Abstract
Background
Metformin has been available since 1957. Over 50 years later, one can legitimately question whether a clear definition of its “therapeutic concentrations” is available.
Objective
The objective of this systematic review was to establish whether or not there is a literature consensus on the “therapeutic concentrations” of metformin.
Methods
We systematically searched the scientific literature with the keywords “metformin”, “therapeutic concentration”, “therapeutic level”, and “therapeutic range”. When the suggested values were defined by citing a literature reference, the types of studies in cited references and the concordance of data between the citations and theirs sources were studied.
Results
We identified 120 documents that reported or cited 65 different “therapeutic” plasma metformin concentrations or ranges. The values ranged from 0.129 to 90 mg/L, and the lowest and highest boundaries were 0 and 1800 mg/L. Only four original research studies determined a “therapeutic concentration”. Fifty-four publications cited previous studies as defining the therapeutic concentrations, whereas 62 publications mentioned “therapeutic concentrations” but did not even cite a supporting reference. The supporting references were mostly reviews, pharmacokinetic studies and in vitro studies. In the 54 publications that cited references, concordance between the wording of the citation and the true nature of the source data was observed in only 23 cases (42.6 %).
Limitations
Given the nature of a systematic literature search, the only possible limitation would be incomplete identification and retrieval of publications on therapeutic concentrations. An extensive study of the literature has, however, been performed by examining nearly 1000 potentially relevant publications.
Guidance for Clinical Practice
The only valid way of defining the therapeutic concentration window for metformin would be to relate dose efficacy (in terms of blood glucose control) to the corresponding plasma concentration in long-term treated patients.
Conclusions
Although metformin has been available for over 50 years and it is the key medication in first-line treatment of type 2 diabetes mellitus, major methodological and/or conceptual errors have confounded the literature on its therapeutic concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We identified 120 documents that reported or cited 65 different “therapeutic” plasma metformin concentrations or ranges. |
Although metformin has been available for over 50 years, major methodological and/or conceptual errors have confounded the literature on its therapeutic concentrations. |
A dose-efficacy study with measurement of the corresponding plasma metformin concentrations is therefore needed for defining the therapeutic concentration window for metformin. |
1 Introduction
For any given drug, the physicians should always prescribe the “therapeutic dose”—a dosage corresponding to the “therapeutic range” that provides sufficient efficacy whilst avoiding overdosing. The “therapeutic range” can also be defined as “an approximation of the average plasma drug concentrations that are safe and efficacious in most patients” [1]. However, when studying the dose–response relationship for various dosage regimens, the relationship between blood concentrations and clinical effects may be evaluated in several different ways: (1) in clinical studies from which pharmacological parameters are measured; (2) by deducing the therapeutic range on the basis of pharmacological data; and (3) by combining clinical and pharmacological data on drug efficacy and concentrations. Once a therapeutic range has been defined, one can subsequently define subtherapeutic and supratherapeutic conditions.
Although metformin has been available since 1957 and is recommended as a first-line treatment for type 2 diabetes mellitus [2], this drug provides an excellent example of why therapeutic concentrations must be characterized. On one hand, metformin should be prescribed more widely because of its beneficial, pleiotropic effects. On the other, it should be prescribed less widely because an increasing proportion of diabetic patients have poor renal function—a contraindication to metformin use because of the risk of so-called “metformin-associated lactic acidosis” [3–5].
The first reports on a therapeutic dose and therapeutic concentrations for metformin were published in 1962 [6] and 1972 [7], respectively. Over 50 years later, one can legitimately question whether a clear definition of the therapeutic concentrations of metformin is available. To address this issue, we systematically searched the literature for publications that mention “therapeutic concentrations” of metformin.
2 Research Design and Methods
2.1 Data Sources
We performed a systematic search of the scientific literature recorded in the MEDLINE, Scopus, ScienceDirect, and Wiley-Blackwell electronic databases between January 1957 and November 2014. We also searched the Internet using the Google search engine and performed a manual search in our personal libraries (including documents that are not referenced in the aforementioned electronic databases).
2.2 Data Selection
We used the keywords “metformin”, “therapeutic concentration”, and “therapeutic range” but also searched for allusions and related wordings such as “therapeutic level”, “plasma concentration”, and “normal value” with regard to metformin’s antidiabetic effects.
2.3 Data Extraction
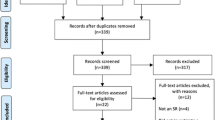
We extracted values or ranges described as “therapeutic concentrations” from the retrieved documents and their cited references (Fig. 1). When a blood metformin value or range was not defined exactly as a “therapeutic concentration” or “therapeutic range”, we cite the original wording of the text. When suggested therapeutic values were defined by citing a literature reference, the types of studies in cited references and the concordance of data between the citations and their sources were studied.
2.4 Data Presentation
All of the collected data are presented in a single table (Table 1), which is rather large but it was impossible to subdivide it into study categories (e.g., in vitro studies, animal studies, clinical studies, etc.). Indeed, this type of categorization might have erroneously suggested that the mentioned “therapeutic concentrations” were deduced from the results of the studies themselves. In fact, most of the “therapeutic concentrations” were suggested by citing other data (either personal data or, more often, a previous publication) and thus were not based on the study’s design and results. Furthermore, Fig. 2 serves as a guide to reading Table 1.
A guide to reading and interpreting Table 1
In some papers, metformin concentrations were noted in molar or gram units. Here, the concentrations were converted into mg/L if necessary. The exact molecular weight of metformin is 129.1636 [8] (rounded down here to 129 g/mol), and so the equivalences are 10−5 mol/L = 1.29 mg/L and 1 mg/L = 7.75 μmol/L.
3 Results
We identified a total of 120 publications, which reported or cited 65 different therapeutic plasma metformin concentrations or ranges. The individual values ranged from 0.129 to 90 mg/L. When considering concentration ranges, the lowest and highest boundaries were 0 and 1800 mg/L. The narrowest range was 0.000225–0.003 mg/L and the broadest was 150–1800 mg/L. Most (77 %) of the values or ranges proposed were between 0.1 and 4 mg/L.
