Abstract
Uncertainties abound in clinical management of type 2 diabetes. Sources of uncertainty specific to type 2 diabetes originate from the panoply of glycemic (HbA1c) targets, the complexity of drug therapy, the ideal sequence of drugs after metformin failure, the possible harms of anti-hyperglycemic drugs, the outcomes of treatment (surrogate versus clinical) and the hierarchy of risk factors to treat in order to prevent the vascular complications. Ironically, multiple treatment guidelines and algorithms periodically released to improve guidance may generate confusion into clinicians. Moreover, treatment algorithms cannot be truly evidence-based because of a lack of studies comparing all available treatment combination options. Personalized therapy essentially identifies patients who could have major benefits from the therapy as compared with other patients. Personalized medicine for type 2 diabetic has the potential to improve the quality health-care practice of diabetes management, but specific research is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Uncertainties abound in healthcare [1, 2]. Clinical uncertainty has the potential to contribute to clinical inertia, a condition supposed to be implicated in delaying initiation or intensification of therapy when indicated [3]. In 2011, there were 366 million people with diabetes worldwide, and this is expected to rise to 552 million by 2030, rendering previous estimates very conservative [4]. Uncertainty also abounds in therapeutic management of type 2 diabetes.
What is the evidence of uncertainty?
Uncertainty about the HbA1c goal. The dogma of “one size fits all” has been disputed for the HbA1c target of <7 % for all diabetic patients [5]. It will be surprising how less frequently a clinician has the occasion to realize that a HbA1c value of 7 % corresponds to a mean daily glycemia of about 160 mg/dl: Paradoxically, the golden goal of less than 7 %, still a difficult task for many individuals with type 2 diabetes [6–8], does not mean normal glucose levels.
Uncertainty about drug sequences The optimal drug sequence after metformin use is an area of increasing uncertainty [9], given the panoply of diabetes medications so far available (more tomorrow) for treatment of hyperglycemia (dual, triple, or quadruple therapy) [10, 11].
Uncertainty about harm of treatment As drugs, all diabetes medications have some problem with tolerability and safety. Moreover, antihyperglycemic agents also have the potential to contribute to therapeutic inertia, via factors inherent to the drugs themselves, such as treatment-related adverse effects (e.g., hypoglycemia, weight gain, edema, gastrointestinal symptoms, and cancer risk), perception of long-term safety profiles, and the complexity of the treatment regimen. Adherence to oral antidiabetes therapy may be as low as 50 % after 2 years [12].
Uncertainty about cardiovascular outcomes Not every subject with type 2 diabetes benefits from intensive glycemic control: The main lesson we learned from recent trials is that surrogate improvements (more intensive lowering of HbA1c) appear to produce marginal benefit for macrovascular disease. The most recent meta-analyses conclude for limited and inconclusive evidence of risk reduction for cardiovascular end points, while confirming a substantial increase of severe hypoglycemia [13, 14].
Uncertainty about risk factors Uncertainty also exists about the most important factor in preventing all diabetes complications. Among US adults with diabetes in 2005–2006, there was nearly universal antidiabetes medication use, but only 78 and 51 % antihypertensive and statin medication use, respectively [15]. This pointed to a disproportionate emphasis being placed on controlling hyperglycemia at the expense of the more evidence-based cardiovascular risk reduction strategies of controlling hypertension and high cholesterol level.
Is ongoing research likely to provide relevant evidence?
Ironically, multiple treatment guidelines, algorithms, and goals periodically released to improve guidance may generate uncertainty. Treatment algorithms cannot be truly evidence-based because the rising number of diabetes medications makes it hard to explore all possible combinations and sequences of combinations that could be recommended. As an example, the guidelines released by the American College of Physicians recommend adding a second agent when lifestyle modifications and monotherapy with metformin fail to control hyperglycemia without any further suggestion as no good evidence supports one combination therapy over another [16]. At the other extreme, tailored therapeutic algorithms for some of the most common type 2 diabetes phenotypes have been developed, taking into consideration age, body mass index, the presence of micro- and macrovascular complications, hypoglycemia risk, and the coexistence of chronic renal failure (www.aemmedi.it/algoritmi/). So, the guidance varies immensely.
