adjunctive lacosamide for the treatment of partial-onset seizures in Chinese and Japanese adults: a randomized, double-blind, placebo-controlled study. Epilepsy Res. 2016;127:267–75.
Yamamoto Y, Terada K, Takahashi Y, et al. Therapeutic drug monitoring for lacosamide in Japanese patients with epilepsy. Eur J Clin Pharmacol. 2019;75(Suppl. 1):S40.
Kim SE, Gu N, Kim B-H, et al. Pharmacokinetics of lacosamide in healthy Korean male volunteers. Pharmacology. 2012;89(3–4):172–8.
Mahmoud SH, Yearwood C. Critical Care Assessment. In: Mahmoud SH (ed.) Patient assessment in clinical pharmacy: a comprehensive guide. Springer International Publishing. 2019;353–373.
Mahmoud HS, Shen C. Augmented renal clearance in critical illness: an important consideration in drug dosing. Pharmaceutics. 2017. https://doi.org/10.3390/pharmaceutics9030036.
Cherry S, Judd L, Carlos J, et al. Safety and efficacy of lacosamide in the intensive care unit. Neurocrit Care. 2012;16:294–8.
Luk ME, Tatum WO, Patel AV, et al. The safety of lacosamide for treatment of seizures and seizure prophylaxis in adult hospitalized patients. Neurohospitalist. 2012;2(3):77–81.
Landmark CJ, Svendsen T, Brodtkorb E, et al. Comparison of lacosamide treatment in patients with and without intellectual disability. Epilepsia. 2018;59(Suppl. 3):S173–4.
Berei TJ, Lillyblad MP, Almquist AK. Lacosamide-induced recurrent ventricular tachycardia in the acute care setting. J Pharm Pract. 2018;31(2):222–6.
Deslandes G, Bouquié R, Lorber J, et al. Status epilepticus following self-poisoning of lacosamide and lamotrigine: a case report with follow-up of drug serum concentrations. Toxicol Anal Clin. 2015;27(2):88–90.
Kiernan E, et al. Hemodialysis: the final frontier for acute lacosamide toxicity? J Med Toxicol. 2019;15(2):63.
Payto D, Foldvary-Schaefer N, So N, et al. A sensitive and rapid method for quantification of lacosamide and desmethyl lacosamide by LC-MS/MS. Bioanalysis. 2014;6(23):3161–8.
D’urso A, Ricotta T, de Grazia U, et al. Evaluation of a novel immunoassay for lacosamide therapeutic drug monitoring: comparison with a liquid chromatography-mass spectrometry assay. Ther Drug Monit. 2017;39(6):663–8.
Nikolaou P, Papoutsis I, Spiliopoulou C, et al. A fully validated method for the determination of lacosamide in human plasma using gas chromatography with mass spectrometry: application for therapeutic drug monitoring. J Sep Sci. 2015;38(2):260–6.
Korman E, Bjergum M, Danso D, et al. High-throughput validatedmethod for the quantification of lacosamide in serum using ultra fast SPE-MS/MS. Ther Drug Monit. 2013;35(5):675.
Acknowledgement
Janice Kung, Librarian at John W. Scott Library, for assisting with the keywords selection and search strategy.
Author information
Authors and Affiliations
Contributions
SHM had the idea of the article. LS performed the literature search and data abstraction and summarization. Both authors confirmed data collected and search and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflicts of interest to declare.
Funding
There is no funding associated with this work.
Rights and permissions
About this article
Cite this article
Schultz, L., Mahmoud, S.H. Is Therapeutic Drug Monitoring of Lacosamide Needed in Patients with Seizures and Epilepsy?. Eur J Drug Metab Pharmacokinet 45, 315–349 (2020). https://doi.org/10.1007/s13318-019-00601-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-019-00601-8