The collected data are presented in Table 1 [3, 7–160] and summarized in Table 2.
None of the studies was performed with the specific objective of defining the therapeutic concentrations for metformin. Fifty-four publications (45 %) cited previous studies as providing therapeutic concentrations, whereas 62 publications (51.7 %) mentioned “therapeutic concentrations” but did not even cite a supporting reference.
Only four original research studies (3.3 %) [8, 47, 80, 119] determined a therapeutic concentration. These studies assayed the plasma metformin concentrations (and the erythrocyte metformin concentrations in two studies) in different populations of diabetic subjects (1) undergoing “well-tolerated chronic metformin treatment” [47, 80]; (2) with chronic kidney disease [119]; and (3) “in steady state not having lactic acidosis” [8].
When a literature value was cited, the references were mostly reviews, pharmacokinetic studies, and in vitro studies. However, none of these cited studies was performed with the specific objective of defining the therapeutic concentration for metformin. In the 54 publications that cited supporting references, concordance between the wording of the citation and the true nature of the source data was observed in only 23 cases (42.6 %).
4 Discussion
The present study is the first to have systematically analyzed literature reports of therapeutic concentrations and therapeutic ranges for metformin. Although one would expect to find a consensus on a drug that has been available since 1957, at least for the antidiabetic properties of metformin, major methodological and/or conceptual errors have confounded this subject.
Firstly, the difficulty of characterizing therapeutic concentrations lies in the fact that a very large number of either values or ranges were identified (n = 65) and that, moreover, there was a very large variation between the different proposals. Indeed, the proposed single values varied from 0.129 to 90 mg/L, and the lowest and highest range boundaries were 0 and 1800 mg/L, respectively.
In this respect, it is surprising (from a biological and physicochemical point of view) that a value of 0 mg/L (i.e., the absence of the drug) could be suggested as the lower limit of a therapeutic range. Likewise, the highest boundary suggested (i.e., 1800 mg/L) is far above the values usually reported for metformin intoxication (i.e., up to 267 mg/L) [149]. Of course, one can consider the putative occurrence of arithmetical errors concerning the format (with a value of zero quoted as a lower limit on a purely statistical basis) and/or the calculation (when converting units into mg/L). However, even the majority of the most frequently cited values were between 0.1 and 4 mg/L, a range that already encompasses a 40-fold variation. In other words, some of these disparate values may be due to procedural (rather than conceptual) errors.
Secondly, most of the definitions of therapeutic concentrations were conceptually flawed. The vast majority of the documents studied (116 of 120) did not directly establish a therapeutic concentration for metformin and therefore cited previous studies. This would not be a problem per se, provided that the cited study provides reliable information. In fact, almost half the 120 publications did not cite a reference when referring to therapeutic concentrations, and when a reference was cited, it was not usually concordant. Even more importantly, the cited references provided were mostly reviews or pharmacokinetic studies: none were studies performed with the specific objective of defining the therapeutic concentration for metformin. The criteria used to define a therapeutic concentration of metformin were predominantly pharmacokinetic parameters [e.g., maximum plasma concentration (C max)]. Furthermore, the use of peak values is flawed: C max refers to a drug’s maximum concentration in a specified compartment (e.g., the blood) after a dose of the drug has been administered, whereas definitions of a therapeutic concentration usually refer to the trough steady-state level achieved by the prescribed dosing regimen [56]. Moreover, the above-mentioned pharmacokinetic parameters were mostly measured after the administration a single dose of metformin (in either diabetic or non-diabetic subjects) rather than during long-term metformin therapy (i.e., a multiple-dosage regimen).
Lastly, only four studies (including two from the same research group) provided putative steady-state plasma metformin concentrations determined in patients receiving long-term metformin treatment [8, 47, 80, 119]. Only two studies also quoted erythrocyte metformin concentrations [47, 80]. This is another critical issue, since plasma values do not necessarily reflect tissue concentrations [3, 80] and thus a drug’s true metabolic effects. In this respect, it has been suggested that erythrocyte metformin concentrations correspond to a deep compartment [80]. However, data on metformin concentrations in other putative deep, major compartments of metformin action (such as the intestine, liver, muscle and adipose tissue), which would be even more informative, are evidently not available in clinical practice.
In addition to the huge variation in the therapeutic concentrations due to flaws, significant “natural”, inter-individual variations in the blood metformin concentration should also be taken into consideration: (1) the various definitions of therapeutic concentrations do not appear to take account of the widely varying metformin dosages used in clinical practice; and (2) more importantly, variations in the genes coding for solute carriers significantly modulate metformin’s pharmacodynamics and pharmacokinetics [161–163].
The clinical impact of this type of polymorphism was confirmed in a cohort of type 2 diabetes patients taking 1 g of metformin twice daily over a 24-month period [164]. An 80-fold inter-individual variation in plasma metformin concentrations was documented. Ultimately, this implies that in a particular patient, a given metformin concentration might be therapeutic, subtherapeutic or supratherapeutic, or might even correspond to metformin accumulation. The variations observed thus argue in favor of personalized therapy, as currently recommended by many authors or authorities [165–170].
One must also consider whether the present study had any limitations. However, given the nature of our systematic literature search, the only possible limitation would be incomplete identification and retrieval of publications on therapeutic concentrations. In fact, we are confident that we performed an extensive study of the literature by examining nearly 1000 potentially relevant publications. Lastly (in order to avoid subjective interpretations), we also quoted the original wording of the text referring or alluding to therapeutic concentrations.
