Abstract
Three decades after its introduction, pharmacokinetic population approaches have become a reference method for drug modelling, particularly in paediatrics. The main practical limitation in this specific population is the collected blood volume. Pharmacokinetic population approaches using sparse sampling may resolve this issue. The pharmacokinetics of many drugs have been studied during the last 25 years using such methods. This review summarizes all of the published studies concerning population pharmacokinetic approaches in paediatric subjects from neonate to 2 years old. A literature search was conducted using the PubMed database, from 1985 to December 2010, using the following terms: pharmacokinetic(s), population, paediatric/pediatric and neonate(s). Articles were excluded if they were not pertinent according to our criteria. References of all relevant articles were also evaluated. Ninety-eight studies were included in this review. The following information was extracted from the articles: drug name, therapeutic class, population size, age of patients, number of samples per patient, covariates used for clearance and volume of distribution estimates, software used for modelling and validation methods. An increasing rate of publications over the years was observed; 44 different drugs were studied using a pharmacokinetic population approach. Antibacterials were the most studied class of drugs, including a large number of studies devoted to vancomycin and gentamicin. It must be underlined that few studies have been performed on anticonvulsant drugs and anaesthetics used in clinical daily practice conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Medications used in newborns are rarely evaluated; drug labelling commonly includes disclaimers that safety and effectiveness have not been established in newborns [1, 2]. Most paediatric practices, particularly in inpatient subjects, involve ‘off-label’ use of medications [3–5]. Indeed, two thirds of drugs prescribed to inpatient newborns are unlicensed or off-label. This proportion reaches 90 % in intensive care units.
It is well-known that drug kinetics in children are very different to those in adults as far as drug absorption, distribution, metabolism and elimination are concerned. Indeed, growth and development are two features of children that are not observed in adults. The first few years of life are characterized by growth and maturation of enzymatic processes [6]. Concerning absorption, gastric pH is increased in neonates, infants and young children and reaches adult pH values at around 2 years of age. Gastrointestinal motility is decreased in neonates and reaches adult levels in infants. After absorption, drugs are distributed to various body compartments according to their physicochemical properties. In neonates and infants, total body water is increased, which contributes to an increase in the volume of distribution for hydrophilic drugs. For metabolism, enzymatic activity of metabolic enzymes, such as the cytochrome P450 (CYP) or uridine diphosphate glucuronosyltransferase (UGT) families, depends on genetic, physiological and environmental factors. For example, expression of CYP1A2 at birth is negligible, reaching 50 % of adult expression by 0.9 years of age, activity of CYP2C9 is close to 20 % of adult values at birth and reaches 50 % by 1 month of age, CYP2C19 activity is approximately 30 % of adult activity at birth and adult levels are achieved by 1 year of age, and activity of UGTs is deficient at birth and reaches adult levels at 2–4 years of age [6]. Concerning elimination and renal clearance, the glomerular filtration rate (GFR) increases steadily to 50–75 % of adult function by 6 months and tubular secretion lags behind maturation of glomerular filtration by 7 months to 1 year. Renal function fully matures by around 1 year of age. Also, drug dynamics, including desired and undesired side effects, may be very different in newborns as the amplitude and the nature of the response may be different to that in adults. The contribution of pharmacodynamic variability due to distribution from the blood to the site of action will depend largely on changes in target tissue perfusion. Receptor sensitivity and efficacy may also vary. The observed response may not be explained by a direct consequence of drug receptor binding but rather through intermediate physiological mechanisms. Disease states may also be different in newborns, compared with infants or adults, some of which are only observed in newborns. In addition, neonates and young infants may suffer from permanent effects resulting from stimulus applied at a sensitive point in development [7].
For all of these reasons, the pharmacokinetic differences between newborns and adults justify specific pharmacokinetic studies in newborns. After introduction of the non-linear mixed-effects modelling methodology to clinical pharmacology, the population pharmacokinetic approaches became a reference technique in the newborn population. This method allows pharmacokinetic studies with sparse data (rich pharmacokinetic data might be difficult or impossible to obtain in the paediatric population).
Population modelling is a relatively new pharmacological tool, the development of which has largely been stimulated by the need for accurate pharmacokinetic models of numerous drugs. Non-linear mixed-effects modelling, a commonly used population-based modelling approach, estimates intra- and inter-individual variability and allows simulations of drug delivery regimens. In addition, covariates such as bodyweight, age and disease state may be taken into account in the same pharmacokinetic modelling analysis.
This paper provides an overview of the current literature on population pharmacokinetic studies in paediatrics from neonates to 2 years old.
2 Search Strategy and Selection Criteria
2.1 Inclusion Criteria
Articles were included if they met the following criteria:
-
Populations: neonates to 2 years old
-
Treatment: all drugs, all routes of administration
-
Pharmacokinetic analysis: modelling by population approach.
2.2 Exclusion Criteria
Articles were excluded if they met the following criteria:
-
Populations: subjects of more than 2 years old
-
Papers not written in English.
2.3 Data Extraction
A literature search of original articles was conducted using the PubMed database, from 1985 to December 2010, using the following keywords: [population AND pharmacokinetics AND neonate(s)] OR (population AND pharmacokinetics AND paediatric (pediatric)). Then, based on the abstract, articles were selected according to our inclusion criteria, i.e. articles describing a population pharmacokinetic model of one or several drugs in neonates to 2 years old. A study was considered to be a population study if a mixed-effects model fitted the data, whatever the population size.