What should we do in the light of the uncertainty?
Personalized medicine, the newest trend based on patient-centered care [17], may be seen as a promising tool to manage therapeutic uncertainty in type 2 diabetes. Translated to type 2 diabetes, a patient-centered approach essentially identifies patients who could have major benefits from a particular drug as compared with other drugs. Perhaps it is not by chance that the position statement from the ADA/EASD on the management of hyperglycemia in type 2 diabetes is subtitled a patient-centered approach [7]. This statement highlights the clinical judgment (a mixture of clinical experience, knowledge, and skill) of physicians together with the patient values and preferences. However, physician feeling and conviction about the willingness to reach the HbA1c target (now tailored on the patient) remain paramount to reduce unnecessary therapeutic inertia.
Individualizing HbA1c target and personalizing treatment
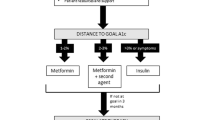
Some practical aspects that may help clinicians to individualize the specific HbA1c target and personalize diabetes therapy in type 2 diabetic patients are given in the Table 1.
-
Individuals with type 2 diabetes should seek HbA1c <7 % in the absence of warnings (long duration of diabetes, severe vascular complications, short life expectancy, prior severe hypoglycemia) [7]; on-treatment failure to reduce HbA1c by at least 0.5 % from the baseline in 4–12 months, associated with hypoglycemia requiring assistance, also suggests abandoning the HbA1c target of <7 % [18]. The suggested target range is 7–8 % HbA1c.
-
Therapeutic tactics may be adapted to patient characteristics in order to magnify the benefits, while reducing the risks of therapy.
-
The distance from the target, i.e., the difference between the current HbA1c value of the patient and the individualized HbA1c target, may be useful as a predictor of therapeutic success. The expected reduction in HbA1c after failure of the first drug (metformin) ranges between 0.62 and 1.0 % in patients treated with various adjunctive drugs (excluding insulin), while after failure of two drugs (mainly metformin and sulfolylureas), the HbA1c decrease ranges between 0.6 and 1.15 % [19, 20]. So, on the average, the expected HbA1c decrease after failure of one or two oral drugs is about 1 %.
-
Among the many antidiabetes drugs added to the previous treatment (either monotherapy or dual therapy), insulin has the best chance to reach the prefixed HbA1c target [20]. Unlike noninsulin drugs, the relationship between baseline HbA1c and the decrease of HbA1c after insulin therapy remains linear at higher baseline HbA1c levels [21]. So, consider insulin if the distance from the target is >1.5 %.
-
The greater the baseline HbA1c level of the patient, the greater the HbA1c reduction following therapy. For noninsulin drugs, it has been estimated that for each 0.5 % increase in baseline HbA1c level (from 7.5 to 9.5 %), there is 0.2 % more HbA1c reduction after therapy with any drug [21].
Conclusions
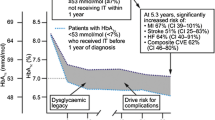
Medicine is a complex mixture of art and science: More than a century ago, Osler described medicine as a “science of uncertainty and art of probability.” Little improvement in glycemic control occurred in the UK between 1997 and 2007 among type 2 diabetic patients [22] and, at the best, half or less than half of the people with type 2 diabetes reach the HbA1c goal of <7 %. Personalized diabetology has the potential to improve the quality healthcare practice of diabetes management, including avoidance of unnecessary therapeutic inertia, but specific research is needed.