4.1 Guidance for Clinical Practice
Given the above pitfalls, one can legitimately ask whether any of the literature information on metformin concentrations is of use in clinical practice. In a recent review, Dowling et al. [143] stated that in vivo preclinical and in vitro studies often involve extremely high, non-physiological concentrations of metformin that are far in excess of the doses used in clinical and epidemiological studies. Indeed, in vitro studies have typically featured a metformin dose between 25 and 1000 times higher than that used in clinical studies. For in vivo studies, the values are still between 2 and 45 times higher than in clinical studies. This is of crucial importance when seeking to distinguish between therapeutic and supratherapeutic concentrations and to characterize mechanisms of action. In studies of animal hepatocytes in vitro, metformin concentrations below 50 μmol/L activate AMPK (5′ adenosine monophosphate-activated protein kinase) and suppress dibutyryl-cAMP (cyclic adenosine monophosphate)-stimulated gluconeogenic gene expression (and therefore hepatic glucose production) [171], whereas much higher metformin concentrations (5 mmol/L) are required to inhibit the respiratory chain complex 1 [172]. In an in vitro study in humans, inhibition of the respiratory chain complex 1 [concentration of drug producing 50 % inhibition (IC50)] was obtained at metformin concentrations of between 0.45 and 1.2 mmol/L (i.e., 58–155 mg/L) [173]. These figures should be compared with metformin concentrations reflecting pharmacologic or toxic effects in humans: C max for metformin in healthy subjects is ~8–16 μmol/L (1–2 mg/L) [6] and the metformin concentrations measured in cases of metformin overdose go up to 2 mmol/L (i.e., 210 mg/L) [149].
The only valid way of defining the therapeutic concentration window for metformin would be to relate dose efficacy (in terms of blood glucose control [174]) to the corresponding plasma metformin concentrations in long-term therapy. Since this procedure has never been attempted, the question arises as to what extent it is possible to extract at least some literature data of value for guiding clinical practice (i.e., after avoiding the aforementioned flaws). A pertinent basis for defining the therapeutic concentrations of metformin would be the range of plasma metformin trough concentrations produced by well-tolerated doses. This would also take account of the time interval following metformin administration [175]. On the basis of the very few studies that have actually done this [8], a value of 2.5 mg/L probably corresponds to the upper limit of the therapeutic range. Establishing the lower concentration is more problematic. However, this type of approach cannot answer an additional question: what is the metformin threshold for adverse effects? This is a very difficult question because even major metformin accumulation does not necessarily lead to lactic acidosis [47, 58, 75, 176]. Conversely, some patients may display an idiosyncratic, hyperlactatemic response to metformin therapy—even in the absence of metformin accumulation [60, 176].
It is therefore not possible to define the therapeutic concentrations for metformin purely on a safety basis, i.e., by considering metformin concentrations associated with adverse effects. Nevertheless, Table 3 differentiates between excessive metformin concentrations on one hand and concentrations obtained in patients at therapeutic doses (with no risk factors for metformin accumulation) on the other. On this basis, we suggest that metformin concentrations can be categorized as follows: non-therapeutic (i.e., ineffective), therapeutic and safe (for the great majority of patients), intermediate (with a risk of hyperlactatemia in some individuals), and excessive (with a high risk of hyperlactatemia in most patients).
5 Conclusion
The huge differences between the various therapeutic metformin concentrations suggested in the literature far exceed the inter-individual variations due to genetic factors (which are already quite large) and thus mainly reflect methodological and/or conceptual errors. Accordingly, it must be acknowledged that the therapeutic concentrations reported to date are of no value in (1) guiding the clinically useful, safe dosage of metformin; (2) distinguishing between therapeutic, supratherapeutic, and subtherapeutic concentrations; and (3) attributing responsibility to metformin in the genesis of so-called “metformin-associated lactic acidosis”.
A dose-efficacy study with measurement of the corresponding plasma metformin concentrations is urgently needed with a view to defining the therapeutic concentration window for metformin.
References
Shargel L, Wu-Pong S, Yu ABC. Applied biopharmaceutics and pharmacokinetics. 5th ed. New York: McGraw-Hill; 2005. p. 613–72.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9.
Lalau JD, Arnouts P, Sharif A, De Broe ME. Metformin and other antidiabetic agents in renal failure patients. Kidney Int. 2015;87(2):308–22.
Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312(24):2668–75.
Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
Gottlieb B, Auld WH. Metformin in treatment of diabetes mellitus. Br Med J. 1962;1(5279):680–2.
Frayn KN, Adnitt PI. Effects of metformin on glucose uptake by isolated diaphragm from normal and diabetic rats. Biochem Pharmacol. 1972;21(23):3153–62.
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98.
Pignard P. Spectrophotometric determination of N,N-dimethylbiguanide in blood and urine [in French]. Ann Biol Clin (Paris). 1962;20:325–33.
Adnitt PI, Frayn KN. Effects of metformin on glucose uptake by the isolated rat diaphragm. Br J Pharmacol. 1972;45(1):152P–3P.
Clarke BF, Ducan LJP. Biguanide treatment in the management of insulin independent (maturity-onset) diabetes: clinical experience with metformin. Res Clin Forums. 1979;1:53–63.
Sirtori CR, Franceschini G, Galli-Kienle M, Cighetti G, Galli G, Bondioli A, et al. Disposition of metformin (N,N-dimethylbiguanide) in man. Clin Pharmacol Ther. 1978;24(6):683–93.
Isnard F, Laviuville M, Gut M. Pharmacocinétique de la metformine. In: Journées de Diabétologie de l’Hôtel-Dieu. Paris: Flammarion Médecine-Sciences; 1980. p. 305–12.
Bruneder H, Klein HJ, Isnard F, Noel M. Fasting metformin levels in ambulatory treated diabetic patients [abstract]. Excerpta Medica 1979;ICS 481:33,82P.
Mountjoy KG, Finlay GJ, Holdaway IM. Effects of metformin and glibenclamide on insulin receptors in fibroblasts and tumor cells in vitro. J Endocrinol Invest. 1987;10(6):553–7.