The following information was extracted from the articles: drug name, therapeutic class, population size, age of patients, number of samples per patient, software used for modelling, evaluation methods, covariates used for clearance and volume of distribution estimates, study design (retrospective study with therapeutic drug monitoring data, prospective study or prospective study with optimal sampling) and conclusion of the study (estimation of pharmacokinetic parameters or dosing recommendations).
Following Brendel et al. [8] and Tod et al. [9], the evaluation methods were divided into three categories according to increasing order of quality: basic internal methods (goodness-of-fit plots), advanced internal methods (bootstrap, cross-validation, Monte-Carlo simulations, etc.) and external model evaluation.
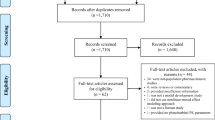
A total of 106 citations were produced after the search was performed, eight of which were excluded in relation to our exclusion criteria. The 98 remaining articles were then analysed [10–104].
3 Study Characteristics
The 98 studies analysed described a pharmacokinetic population model in a newborn population and were published between 1985 and 2010 (Table 1). Studied populations consisted of paediatrics from neonates to 2 years old. Drugs were administered by intravenous, oral and rectal routes.
4 Data Synthesis
There was an increasing rate of publications over the years, reaching about 40 in the most recent period between 2005 and 2010. Regarding the software involved in model building, NONMEM® was used in 83 % of the studies. Advanced validation and external validation methods of the population model were carried out in 37 and 11 % of articles, respectively. The number of studies classified by therapeutic class shows that antibacterial and anaesthetic classes were the most frequently evaluated. A more detailed analysis for the antibacterial class shows that 14 different drugs were studied, with most studies concerning gentamicin (aminoglycoside) and vancomycin (glycopeptide) (Fig. 1). Most studies were conducted with a population of between 25 and 100 patients. The studies were realized with a median post-natal age (PNA) ranging from the first day of life to 1 month (Fig. 2). The number of samples per patient, which was not often reported, did not exceed five samples per patient. Concerning the study design, 39 studies were performed using data from therapeutic drug monitoring, 57 were prospective studies and only two were prospective studies using the optimization of sampling (Fig. 3). Summarizing the conclusions of these studies, 50 consisted only of an estimation of pharmacokinetic parameters of the studied drug and the remaining 48 concluded with dosing recommendations (Fig. 4). However, none of these dosing recommendations were followed by a new study devoted to their clinical evaluation.
Concerning clearance and volume of distribution modelling, three covariates were frequently used to estimate the clearance while two were used for volume of distribution. Indeed, bodyweight was the most used covariate to estimate clearance or volume of distribution. To estimate clearance among the 67 studies using bodyweight, 65 studies directly used bodyweight, including 19 allometric functions, while two studies use body surface area (BSA). To estimate the volume of distribution among the 64 studies using bodyweight, 63 studies directly used bodyweight, including 18 allometric functions, and one study used BSA.
The second covariate used was age; 59 studies included this covariate in the equation of the clearance and eight studies included it in the equation for the volume of distribution. Age was variously expressed as post-conceptional age (PCA) or post-menstrual age (PMA), PNA and gestational age (GA). For the estimation of clearance, 29 studies used the PNA, 17 studies used the PCA or PMA, and 13 studies used the GA. As for clearance, PNA was mainly used to estimate the volume of distribution, with five studies using the PNA, two studies using GA and one study using PCA. The other covariate frequently used was renal function. To estimate clearance, this covariate was expressed using three parameters: creatinine clearance (CLCR), serum creatinine (SCr or Cr) or GFR. Thirteen studies used this covariate: nine studies the SCr, four studies the CLCR and one study GFR (one study used GFR and SCr [acyclovir]).
5 Discussion
This survey confirmed that population pharmacokinetic studies in paediatrics are increasing. Indeed, we observed an increasing rate of publications over the years, reaching about eight per year in the most recent period between 2008 and 2010. We also note that the number of studies doubles every 5 years. This can be explained by the fact that such studies can be realized with sparse data, reducing the invasiveness, and making neonates a population of choice for this type of study. The increasing number of studies is also in parallel with population pharmacokinetic software development. Several programs have been created over the past 20 years but NONMEM® software is the most used. Created in the 1980s by L. Sheiner and S. Beal, it was the first software that allowed this type of analysis and is considered to be the gold standard in the field of pharmacokinetic modelling. Whatever the software used, a robust evaluation of the pharmacokinetic model defined is required. This review describes the methods as proposed by Brendel et al. [8]. Only 10 % of the described models were evaluated by an external evaluation. Concerning study design, the number of patients per study was between 25 and 100 with 3–5 blood samples per patient. The conditions of the studies could not be clearly evaluated because some aspects of the study design were not described in sufficient detail. However, the majority of the studies were prospective (60 %), while 40 % were retrospective studies with data from therapeutic drug monitoring. It must be noted that only 2 % of the prospective studies used the ‘optimal sampling’. Among the 58 % of prospective studies remaining, in 28 % the number of samples per child was not referenced and 44 % were achieved with less than five samples per child; this confirms that the volume of blood collected in this population is a limiting factor despite the emergence of new assay techniques such as mass spectrometry. These new techniques make it possible to work with micro-volumes and therefore are indicated in the paediatric population, even if they are not yet available for all drugs. This information therefore explains why a majority of the studies were conducted with a limited number of samples per patient whether they were retrospective or prospective studies. Concerning the conclusions of the studies, 51 % conclude with an estimate of pharmacokinetic parameters and 49 % conclude on dosing recommendations. When looking specifically at studies recommending new dosages, it appears that none of these studies have made a clinical evaluation of a new proposed dosing. The growing interest for this type of pharmacokinetic study in this population shows the necessity to better describe the pharmacokinetics of drugs administered to infants. However, these studies only propose a simple pharmacokinetic parameter estimation, which is not actually used in clinical practice conditions by physicians. Dosing recommendations seem to be much easier to use clinically, the only constraint being that the non-clinical evaluation remains an obstacle for clinicians. A collaboration between pharmacologists and clinicians should be implemented when modelling data in order to clinically evaluate dosing data proposed by this modelling.