References
J. Wennberg, On patient need, equity, supplier-induced demand, and the need to assess the outcome of common medical practice. Med. Care 23, 512–520 (1985)
D. Gianakos, Accepting limits. Arch. Intern. Med. 158, 1059–1061 (1998)
D. Giugliano, K. Esposito, Clinical inertia as a clinical safeguard. JAMA 305, 1591–1592 (2011)
D.R. Whiting, L. Guariguata, C. Weil, J. Shaw, IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 94, 311–321 (2011)
W.T. Cefalu, American Diabetes Association-European Association for the study of diabetes position statement: due diligence was conducted. Diabetes Care 35, 1201–1203 (2012)
T.J. Hoerger, J.E. Segel, E.W. Gregg, J.B. Saaddine, Is glycemic control improving in U.S. adults? Diabetes Care 31, 81–86 (2008)
S.E. Inzucchi, R.M. Bergenstal, J.B. Buse, M. Diamant, E. Ferrannini, M. Nauck, A.L. Peters, A. Tsapas, R. Wender, D.R. Matthews, Management of Hyperglycemia in Type 2 Diabetes: a Patient-Centered Approach: position Statement of the American Diabetes Association (ADA) and the European Association for the study of diabetes (EASD). Diabetes Care 35, 1364–1379 (2012)
American Diabetes Association, Standards of medical care in diabetes-2013. Diabetes Care 36(Suppl. 1), S11–S66 (2013)
K. Esposito, G. Bellastella, D. Giugliano, When metformin fails in type 2 diabetes mellitus. Arch. Intern. Med. 171, 365–366 (2011)
D. Giugliano, A. Ceriello, K. Esposito, HbA1c targets for type 2 diabetes: how many, how far! Diabetes Res. Clin. Pract. 96, 414–415 (2012)
S. Colagiuri, Optimal management of type 2 diabetes: the evidence S. Diabetes Obes. Metab. 14(Suppl. 1), 3–8 (2012)
J. Yeaw, J.S. Benner, J.G. Walt, S. Sian, D.B. Smith, Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 15, 728–740 (2009)
B. Hemmingsen, S.S. Lund, C. Gluud, A. Vaag, T. Almdal, C. Hemmingsen, J. Wetterslev, Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ 343, d6898 (2011)
R. Boussageon, T. Bejan-Angoulvant, M. Saadatian-Elahi, S. Lafont, C. Bergeonneau, B. Kassaï, S. Erpeldinger, J.M. Wright, F. Gueyffier, C. Cornu, Effect of intensive glucose lowering treatment on all-cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 343, d4169 (2011)
D.M. Mann, M. Woodward, F. Ye, M. Krousel-Wood, P. Muntner, Trends in medication use among US adult with diabetes mellitus: glycemic control at the expense of controlling cardiovascular risk factors. Arch. Intern. Med. 169, 1718–1720 (2009)
A. Qaseem, L.L. Humphrey, D.E. Sweet, M. Starkey, P. Shekelle, For the Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 156, 218–231 (2012)
M.J. Barry, S. Edgman-Levital, Shared decision making—the pinnacle of patient-centered care. N. Engl. J. Med. 366, 780–781 (2012)
M.C. Riddle, D.M. Karl, Individualizing targets and tactics for high-risk patients with type 2 diabetes. Practical lessons from ACCORD and other cardiovascular trials. Diabetes Care 35, 2100–2107 (2012)
O.J. Phung, J.M. Scholle, M. Talwar, G.I. Coleman, Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 303, 1410–1418 (2010)
J.L. Gros, C.K. Kramer, C.B. Leitão, N. Hawkins, L.V. Viana, B.D. Schaan, L.C. Pinto, T.C. Rodrigues, M.J. Azevedo, For the Diabetes and Endocrinology Meta-analysis Group (DEMA). Effect of antihyperglycemic agents added to metformin and a sulfonylurea on glycemic control and weight gain in type 2 diabetes: a network meta-analysis. Ann. Intern. Med. 154, 672–679 (2011)
K. Esposito, P. Chiodini, G. Bellastella, M.I. Maiorino, D. Giugliano, Proportion of patients at HbA1c target < 7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes. Metab. 14, 228–233 (2012)
C.J. Currie, E.A. Gale, C.D. Poole, Estimation of primary care treatment costs and treatment efficacy for people with Type 1 and Type 2 diabetes in the United Kingdom from 1997 to 2007. Diabet. Med. 27, 938–948 (2010)
Acknowledgments
Nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esposito, K., Ceriello, A. & Giugliano, D. Does personalized diabetology overcome clinical uncertainty and therapeutic inertia in type 2 diabetes?. Endocrine 44, 343–345 (2013). https://doi.org/10.1007/s12020-013-9918-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-9918-x