Lambert H, Isnard F, Delorme N, Claude D, Bollaert PE, Straczek J, Larcan A. Physiopathological approach to pathological hyperlactatemia in the diabetic patient. Value of blood metformin [in French]. Ann Fr Anesth Reanim. 1987;6(2):88–94.
Tymms DJ, Leatherdale BA. Lactic acidosis due to metformin therapy in a low risk patient. Postgrad Med J. 1988;64(749):230–1.
Wollen N, Bailey CJ. Metformin potentiates the antigluconeogenic action of insulin. Diabete Metab. 1988;14(2):88–91.
Beckmann R. Biguanide (Experimenteller Teil). In: Maske H, editor. Handbook of experimental pharmacology, vol. 29. Berlin: Springer; 1971. p. 439–596.
Noel M. Kinetic study of normal and sustained release dosage forms of metformin in normal subjects. Res Clin Forums. 1979;1:33–44.
Caporicci D, Mori A, Pepi R, Lapi E. Effects of dimethylbiguanide (metformin) on peripheral insulin clearance and lipid biosynthesis in obese dyslipidemic subjects with and without diabetes mellitus [in Italian. Clin Ter. 1979;88(4):372–86.
De Lorenzi F. La farmacocinetica e la farmacodinamica delle biguanide [in Italian]. Omnia Med Ther. 1975;56.
Wollen N, Bailey CJ. Inhibition of hepatic gluconeogenesis by metformin. Synergism with insulin. Biochem Pharmacol. 1988;37(22):4353–8.
Bailey CJ, Nattrass M. Treatment–metformin. Baillieres Clin Endocrinol Metab. 1988;2(2):455–76.
Gregorio F, Filipponi P, Ambrosi F, Cristallini S, Marchetti P, Calafiore R, et al. Metformin potentiates B-cell response to high glucose: an in vitro study on isolated perfused pancreas from normal rats. Diabete Metab. 1989;15(3):111–7.
Benzi L, Marchetti P, Cecchetti P, Navalesi R. Determination of metformin and phenformin in human plasma and urine by RP-HPLC. J Chromatgr Biomed Appl. 1986;48:184–9.
Wilcock C, Bailey CJ. Sites of metformin-stimulated glucose metabolism. Biochem Pharmacol. 1990;39(11):1831–4.
Lalau JD, Vermersch A, Hary L, Andrejak M, Isnard F, Quichaud J. Type 2 diabetes in the elderly: an assessment of metformin. Int J Clin Pharmacol Ther Toxicol. 1990;28(8):329–32.
Gregorio F, Ambrosi F, Cristallini S, Marchetti P, Navalesi R, Brunetti P, et al. Do metformin and phenformin potentiate differently B-cell response to high glucose? An in vitro study on isolated rat pancreas. Diabete Metab. 1991;17(1):19–28.
Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12(2):235–46.
Sarabia V, Lam L, Burdett E, Leiter LA, Klip A. Glucose transport in human skeletal muscle cells in culture. Stimulation by insulin and metformin. J Clin Invest. 1992;90(4):1386–95.
Pentikäinen PJ, Neuvonen PJ, Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979;16(3):195–202.
Freisleben HJ, Ruckert S, Wiernsperger N, Zimmer G. The effects of glucose, insulin and metformin on the order parameters of isolated red cell membranes. An electron paramagnetic resonance spectroscopic study. Biochem Pharmacol. 1992;43(6):1185–94.
Bailey CJ, Wilcock C, Day C. Effect of metformin on glucose metabolism in the splanchnic bed. Br J Pharmacol. 1992;105(4):1009–13.
Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15(6):755–72.
Sum CF, Webster JM, Johnson AB, Catalano C, Cooper BG, Taylor R. The effect of intravenous metformin on glucose metabolism during hyperglycaemia in type 2 diabetes. Diabet Med. 1992;9(1):61–5.
Hermann LS, Melander A. Biguanides: Basic aspects and clinical uses. In: Alberti KGM, DeFronzo RA, Keen H, Zimmet P, editors. International textbook of diabetes mellitus, vol. 1. London: Wiley; 1992. p. 773–95.
Marchetti P, Benzi L, Cecchetti P, Giannarelli R, Boni C, Ciociaro D, et al. Plasma biguanide levels are correlated with metabolic effects in diabetic patients. Clin Pharmacol Ther. 1987;41(4):450–4.
Marchetti P, Benzi L, Gregorio F, Giannarelli R, Cecchetti P, Di Cianni G, et al. New findings on the metabolic effects of biguanides. In vitro and in vivo studies. Minerva Endocrinol. 1988;13(3):173–80.
Chalmers J, McBain AM, Brown IRF, Campbell IW. Metformin: Is its use contraindicated in the elderly? Pract Diab Int. 1992;9:51–3.
Bailey CJ. Metformin revisited: its actions and indications for use. Diabetic Med. 1988;5:315–20.
Galuska D, Nolte LA, Zierath JR, Wallberg-Henriksson H. Effect of metformin on insulin-stimulated glucose transport in isolated skeletal muscle obtained from patients with NIDDM. Diabetologia. 1994;37(8):826–32.
Denno KM, Sadler TW. Effects of the biguanide class of oral hypoglycemic agents on mouse embryogenesis. Teratology. 1994;49(4):260–6.
Marchetti P, Navalesi R. Pharmacokinetic-pharmacodynamic relationships of oral hypoglycaemic agents. An update. Clin Pharmacokinet. 1989;16(2):100–28.
Fischer Y, Thomas J, Rösen P, Kammermeier H. Action of metformin on glucose transport and glucose transporter GLUT1 and GLUT4 in heart muscle cells from healthy and diabetic rats. Endocrinology. 1995;136(2):412–20.
Bailey CJ. Metformin—an update. Gen Pharmacol. 1993;24(6):1299–309.
Lalau JD, Lacroix C, Compagnon P, de Cagny B, Rigaud JP, Bleichner G, et al. Role of metformin accumulation in metformin-associated lactic acidosis. Diabetes Care. 1995;18(6):779–84.