Pharmacokinetic modelling identifies a number of covariates, thus explaining some of the pharmacokinetic variability. This review identified the significant covariates, i.e. the use of these covariates improves prediction of the time–concentration profile in the individual infant. Bodyweight, age and renal function are the three major covariates in neonates and young infants. Bodyweight is the most common covariate used to determine dose in a paediatric population. The change in bodyweight with age is significant up to 1 year: bodyweight increases approximately three- to fourfold from birth to 1 year [105]. Allometric size modelling is used with increasing frequency in paediatric pharmacokinetic population analyses. It is now widely recognised that there is a non-linear relationship between bodyweight and drug elimination capacity. It is possible to show that the log of the basal metabolic rate plotted against the log of the bodyweight produces a straight line with a slope of 0.75. These allometric ‘1/4 power’ models can be applied to pharmacokinetic parameter estimates in infants, e.g. clearance (0.75) and volume of distribution (1) [106]. The use of these coefficients is supported by fractal geometric concepts and observations from diverse areas in biology [107]. Allometric scaling also allows the direct comparison of paediatric estimates with adults when a bodyweight standard of 70 kg is used. Nevertheless, bodyweight is insufficient to predict clearance in neonates and infants from adult estimates. By choosing bodyweight as the primary covariate and by using this exponent of 0.75, secondary covariates can be investigated within a given dataset describing time–concentration profiles in a population.
Age is the second covariate most used. Indeed, the first few years of life are a time of growth and maturation of enzymatic processes. This maturation factor cannot be explained by allometry. The addition of a model describing maturation is required. The sigmoid hyperbolic or Hill model has been found to be useful for describing this maturation process but this model is seldom used [108]. Maturation of clearance begins before birth, suggesting that covariates such as PMA or GA would be a better predictor of drug elimination than PNA. Indeed, the impact of ontogeny on the expression and functional activity of the major drug-metabolizing enzymes may be important. However, this review shows that in daily practice the PNA covariate is more often used. This can be explained by the fact that PMA and GA are more difficult to retrieve, particularly with regard to retrospective studies but also when the study involves older children. Whatever the definition of age (PCA or PMA, GA or PNA), this factor largely contributes to the variability of drugs given to neonates and young infants, but the impact will depend on the speed of maturation and the subpopulation studied.
The third covariate is renal function, which is often estimated by SCr, CLCR or GFR. With this covariate, especially regarding CLCR, we might expect to reflect the influences of size, maturation and organ function. Impaired renal function alters the ability of this organ to clear drugs from the body. For example, GFR is reduced in neonates and matures over the first few years of life [109]. But this covariate is of interest only if the studied drug is excreted renally as is the case in 13 studies including this covariate. Therefore, bodyweight and age are the baseline covariates among neonates and infants that can significantly reduce inter-individual variability.
Among the covariates used to estimate the pharmacokinetic parameters some relationships seem unusual, e.g. the relationship between the clearance of midazolam and time after cannulation, between the clearance of vancomycin and the concomitant administration of amoxicillin, and between the clearance of gentamicin and the Apgar score [12, 97, 98]. Regarding the study of midazolam [97], the authors explain a collinearity between the PNA, PMA and extracorporeal membrane oxygenation (ECMO) time (Tecmo) because most patients are placed on ECMO in a short timeframe and because only neonates with a GA of at least 34 weeks are eligible. Therefore, the authors selected an appropriate combination of temporal covariates based on the best improvement in the goodness of fit and statistical significance at the 95 % confidence level. For the study on vancomycin [98], the authors propose as an explanation a possible inhibition of tubular reabsorption of vancomycin caused by amoxicillin. This hypothesis can therefore explain the increased clearance of vancomycin in patients receiving amoxicillin. For the last unusual relationship between the clearance of gentamicin and the Apgar score, the authors provide no information to explain this relationship [12]. Concerning the volume of distribution, the relationship between the volume of distribution of meropenem and CLCR, between the volume of distribution of vancomycin and the concomitant administration of spironolactone, and between the volume of distribution of phenobarbital and the Apgar score are unexpected [10, 93, 98]. Regarding the study on meropenem, the authors explain this relationship simply by the fact that changes in body water and the development of renal function influences the distribution of meropenem [93]. For the study of vancomycin, the authors postulate that spironolactone could decrease vancomycin volume of distribution as a consequence of changes in the total body water [98]. As for the final unusual relationship cited, the explanation is not detailed; the authors associate an increased distribution of phenobarbital in patients with asphyxia [10].
These unusual relationships between pharmacokinetic parameters and covariates recall the importance of testing clinically relevant covariates in order to decrease the risk of false positives. The objective of including a covariate in a model is not only for decreasing inter-individual variability but also for explaining it. This is essential before deciding if a drug adjustment based on this covariate is required.