Miao J, Smoak IW. In vitro effects of the biguanide metformin on early-somite mouse embryos. Toxic Subst Mech. 1995;14(3):185–92.
Wiernsperger N. Biguanides: preclinical pharmacology. In: Kuhlman J, editor. Handbook of experimental pharmacology, vol. 119. New York: Springer; 1996. p. 305–58.
Stith BJ, Goalstone ML, Espinoza R, Mossel C, Roberts D, Wiernsperger N. The antidiabetic drug metformin elevates receptor tyrosine kinase activity and inositol 1,4,5-trisphosphate mass in Xenopus oocytes. Endocrinology. 1996;137(7):2990–9.
Sasson S, Gorowits N, Joost HG, King GL, Cerasi E, Kaiser N. Regulation by metformin of the hexose transport system in vascular endothelial and smooth muscle cells. Br J Pharmacol. 1996;117(6):1318–24.
Marchetti P, Gregorio F, Benzi L, Giannarelli R, Cecchetti P, Villani G, et al. Diurnal pattern of plasma metformin concentrations and its relation to metabolic effects in type 2 (non-insulin-dependent) diabetic patients. Diabete Metab. 1990;16(6):473–8.
Caillé G, Lacasse Y, Raymond M, Landriault H, Perrotta M, Picirilli G, et al. Bioavailability of metformin in tablet form using a new high pressure liquid chromatography assay method. Biopharm Drug Dispos. 1993;14(3):257–63.
Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30(5):359–71.
Radziuk J, Zhang Z, Wiernsperger N, Pye S. Effects of metformin on lactate uptake and gluconeogenesis in the perfused rat liver. Diabetes. 1997;46(9):1406–13.
Schulz M, Schmoldt A. Therapeutic and toxic blood concentrations of more than 500 drugs. Pharmazie. 1997;52(12):895–911.
Stith BJ, Woronoff K, Wiernsperger N. Stimulation of the intracellular portion of the human insulin receptor by the antidiabetic drug metformin. Biochem Pharmacol. 1998;55(4):533–6.
Lalau JD, Mourlhon C, Bergeret A, Lacroix C. Consequences of metformin intoxication. Diabetes Care. 1998;21(11):2036–7.
Lalau JD, Race JM, Brinquin L. Lactic acidosis in metformin therapy. Relationship between plasma metformin concentration and renal function. Diabetes Care. 1998;21(8):1366–7.
al-Jebawi AF, Lassman MN, Abourizk NN. Lactic acidosis with therapeutic metformin blood level in a low-risk diabetic patient. Diabetes Care. 1998;21(8):1364–5.
Detaille D, Wiernsperger N, Devos P. Potentiating effect of metformin on insulin-induced glucose uptake and glycogen metabolism with Xenopus oocytes. Diabetologia. 1998;41(1):2–8.
Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica. 1994;24(1):49–57.
Detaille D, Wiernsperger N, Devos P. Cellular and molecular mechanisms involved in insulin’s potentiation of glycogen synthase activity by metformin. Biochem Pharmacol. 1999;58(9):1475–86.
Lupi R, Del Guerra S, Tellini C, Giannarelli R, Coppelli A, Lorenzetti M, et al. The biguanide compound metformin prevents desensitization of human pancreatic islets induced by high glucose. Eur J Pharmacol. 1999;364(2–3):205–9.
Wiernsperger NF. Membrane physiology as a basis for the cellular effects of metformin in insulin resistance and diabetes. Diabetes Metab. 1999;25(2):110–27.
Lalau JD, Race JM. Lactic acidosis in metformin therapy. Drugs. 1999;58(Suppl 1):55–60.
Nelson RW. Oral medications for treating diabetes mellitus in dogs and cats. J Small Anim Pract. 2000;41(11):486–90.
Sambol NC, Chiang J, O’Conner M, Liu CY, Lin ET, Goodman AM, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol. 1996;36(11):1012–21.
Desel H, Stedtler U, Behrens A, Neuratz H. Mischintoxikation mit Metformin. Toxichem Krimtech [in German]. 2000;67:4–8.
Reeker W, Schneider G, Felgenhauer N, Tempel G, Kochs E. Metformin-inducedlactic acidosis [in German]. Dtsch Med Wochenschr. 2000;125:249–51.
Lipha Pharmaceuticals Inc. Package insert of Glucophage [FDA approved]. France: Lipha Pharmaceuticals Inc.; 1997.
Lalau JD, Race JM. Metformin and lactic acidosis in diabetic humans. Diabetes Obes Metab. 2000;2(3):131–7.
Mueller WM, Stanhope KL, Gregoire F, Evans JL, Havel PJ. Effects of metformin and vanadium on leptin secretion from cultured rat adipocytes. Obes Res. 2000;8(7):530–9.
Lalau JD, Race JM, Andreelli F, Lacroix C, Canarelli JP. Metformin retention independent of renal failure in intestinal occlusion. Diabetes Metab. 2001;27(1):24–8.
Kruse JA. Metformin-associated lactic acidosis. J Emerg Med. 2001;20(3):267–72.
Lalau JD, Race JM. Lactic acidosis in metformin therapy: searching for a link with metformin in reports of ‘metformin-associated lactic acidosis’. Diabetes Obes Metab. 2001;3(3):195–201.
Yuan L, Ziegler R, Hamann A. Inhibition of phosphoenolpyruvate carboxykinase gene expression by metformin in cultured hepatocytes. Chin Med J. 2002;115(12):1843–8.
Lupi R, Del Guerra S, Fierabracci V, Marselli L, Novelli M, Patanè G, et al. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes. 2002;51(Suppl 1):S134–7.
Barrueto F, Meggs WJ, Barchman MJ. Clearance of metformin by hemofiltration in overdose. J Toxicol Clin Toxicol. 2002;40(2):177–80.