The studied therapeutic classes represent a panel of drugs most frequently administered in paediatrics: antibacterials, anaesthetics, NSAIDs, cardiovascular drugs, anti-asthmatics, antiretrovirals, antivirals, anticonvulsants, antifungals, analgesics, drugs affecting gastrointestinal function, caffeine and allopurinol. Even though 44 different drugs were studied in neonates, numerous other drugs remain to be investigated.
Antibacterials were the most studied drug class, representing 44 % of the studies. The two types of infections affecting neonates are maternal–fetal infections or post-natal infections such as nosocomial infections: in both cases, treatment is usually a bi- or tri-therapy consisting of an aminoglycoside, β-lactam and penicillin. Indeed, the potential severity of the infection requires a quick, effective initiation of antibacterial treatment. The initial choice of antibacterials depends on several criteria including pharmacokinetic characteristics. The two most studied antibacterials were vancomycin and gentamicin, representing more than 50 % of the published studies. It can be noted that only two studies were conducted on amoxicillin while no study was performed on cefotaxime. This is surprising since these two drugs are extensively used in paediatric units and are part of the WHO Model List of Essential Medicines for Children [110]. Thus, a priority for future pharmacokinetic studies on antibacterials should be the study of cefotaxime and/or amoxicillin.
The second most studied therapeutic class, i.e. 10 % of realized studies, was anaesthetics. Again, anaesthetics are commonly used in intensive care units, especially for their analgesic and sedative properties. Appropriate sedation reduces stress and avoids complications during surgical interventions such as mechanical ventilation. Midazolam is a widely used benzodiazepine in intensive care units that represents more than 50 % of listed studies. Other molecules such as fentanyl and sufentanil are frequently used in intensive care units. Despite important haemodynamic (hypotension) and breathing (apnoea) risks, no population pharmacokinetic study has been realized for these two drugs [111]. Clinicians should adapt treatments for neonates by referring to the available studies conducted in adults, despite the well-known differences between these two populations.
Anticonvulsant drugs are also commonly used in paediatric units but only three studies have been conducted. The main indication for use of these drugs is the treatment of neonatal seizures but they can also be used in premature newborns to prevent intraventricular haemorrhage [112]. Indeed, the risk of seizures is highest in the neonatal period. In this population, a broad range of systemic and CNS disorders can increase the risk of seizures. Most neonatal seizures can lead to long-term neurological consequences. The main difficulty of this therapeutic class is due to their pharmacokinetic properties: as an example, phenobarbital is metabolized by CYP, which is not maturated in newborns; phenytoin pharmacokinetics are known to be non-linear with a narrow therapeutic index [113, 114]. This clearly indicates that future investigations in neonates should particularly target these two drugs.
6 Conclusion
The present review clearly demonstrates that 30 years after their introduction, population pharmacokinetic approaches have become a reference method for drug evaluation in neonatology. The applications of paediatric pharmacokinetic population modelling have greatly expanded in the past decade.
Population pharmacokinetic modelling offers many advantages for neonates. Indeed, one study can be achieved with different doses, times of sampling, numbers of samples and occasions. Moreover, these studies are conducted under ‘daily life’ conditions. Also, such methods allow for exploration of the available co-factors (physiological or pathological) in order to explain the inter-individual variabilities. Such studies can be performed with a reduced number of samples, limiting the invasiveness of the study, which is a major benefit to this population. The only disadvantage of this approach is the need for a significant number of patients, a disadvantage that can be easily countered with the development of biological collections or with the realization of multicentre studies. Neonates have benefitted and will continue to benefit from this approach.
References
Steinbrook R. Testing medications in children. N Engl J Med. 2002;347:1462–70.
Rosato J. The ethics of clinical trials: a child’s view. J Law Med Ethics. 2000;28:362–78.
Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ. 2000;320:79–82.
Treluyer JM, Berger JF, Leclerc F, et al. Use of off-label and unlicensed drugs in neonatal and paediatric intensive care in France [abstract no. 46A]. Pediatric Academic Societies Annual Meeting; 1–4 May 1999; San Francisco.
Chalumeau M, Treluyer JM, Salenave B, et al. Off label and unlicensed drug use among office-based paediatricians. Arch Dis Child. 2000;82:502–5.
Anderson GD, Lynn AM. Optimizing pediatric dosing: a developmental pharmacologic approach. Pharmacotherapy. 2009;29(6):680–90.
Anderson BJ. My child is unique; the pharmacokinetics are universal. Paediatr Anaesth. 2012;22(6):530–8.
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, et al. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007;46(3):221–34.
Tod M, Jullien V, Pons G. Facilitation of drug evaluation in children by population methods and modelling. Clin Pharmacokinet. 2008;47(4):231–43.
Grasela TH Jr, Donn SM. Neonatal population pharmacokinetics of phenobarbital derived from routine clinical data. Dev Pharmacol Ther. 1985;8(6):374–83.
Schaible DH, Rocci ML Jr, Alpert GA, Campos JM, Paul MH, Polin RA, et al. Vancomycin pharmacokinetics in infants: relationships to indices of maturation. Pediatr Infect Dis. 1986;5(3):304–8.
Thomson AH, Way S, Bryson SM, McGovern EM, Kelman AW, Whiting B. Population pharmacokinetics of gentamicin in neonates. Dev Pharmacol Ther. 1988;11(3):173–9.
Moore ES, Faix RG, Banagale RC, Grasela TH. The population pharmacokinetics of theophylline in neonates and young infants. J Pharmacokinet Biopharm. 1989;17(1):47–66.
Wiest DB, Pinson JB, Gal PS, Brundage RC, Schall S, Ransom JL, et al. Population pharmacokinetics of intravenous indomethacin in neonates with symptomatic patent ductus arteriosus. Clin Pharmacol Ther. 1991;49(5):550–7.
Karlsson MO, Thomson AH, McGovern EM, Chow P, Evans TJ, Kelman AW. Population pharmacokinetics of rectal theophylline in neonates. Ther Drug Monit. 1991;13(3):195–200.
Fattinger K, Vozeh S, Olafsson A, Vlcek J, Wenk M, Follath F. Netilmicin in the neonate: population pharmacokinetic analysis and dosing recommendations. Clin Pharmacol Ther. 1991;50(1):55–65.
Izquierdo M, Lanao JM, Cervero L, Jimenez NV, Domínguez-Gil A. Population pharmacokinetics of gentamicin in premature infants. Ther Drug Monit. 1992;14(3):177–83.
Jensen PD, Edgren BE, Brundage RC. Population pharmacokinetics of gentamicin in neonates using a nonlinear, mixed-effects model. Pharmacotherapy. 1992;12(3):178–82.
Karna P, Lee C, Kumar A, Dyke J, Gooch WM 3rd. Population pharmacokinetics of ceftizoxime in premature newborns. Dev Pharmacol Ther. 1993;20(3–4):135–43.
Weber W, Kewitz G, Rost KL, Looby M, Nitz M, Harnisch L. Population kinetics of gentamicin in neonates. Eur J Clin Pharmacol. 1993;44(Suppl 1):S23–5.
Asbury WH, Darsey EH, Rose WB, Murphy JE, Buffington DE, Capers CC. Vancomycin pharmacokinetics in neonates and infants: a retrospective evaluation. Ann Pharmacother. 1993;27(4):490–6.
Burtin P, Jacqz-Aigrain E, Girard P, Lenclen R, Magny JF, Betremieux P, et al. Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther. 1994;56(6 Pt 1):615–25.
Seay RE, Brundage RC, Jensen PD, Schilling CG, Edgren BE. Population pharmacokinetics of vancomycin in neonates. Clin Pharmacol Ther. 1994;56(2):169–75.
Lee TC, Charles BG, Steer PA, Flenady VJ, Grant TC. Theophylline population pharmacokinetics from routine monitoring data in very premature infants with apnoea. Br J Clin Pharmacol. 1996;41(3):191–200.
Zhou XJ, Gruber W, Demmler G, Jacobs R, Reuman P, Adler S, et al. Population pharmacokinetics of ganciclovir in newborns with congenital cytomegalovirus infections: NIAID Collaborative Antiviral Study Group. Antimicrob Agents Chemother. 1996;40(9):2202–5.
Thomson AH, Kerr S, Wright S. Population pharmacokinetics of caffeine in neonates and young infants. Ther Drug Monit. 1996;18(3):245–53.
Harte GJ, Gray PH, Lee TC, Steer PA, Charles BG. Haemodynamic responses and population pharmacokinetics of midazolam following administration to ventilated, preterm neonates. J Paediatr Child Health. 1997;33(4):335–8.
Charles BG, Preechagoon Y, Lee TC, Steer PA, Flenady VJ, Debuse N. Population pharmacokinetics of intravenous amoxicillin in very low birth weight infants. J Pharm Sci. 1997;86(11):1288–92.
de Hoog M, Schoemaker RC, Mouton JW, van den Anker JN. Tobramycin population pharmacokinetics in neonates. Clin Pharmacol Ther. 1997;62(4):392–9.
Falcão AC, de Fernández Gatta MM, Delgado Iribarnegaray MF, Santos Buelga D, García MJ, Dominguez-Gil A, et al. Population pharmacokinetics of caffeine in premature neonates. Eur J Clin Pharmacol. 1997;52(3):211–7.
Lee TC, Charles B, Steer P, Flenady V, Shearman A. Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clin Pharmacol Ther. 1997;61(6):628–40.
Botha JH, du Preez MJ, Miller R, Adhikari M. Determination of population pharmacokinetic parameters for amikacin in neonates using mixed-effect models. Eur J Clin Pharmacol. 1998;53(5):337–41.
Yasuhara M, Iga T, Zenda H, Okumura K, Oguma T, Yano Y, Hori R. Population pharmacokinetics of vancomycin in Japanese pediatric patients. Ther Drug Monit. 1998;20(6):612–8.
Silva R, Reis E, Bispo MA, Almeida AM, Costa IM, Falcão F, et al. The kinetic profile of vancomycin in neonates. J Pharm Pharmacol. 1998;50(11):1255–60.
Lee TC, Charles BG, Harte GJ, Gray PH, Steer PA, Flenady VJ. Population pharmacokinetic modeling in very premature infants receiving midazolam during mechanical ventilation: midazolam neonatal pharmacokinetics. Anesthesiology. 1999;90(2):451–7.
du Preez MJ, Botha JH, McFadyen ML, Holford NH. The pharmacokinetics of theophylline in premature neonates during the first few days after birth. Ther Drug Monit. 1999;21(6):598–603.
Vervelde ML, Rademaker CM, Krediet TG, Fleer A, van Asten P, van Dijk A. Population pharmacokinetics of gentamicin in preterm neonates: evaluation of a once-daily dosage regimen. Ther Drug Monit. 1999;21(5):514–9.