Lalau JD, Lacroix C. Measurement of metformin concentration in erythrocytes: clinical implications. Diabetes Obes Metab. 2003;5(2):93–8.
Schulz M, Schmoldt A. Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie. 2003;58(7):447–74.
Nisse P, Mathieu-Nolf M, Deveaux M, Forceville X, Combes A. A fatal case of metformin poisoning. J Toxicol Clin Toxicol. 2003;41(7):1035–6.
Dawson D, Conlon C. Case study: metformin-associated lactic acidosis: could orlistat be relevant? Diabetes Care. 2003;26(8):2471–2.
Sweeney D, Raymer ML, Lockwood TD. Antidiabetic and antimalarial biguanide drugs are metal-interactive antiproteolytic agents. Biochem Pharmacol. 2003;66(4):663–77.
Howlett HC, Bailey C. A risk-benefit analysis of metformin in type-2 diabetes mellitus. Drug Saf. 2001;6:489–503.
Sirtori CR, Pasik C. Re-evaluation of a biguanide, metformin: mechanism of action and tolerability. Pharmacol Res. 1994;30(3):187–228.
Moore KA, Levine B, Titus JM, Fowler DR. Analysis of metformin in antemortem serum and postmortem specimens by a novel HPLC method and application to an intoxication case. J Anal Toxicol. 2003;27(8):592–4.
Nelson R, Spann D, Elliott D, Brondos A, Vulliet R. Evaluation of the oral antihyperglycemic drug metformin in normal and diabetic cats. J Vet Intern Med. 2004;18(1):18–24.
Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, et al. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J. 2004;382(Pt 3):877–84.
Feng SY, Lai EP, Dabek-Zlotorzynska E, Sadeghi S. Molecularly imprinted solid-phase extraction for the screening of antihyperglycemic biguanides. J Chromatogr A. 2004;1027(1–2):155–60.
Stades AM, Heikens JT, Erkelens DW, Holleman F, Hoekstra JB. Metformin and lactic acidosis: cause or coincidence? A review of case reports. J Intern Med. 2004;255(2):179–87.
Holland W, Morrison T, Chang Y, Wiernsperger N, Stith BJ. Metformin (glucophage) inhibits tyrosine phosphatase activity to stimulate the insulin receptor tyrosine kinase. Biochem Pharmacol. 2004;67(11):2081–91.
Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, et al. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab. 2004;89(11):5535–41.
Patanè G, Piro S, Rabuazzo AM, Anello M, Vigneri R, Purrello F. Metformin restores insulin secretion altered by chronic exposure to free fatty acids or high glucose: a direct metformin effect on pancreatic beta-cells. Diabetes. 2000;49(5):735–40.
Lalau JD, Masmoudi K. Unexpected recovery from prolonged hypoglycemic coma: a protective role of metformin? [letter]. Intensive Care Med. 2005;31(3):493.
Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, Leverve X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54(7):2179–87.
Kimura N, Okuda M, Inui K. Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm Res. 2005;22(2):255–9.
Davidson MB, Peters AL. An overview of metformin in the treatment of type 2 diabetes mellitus. Am J Med. 1997;102(1):99–110.
Arafat T, Kaddoumi A, Shami M, Yassin M. Pharmacokinetics and pharmacodynamics of two oral formulations of metformin hydrochloride. Adv Ther. 1994;11:21–33.
Brookes LG, Sambol NC, Lin ET, et al. Effect of dosage for dose and food on the pharmacokinetics of metformin [abstract]. Pharm Res. 1991;8(Suppl.):S320.
Sambol NC, Chiang J, Lin ET, Goodman AM, Liu CY, Benet LZ, et al. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol. 1995;35(11):1094–102.
Lacher M, Hermanns-Clausen M, Haeffner K, Brandis M, Pohl M. Severe metformin intoxication with lactic acidosis in an adolescent. Eur J Pediatr. 2005;164(6):362–5.
Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, et al. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26(3):611–7.
Friesecke S, Abel P, Kraft M, Gerner A, Runge S. Combined renal replacement therapy for severe metformin-induced lactic acidosis. Nephrol Dial Transplant. 2006;21(7):2038–9.
Vigersky RA, Filmore-Nassar A, Glass AR. Thyrotropin suppression by metformin. J Clin Endocrinol Metab. 2006;91(1):225–7.
Del Prato S, Bianchi C, Marchetti P. beta-cell function and anti-diabetic pharmacotherapy. Diabetes Metab Res Rev. 2007;23(7):518–27.
Wessler I, Herschel S, Bittinger F, Kirkpatrick CJ. Release of non-neuronal acetylcholine from the isolated human placenta is affected by antidepressants. Life Sci. 2007;80(24–25):2210–3.
Prikis M, Mesler EL, Hood VL, Weise WJ. When a friend can become an enemy! Recognition and management of metformin-associated lactic acidosis. Kidney Int. 2007;72(9):1157–60.
Galea M, Jelacin N, Bramham K, White I. Severe lactic acidosis and rhabdomyolysis following metformin and ramipril overdose. Br J Anaesth. 2007;98(2):213–5.
Bruijstens LA, van Luin M, Buscher-Jungerhans PM, Bosch FH. Reality of severe metformin-induced lactic acidosis in the absence of chronic renal impairment. Neth J Med. 2008;66(5):185–90.
Seidowsky A, Nseir S, Houdret N, Fourrier F. Metformin-associated lactic acidosis: a prognostic and therapeutic study. Crit Care Med. 2009;37(7):2191–6.
Dell’Aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009;54(6):818–23.
Stambolic V, Woodgett JR, Fantus IG, Pritchard KI, Goodwin PJ. Utility of metformin in breast cancer treatment, is neoangiogenesis a risk factor? Breast Cancer Res Treat. 2009;114(2):387–9.
Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, Thor AD. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8(13):2031–40.