Grimsley C, Thomson AH. Pharmacokinetics and dose requirements of vancomycin in neonates. Arch Dis Child Fetal Neonatal Ed. 1999;81(3):F221–7.
Preechagoon Y, Charles B, Piotrovskij V, Donovan T, Van Peer A. Population pharmacokinetics of enterally administered cisapride in young infants with gastro-oesophageal reflux disease. Br J Clin Pharmacol. 1999;48(5):688–93.
Mirochnick M, Capparelli E, Connor J. Pharmacokinetics of zidovudine in infants: a population analysis across studies. Clin Pharmacol Ther. 1999;66(1):16–24.
Wang J, Liang WQ, Wu JJ, Pan CM. Population pharmacokinetic analysis of amikacin and validation on neonates using Monte Carlo method. Acta Pharmacol Sin. 2000;21(10):954–60.
de Hoog M, Schoemaker RC, Mouton JW, van den Anker JN. Vancomycin population pharmacokinetics in neonates. Clin Pharmacol Ther. 2000;67(4):360–7.
Hansen TG, Ilett KF, Reid C, Lim SI, Hackett LP, Bergesio R. Caudal ropivacaine in infants: population pharmacokinetics and plasma concentrations. Anesthesiology. 2001;94(4):579–84.
Bleyzac N, Varnier V, Labaune JM, Corvaisier S, Maire P, Jelliffe RW, et al. Population pharmacokinetics of amikacin at birth and interindividual variability in renal maturation. Eur J Clin Pharmacol. 2001;57(6–7):499–504.
Kimura T, Kokubun H, Nowatari M, Matsuura N, Sunakawa K, Kubo H. Population pharmacokinetics of panipenem in neonates and retrospective evaluation of dosage. J Antimicrob Chemother. 2001;47(1):51–9.
Falcão AC, Buelga DS, Méndez ME, García MJ, Pardo M. Population kinetics of tobramycin in neonates. Ther Drug Monit. 2001;23(3):202–8.
Capparelli EV, Lane JR, Romanowski GL, McFeely EJ, Murray W, Sousa P, et al. The influences of renal function and maturation on vancomycin elimination in newborns and infants. J Clin Pharmacol. 2001;41(9):927–34.
Tod M, Lokiec F, Bidault R, De Bony F, Petitjean O, Aujard Y. Pharmacokinetics of oral acyclovir in neonates and in infants: a population analysis. Antimicrob Agents Chemother. 2001;45(1):150–7.
Suematsu F, Yukawa E, Yukawa M, Minemoto M, Ohdo S, Higuchi S, et al. Population-based investigation of relative clearance of digoxin in Japanese neonates and infants by multiple-trough screen analysis. Eur J Clin Pharmacol. 2001;57(1):19–24.
Stolk LM, Degraeuwe PL, Nieman FH, de Wolf MC, de Boer A. Population pharmacokinetics and relationship between demographic and clinical variables and pharmacokinetics of gentamicin in neonates. Ther Drug Monit. 2002;24(4):527–31.
de Hoog M, Schoemaker RC, van den Anker JN, Vinks AA. NONMEM and NPEM2 population modeling: a comparison using tobramycin data in neonates. Ther Drug Monit. 2002;24(3):359–65.
Odoul F, Le Guellec C, Henrot A, Saliba E, Levron JC, Saux MC, et al. Population pharmacokinetics of cisapride in neonates. Eur J Clin Pharmacol. 2002;58(8):507–13.
Mulla H, McCormack P, Lawson G, Firmin RK, Upton DR. Pharmacokinetics of midazolam in neonates undergoing extracorporeal membrane oxygenation. Anesthesiology. 2003;99(2):275–82.
Botha JH, du Preez MJ, Adhikari M. Population pharmacokinetics of gentamicin in South African newborns. Eur J Clin Pharmacol. 2003;59(10):755–9.
DiCenzo R, Forrest A, Slish JC, Cole C, Guillet R. A gentamicin pharmacokinetic population model and once-daily dosing algorithm for neonates. Pharmacotherapy. 2003;23(5):585–91.
Gregoire N, Gualano V, Geneteau A, Millerioux L, Brault M, Mignot A, et al. Population pharmacokinetics of ibuprofen enantiomers in very premature neonates. J Clin Pharmacol. 2004;44(10):1114–24.
Smyth JM, Collier PS, Darwish M, Millership JS, Halliday HL, Petersen S, et al. Intravenous indometacin in preterm infants with symptomatic patent ductus arteriosus: a population pharmacokinetic study. Br J Clin Pharmacol. 2004;58(3):249–58.
Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92(2):208–17.
Rapp HJ, Molnár V, Austin S, Krohn S, Gädeke V, Motsch J, et al. Ropivacaine in neonates and infants: a population pharmacokinetic evaluation following single caudal block. Paediatr Anaesth. 2004;14(9):724–32.
Allegaert K, Anderson BJ, Naulaers G, de Hoon J, Verbesselt R, Debeer A, et al. Intravenous paracetamol (propacetamol) pharmacokinetics in term and preterm neonates. Eur J Clin Pharmacol. 2004;60(3):191–7.
Kimura T, Sunakawa K, Matsuura N, Kubo H, Shimada S, Yago K. Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob Agents Chemother. 2004;48(4):1159–67.
Lanao JM, Calvo MV, Mesa JA, Martín-Suárez A, Carbajosa MT, Miguelez F, et al. Pharmacokinetic basis for the use of extended interval dosage regimens of gentamicin in neonates. J Antimicrob Chemother. 2004;54(1):193–8.
Bailey JM, Hoffman TM, Wessel DL, Nelson DP, Atz AM, Chang AC, et al. A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. J Pharmacokinet Pharmacodyn. 2004;31(1):43–59.
Chalkiadis GA, Anderson BJ, Tay M, Bjorksten A, Kelly JJ. Pharmacokinetics of levobupivacaine after caudal epidural administration in infants less than 3 months of age. Br J Anaesth. 2005;95(4):524–9.
Fukuda T, Yukawa E, Kondo G, Maeda T, Shin-o T, Kondo Y, et al. Population pharmacokinetics of theophylline in very premature Japanese infants with apnoea. J Clin Pharm Ther. 2005;30(6):591–6.
Capparelli E, Hochwald C, Rasmussen M, Parham A, Bradley J, Moya F. Population pharmacokinetics of cefepime in the neonate. Antimicrob Agents Chemother. 2005;49(7):2760–6.
Yukawa E, Suematsu F, Yukawa M, Minemoto M. Population pharmacokinetic investigation of phenobarbital by mixed effect modelling using routine clinical pharmacokinetic data in Japanese neonates and infants. J Clin Pharm Ther. 2005;30(2):159–63.
Würthwein G, Groll AH, Hempel G, Adler-Shohet FC, Lieberman JM, Walsh TJ. Population pharmacokinetics of amphotericin B lipid complex in neonates. Antimicrob Agents Chemother. 2005;49(12):5092–8.
Pullen J, Stolk LM, Nieman FH, Degraeuwe PL, van Tiel FH, Zimmermann LJ. Population pharmacokinetics and dosing of amoxicillin in (pre)term neonates. Ther Drug Monit. 2006;28(2):226–31.
Pullen J, de Rozario L, Stolk LM, Degraeuwe PL, van Tiel FH, Zimmermann LJ. Population pharmacokinetics and dosing of flucloxacillin in preterm and term neonates. Ther Drug Monit. 2006;28(3):351–8.
García B, Barcia E, Pérez F, Molina IT. Population pharmacokinetics of gentamicin in premature newborns. J Antimicrob Chemother. 2006;58(2):372–9.
Al Z’aabi M, Lanner A, Xiaonian X, Donovan T, Charles B. Application of routine monitoring data for determination of the population pharmacokinetics and enteral bioavailability of phenytoin in neonates and infants with seizures. Ther Drug Monit. 2006;28(6):793–9.
Zuppa AF, Nicolson SC, Adamson PC, Wernovsky G, Mondick JT, Burnham N, et al. Population pharmacokinetics of milrinone in neonates with hypoplastic left heart syndrome undergoing stage I reconstruction. Anesth Analg. 2006;102(4):1062–9.
van Kesteren C, Benders MJ, Groenendaal F, van Bel F, Ververs FF, Rademaker CM. Population pharmacokinetics of allopurinol in full-term neonates with perinatal asphyxia. Ther Drug Monit. 2006;28(3):339–44.
Al Za’abi M, Donovan T, Tudehope D, Woodgate P, Collie LA, Charles B. Orogastric and intravenous indomethacin administration to very premature neonates with patent ductus arteriosus: population pharmacokinetics, absolute bioavailability, and treatment outcome. Ther Drug Monit. 2007;29(6):807–14.
Allegaert K, Peeters MY, Verbesselt R, Tibboel D, Naulaers G, de Hoon JN, et al. Inter-individual variability in propofol pharmacokinetics in preterm and term neonates. Br J Anaesth. 2007;99(6):864–70.
Muller AE, DeJongh J, Bult Y, Goessens WH, Mouton JW, Danhof M, et al. Pharmacokinetics of penicillin G in infants with a gestational age of less than 32 weeks. Antimicrob Agents Chemother. 2007;51(10):3720–5.
Anderson BJ, Allegaert K, Van den Anker JN, Cossey V, Holford NH. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol. 2007;63(1):75–84.
Acosta EP, Brundage RC, King JR, Sánchez PJ, Sood S, Agrawal V, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group, et al. Ganciclovir population pharmacokinetics in neonates following intravenous administration of ganciclovir and oral administration of a liquid valganciclovir formulation. Clin Pharmacol Ther. 2007;81(6):867–72.
Yukawa E, Akiyama K, Suematsu F, Yukawa M, Minemoto M. Population pharmacokinetic investigation of digoxin in Japanese neonates. J Clin Pharm Ther. 2007;32(4):381–6.
Paradisis M, Jiang X, McLachlan AJ, Evans N, Kluckow M, Osborn D. Population pharmacokinetics and dosing regimen design of milrinone in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F204–9.
Hirt D, Van Overmeire B, Treluyer JM, Langhendries JP, Marguglio A, Eisinger MJ, et al. An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study. Br J Clin Pharmacol. 2008;65(5):629–36.
Gregoire N, Desfrere L, Roze JC, Kibleur Y, Koehne P. Population pharmacokinetic analysis of Ibuprofen enantiomers in preterm newborn infants. J Clin Pharmacol. 2008;48(12):1460–8.
Palmer GM, Atkins M, Anderson BJ, Smith KR, Culnane TJ, McNally CM, et al. I.V. acetaminophen pharmacokinetics in neonates after multiple doses. Br J Anaesth. 2008;101(4):523–30.
Lima-Rogel V, Medina-Rojas EL, Del Carmen Milán-Segovia R, Noyola DE, Nieto-Aguirre K, López-Delarosa A, et al. Population pharmacokinetics of cefepime in neonates with severe nosocomial infections. J Clin Pharm Ther. 2008;33(3):295–306.