Cunha JP, editor. Fortamet (metformin HCl) monograph. RxList [updated 2013 November 20]. 2013 http://www.rxlist.com/cgi/generic/fortamet_cp.htm. Accessed 15 June 2014.
Lalau JD. Lactic acidosis induced by metformin: incidence, management and prevention. Drug Saf. 2010;33(9):727–40.
Friesecke S, Abel P, Roser M, Felix SB, Runge S. Outcome of severe lactic acidosis associated with metformin accumulation. Crit Care. 2010;14(6):R226.
Kane DA, Anderson EJ, Price JW, Woodlief TL, Lin CT, Bikman BT, et al. Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med. 2010;49(6):1082–7.
Frid A, Sterner GN, Löndahl M, Wiklander C, Cato A, Vinge E, Andersson A. Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: clinical recommendations. Diabetes Care. 2010;33(6):1291–3.
Giuliani E, Albertini G, Vaccari C, Barbieri A. pH 6.68—surviving severe metformin intoxication. QJM. 2010;103(11):887–90.
Protti A, Russo R, Tagliabue P, Vecchio S, Singer M, Rudiger A, et al. Oxygen consumption is depressed in patients with lactic acidosis due to biguanide intoxication. Crit Care. 2010;14(1):R22.
Dell’Aglio DM, Perino LJ, Todino JD, Algren DA, Morgan BW. Metformin overdose with a resultant serum pH of 6.59: survival without sequalae. J Emerg Med. 2010;39(1):e77–80.
Sørensen LK. Determination of metformin and other biguanides in forensic whole blood samples by hydrophilic interaction liquid chromatography-electrospray tandem mass spectrometry. Biomed Chromatogr. 2012;26(1):1–5.
The International Association of Forensic Toxicologists (TIAFT). Reference blood level list of therapeutic and toxic substances. London: The Association; 1995–2015. http://www.tiaft.org. Accessed 8 Dec 2010.
Yeung CW, Chung HY, Fong BM, Tsai NW, Chan WM, Siu TS, et al. Metformin-associated lactic acidosis in Chinese patients with type II diabetes. Pharmacology. 2011;88(5–6):260–5.
Lalau JD, Lemaire-Hurtel AS, Lacroix C. Establishment of a database of metformin plasma concentrations and erythrocyte levels in normal and emergency situations. Clin Drug Investig. 2011;31(6):435–8.
de Oliveira Baraldi C, Lanchote VL, de Jesus Antunes N, de Jesus Ponte Carvalho TM, Dantas Moisés EC, Duarte G, et al. Metformin pharmacokinetics in nondiabetic pregnant women with polycystic ovary syndrome. Eur J Clin Pharmacol. 2011;67(10):1027–33.
Correia CS, Bronander KA. Metformin-associated lactic acidosis masquerading as ischemic bowel. Am J Med. 2012;125(5):e9.
Vecchio S, Papa P, Protti A. Metformin and lactic acidosis. In: Vincent JL, editor. Annual update in intensive care and emergency medicine, vol. 1. Heidelberg: Springer Science & Business Media; 2011. p. 685–93.
Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, Makino Y, et al. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1α expression and oxygen metabolism. Diabetes. 2011;60(3):981–92.
Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9.
Roche C, Nau A, Peytel E, Moalic JL, Oliver M. Severe lactic acidosis due to metformin: report of 3 cases. Ann Biol Clin (Paris). 2011;69(6):705–11.
Perrone J, Phillips C, Gaieski D. Occult metformin toxicity in three patients with profound lactic acidosis. J Emerg Med. 2011;40(3):271–5.
Pikwer A, Vernersson E, Frid A, Sterner G. Extreme lactic acidosis type B associated with metformin treatment. NDT Plus. 2011;4(6):399–401.
Cosenza L, Al-Dahir S, Engel LS, Nielsen N. A potentially fatal case of mistaken identity: metformin vs oxycodone-acetaminophen [abstract]. J Investig Med. 2011;59(2):385.
Berstein LM, Yue W, Wang JP, Santen RJ. Isolated and combined action of tamoxifen and metformin in wild-type, tamoxifen-resistant, and estrogen-deprived MCF-7 cells. Breast Cancer Res Treat. 2011;128(1):109–17.
Jagia M, Taqi S, Hanafi M. Metformin poisoning: a complex presentation. Indian J Anaesth. 2011;55(2):190–2.
Briet C, Saraval-Gross M, Kajbaf F, Fournier A, Hary L, Lalau JD. Erythrocyte metformin levels in patients with type 2 diabetes and varying severity of chronic kidney disease. Clin Kidney J. 2012;5(1):65–7.
Schulz M, Iwersen-Bergmann S, Andresen H, Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit Care. 2012;16(4):R136.
Protti A, Fortunato F, Monti M, Vecchio S, Gatti S, Comi GP, et al. Metformin overdose, but not lactic acidosis per se, inhibits oxygen consumption in pigs. Crit Care. 2012;16(3):R75.
Protti A, Lecchi A, Fortunato F, Artoni A, Greppi N, Vecchio S, et al. Metformin overdose causes platelet mitochondrial dysfunction in humans. Crit Care. 2012;16(5):R180.
Lam N, Sekhon G, House AA. Metformin-associated lactic acidosis following intentional overdose successfully treated with tris-hydroxymethyl aminomethane and renal replacement therapy. Case Rep Nephrol. 2012;2012:671595.
Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48(3):R31–43.
Duong JK, Furlong TJ, Roberts DM, Graham GG, Greenfield JR, Williams KM, et al. The role of metformin in metformin-associated lactic acidosis (MALA): case series and formulation of a model of pathogenesis. Drug Saf (Epub 2013 Apr 3).
Kajbaf F, Lalau JD. The criteria for metformin-associated lactic acidosis: the quality of reporting in a large pharmacovigilance database. Diabet Med. 2013;30(3):345–8.
Luft D, Deichsel G, Schmulling R, et al. Definition of clinically relevant lactic acidosis in patients with internal diseases. Am J Clin Pathol. 1983;80(4):484–9.