Bradley JS, Sauberan JB, Ambrose PG, Bhavnani SM, Rasmussen MR, Capparelli EV. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J. 2008;27(9):794–9.
Wade KC, Wu D, Kaufman DA, Ward RM, Benjamin DK Jr. Sullivan JE, et al. National Institute of Child Health and Development Pediatric Pharmacology Research Unit Network. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother. 2008;52(11):4043–9.
Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A. Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Ther Drug Monit. 2008;30(6):709–16.
Dailly E, Drouineau MH, Gournay V, Rozé JC, Jolliet P. Population pharmacokinetics of domperidone in preterm neonates. Eur J Clin Pharmacol. 2008;64(12):1197–200.
Zuppa AF, Mondick JT, Davis L, Cohen D. Population pharmacokinetics of ketorolac in neonates and young infants. Am J Ther. 2009;16(2):143–6.
Sherwin CM, Svahn S, Van der Linden A, Broadbent RS, Medlicott NJ, Reith DM. Individualised dosing of amikacin in neonates: a pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol. 2009;65(7):705–13.
Nielsen EI, Sandström M, Honoré PH, Ewald U, Friberg LE. Developmental pharmacokinetics of gentamicin in preterm and term neonates: population modelling of a prospective study. Clin Pharmacokinet. 2009;48(4):253–63.
van den Anker JN, Pokorna P, Kinzig-Schippers M, Martinkova J, de Groot R, Drusano GL, et al. Meropenem pharmacokinetics in the newborn. Antimicrob Agents Chemother. 2009;53(9):3871–9.
Mukherjee A, Dombi T, Wittke B, Lalonde R. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther. 2009;85(1):56–63.
Hirt D, Urien S, Rey E, Arrivé E, Ekouévi DK, Coffié P, et al. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2009;53(3):1067–73.
Hirt D, Urien S, Ekouévi DK, Rey E, Arrivé E, Blanche S, et al. ANRS 12109. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109). Clin Pharmacol Ther. 2009;85(2):182–9.
Ahsman MJ, Hanekamp M, Wildschut ED, Tibboel D, Mathot RA. Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2010;49(6):407–19.
Marqués-Miñana MR, Saadeddin A, Peris JE. Population pharmacokinetic analysis of vancomycin in neonates: a new proposal of initial dosage guideline. Br J Clin Pharmacol. 2010;70(5):713–20.
Lo YL, van Hasselt JG, Heng SC, Lim CT, Lee TC, Charles BG. Population pharmacokinetics of vancomycin in premature Malaysian neonates: identification of predictors for dosing determination. Antimicrob Agents Chemother. 2010;54(6):2626–32.
Hope WW, Smith PB, Arrieta A, Buell DN, Roy M, Kaibara A, et al. Population pharmacokinetics of micafungin in neonates and young infants. Antimicrob Agents Chemother. 2010;54(6):2633–7.
Blumer J, Rodriguez A, Sánchez PJ, Sallas W, Kaiser G, Hamed K. Single-dose pharmacokinetics of famciclovir in infants and population pharmacokinetic analysis in infants and children. Antimicrob Agents Chemother. 2010;54(5):2032–41.
Xie HG, Cao YJ, Gauda EB, Agthe AG, Hendrix CW, Lee H. Clonidine clearance matures rapidly during the early postnatal period: a population pharmacokinetic analysis in newborns with neonatal abstinence syndrome. J Clin Pharmacol. 2011;51(4):502–11.
Ward RM, Tammara B, Sullivan SE, Stewart DL, Rath N, Meng X, et al. Single-dose, multiple-dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD). Eur J Clin Pharmacol. 2010;66(6):555–61.
Benaboud S, Ekouévi DK, Urien S, Rey E, Arrivé E, Blanche S, TEmAA ANRS 12109 Study Group, et al. Population pharmacokinetics of nevirapine in HIV-1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2011;55(1):331–7.
Kearns GL, Reed MD. Clinical pharmacokinetics in infants and children: a reappraisal. Clin Pharmacokinet. 1989;17:29–67.
Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006;165:819–29.
West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284(5420):1677–9.
Anderson BJ, Holford NH. Mechanistic basic of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36.
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Cole M, Chatelut E, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76.
World Health Organization. WHO model lists of essential medicines. http://whqlibdoc.who.int/hq/2011/a95964_fre.pdf. Accessed 1 Oct 2010.
Jacqz-Aigrain E, Burtin P. Clinical pharmacokinetics of sedatives in neonates. Clin Pharmacokinet. 1996;31(6):423–43.
Rennie JM, Boylan GB. Neonatal seizures and their treatment. Curr Opin Neurol. 2003;6(2):177–81.
Painter MJ, Pippenger C, Wasterlain C, Barmada M, Pitlick W, Carter G, Abern S. Phenobarbital and phenytoin in neonatal seizures: metabolism and tissue distribution. Neurology. 1981;31(9):1107–12.
Tokola RA, Neuvonen PJ. Pharmacokinetics of antiepileptic drugs. Acta Neurol Scand Suppl. 1983;97:17–27.
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marsot, A., Boulamery, A., Bruguerolle, B. et al. Population Pharmacokinetic Analysis during the First 2 Years of Life. Clin Pharmacokinet 51, 787–798 (2012). https://doi.org/10.1007/s40262-012-0015-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-012-0015-8