Kajbaf F, Lalau JD. The prognostic value of blood pH and lactate and metformin concentrations in severe metformin-associated lactic acidosis. BMC Pharmacol Toxicol. 2013;12(14):22.
Bonsignore A, Pozzi F, Fraternali Orcioni G, Ventura F, Palmiere C. Fatal metformin overdose: case report and postmortem biochemistry contribution. Int J Legal Med. 2014;128(3):483–92.
Al-Abri SA, Hayashi S, Thoren KL, Olson KR. Metformin overdose-induced hypoglycemia in the absence of other antidiabetic drugs. Clin Toxicol (Phila). 2013;51(5):444–7.
Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56(9):1898–906.
Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210.
Vecchio S, Giampreti A, Petrolini VM, Lonati D, Protti A, Papa P, Rognoni C, et al. Metformin accumulation: lactic acidosis and high plasmatic metformin levels in a retrospective case series of 66 patients on chronic therapy. Clin Toxicol (Phila). 2014;52(2):129–35.
Moffat AC, Osselton MD, Widdop B, editors. Clarke’s analysis of drugs and poisons. 3rd ed. London: Pharmaceutical Press; 2004. p. 243.
Repetto MR, Repetto M. Tabla de concentraciones de xenobioticos en fluidos humanos como referencia parael diagnostico toxicologico. 2012. http://www.busca-tox.com. Accessed 3 July 2012.
Geerling JJ, Boon MR, van der Zon GC, van den Berg SA, van den Hoek AM, Lombès M, et al. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes. 2014;63(3):880–91.
Launiainen T, Ojanperä I. Drug concentrations in post-mortem femoral blood compared with therapeutic concentrations in plasma. Drug Test Anal. 2014;6(4):308–16.
Fremin K, Owen J. Metformin overdose and early renal replacement therapy. Am J Kidney Dis. 2014;63(5):B47.
Renehan A. Diabetes treatment and cancer—five years after the ‘breaking news’. Diabetes Voice. 2014;59(1):36–9.
Adam WR, O’Brien RC. A justification for less restrictive guidelines on the use of metformin in stable chronic renal failure. Diabet Med. 2014;31(9):1032–8.
Acquistapace G, Rossi M, Garbi M, Cosci P, Canetta C, Manelli A, et al. Acute metformin intoxication: 2012 experience of Emergency Department of Lodi, Italy. Clin Chem Lab Med. 2014;52(10):1489–97.
Maruthur NM, Gribble MO, Bennett WL, Bolen S, Wilson LM, Balakrishnan P, et al. The pharmacogenetics of type 2 diabetes: a systematic review. Diabetes Care. 2014;37(3):876–86.
Tzvetkov MV, Vormfelde SV, Balen D, Meineke I, Schmidt T, Sehrt D, et al. The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2, and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther. 2009;86(3):299–306.
Goswami S, Yee SW, Stocker S, Mosley JD, Kubo M, Castro R, et al. Genetic variants in transcription factors are associated with the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2014;96(3):370–9.
Christensen MM, Brasch-Andersen C, Green H, Nielsen F, Damkier P, Beck-Nielsen H, et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21(12):837–50.
Bailey CJ, Aschner P, Del Prato S, LaSalle J, Ji L, Matthaei S. Global Partnership for Effective Diabetes Management. Individualized glycaemic targets and pharmacotherapy in type 2 diabetes. Diab Vasc Dis Res. 2013;10(5):397–409.
Watanabe RM. Drugs, diabetes and pharmacogenomics: the road to personalized therapy. Pharmacogenomics. 2011;12(5):699–701.
Esposito K, Ceriello A, Giugliano D. Does personalized diabetology overcome clinical uncertainty and therapeutic inertia in type 2 diabetes? Endocrine. 2013;44(2):343–5.
Ceriello A, Gallo M, Candido R, De Micheli A, Esposito K, Gentile S, et al. Personalized therapy algorithms for type 2 diabetes: a phenotype-based approach. Pharmgenomics Pers Med. 2014;19(7):129–36.
Scheen AJ. Personalising metformin therapy: a clinician’s perspective. Lancet Diabetes Endocrinol. 2014;2(6):442–4.
Pratley RE, Kuritzky L, Tenzer P. A patient-centered approach to managing patients with type 2 diabetes. Am J Med. 2014;127(11):e15–6.
Cao J, Meng S, Chang E, Beckwith-Fickas K, Xiong L, Cole RN, et al. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J Biol Chem. 2014;289(30):20435–46.
El-Mir MY, Nogueira V, Fontaine E, Ave´ret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–8.
Piel S, Ehinger JK, Elmér E, Hansson MJ. Metformin induces lactate production in peripheral blood mononuclear cells and platelets through specific mitochondrial complex I inhibition. Acta Physiol (Oxf). 2015;213(1):171–80.
Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35(2):446–54.
Lalau JD, Kajbaf F. Interpreting the consequences of metformin accumulation in an emergency context: impact of the time frame on the blood metformin levels. Int J Endocrinol. 2014;2014:717198.
Lalau JD, Azzoug ML, Kajbaf F, Briet C, Desailloud R. Metformin accumulation without hyperlactataemia and metformin-induced hyperlactataemia without metformin accumulation. Diabetes Metab. 2014;40(3):220–3.
Kajbaf F, Lalau JD, Azzoug M, Lemaire-Hurtel AS, De Broe ME. Metformin therapy at different stages of chronic kidney disease [abstract]. J Am Soc Nephrol. 2014;25(Abstract Edition):76A.
Acknowledgments
The authors thank the Amiens University Library and Dr Kerstin Brand for providing reprints of some articles.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Kajbaf, F., De Broe, M.E. & Lalau, JD. Therapeutic Concentrations of Metformin: A Systematic Review. Clin Pharmacokinet 55, 439–459 (2016). https://doi.org/10.1007/s40262-015-0323-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0323-x