Abstract
Introduction
Population modelling using mixed effects models provides a means to study variability in paediatric drug responses among individuals representative of those in whom the drug will be used clinically.
Discussions
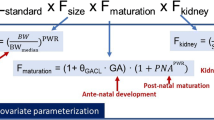
Explanatory covariates explain the predictable part of the between-individual variability. Growth and development are two major aspects of children not seen in adults. These aspects can be investigated by using size and age as covariates. Problems attributable to co-linearity can be approached by using size as the first covariate. Size standardisation is achieved using allometric scaling, a mechanistic approach that has a strong theoretical and empirical basis. Age is used to describe the maturation of clearance. The quantitative models (linear, exponential, first-order, variable slope sigmoidal) used to describe this maturation process vary depending on the span of the ages under investigation. Measures of response are not always straightforward and can be more difficult to quantify in children.
Conclusion
Covariate investigation in children is improving the understanding of developmental aspects of drug disposition and effects in the paediatric population, ultimately leading to more effective use of medications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth and development are two major aspects of children not seen in adults. These aspects can be investigated by using size and age as covariates. One advantage of population modelling programs such as NONMEM [12, 13] is the ability to investigate covariates. Problems exist because covariates can exhibit co-linearity. Clearance, for example, may increase with weight, height, age, body surface area and creatinine clearance. All of these covariates may show a high degree of correlation and they are not mutually exclusive [15]. Any one covariate may or may not predict another. This current paper explores the use of covariates and priors in paediatric pharmacokinetic modelling and introduces some examples of the sigmoidal Emax model used to describe pharmacodynamic effects in children.
Covariate pharmacokinetic considerations in paediatrics
Size adjustment
The range of body weights in children is far greater than that seen in adults and can vary 200-fold (e.g. 0.5–100 kg). Size may be closely linked to age and both covariates may have an impact on the prediction of clearance (Fig. 1a,b). Size, however, is the primary covariate used in the investigation of co-linearity. Debate exists concerning the methodology used to adjust pharmacokinetic (PK) parameters to body size. Empirical approaches that focus on body weight (W) or body surface area (BSA) have traditionally been used. However, the linear per kilogram and surface area models are inappropriate for scaling small children to adults [32]. The linear per kilogram model under-predicts clearance, while the surface area model over-predicts clearance in children. These errors increase with decreasing weight [32, 44]. Linear size models for young children have led to the idea that there is an enhanced capacity of children to metabolise drugs due to proportionally larger livers and kidneys than their adult counterparts [58]. This idea arises because clearance, expressed per kg of body weight, is larger in children than in adults.
Data from studies investigating paracetamol clearance. a Clearance increases with weight. Individual predicted paracetamol clearances (CL), from the NONMEM post hoc step, are plotted against weight. b Clearance also increases with age. Individual predicted paracetamol clearances (CL), from the NONMEM post hoc step, are plotted against postnatal age
It now widely recognised that there is a nonlinear relationship between weight and drug elimination capacity [25]. Body surface area (BSA) was proposed in 1950 to be a more satisfactory index of drug requirements than body weight or age, particularly during infancy and childhood [24]. The surface area is calculated from height and weight [26], but it can also be estimated from weight using a power exponent of 0.67 [18, 32]. However, infants are not morphologically similar to adults—infants have short legs, relatively big heads and large body trunks. The surface area formula is inaccurate in children with a predicted surface area of less than 1.3 m2 (an average 12-year-old) by direct photometric measurement [44]. The mass of empirical evidence suggests that the appropriate scaling factor is significantly different from 0.67 and is compatible with the theoretically expected value of 0.75 [37, 52, 65, 66].
It is possible to show that, in almost all species, including humans, the log of the basal metabolic rate (BMR) plotted against the log of body weight produces a straight line with a slope of 0.75 (Fig. 2). West et al. [65, 66] have used fractal geometry to mathematically explain this phenomenon. The 3/4 power law for metabolic rates was derived from a general model that describes how essential materials are transported through space-filled fractal networks of branching tubes. A great many physiological-, structural- and time-related variables scale predictably within and between species with weight exponents (PWR) of 0.75, 1 and 0.25, respectively. These allometric “1/4 power” models can be applied to pharmacokinetic parameter estimates in children, e.g. clearance (0.75), half-life (0.25), volume of distribution (1):
where Fsize is the factor for size, W is the weight of an individual and W std is the weight standard (e.g. a 70-kg person).
A comparison of the temperature-standardised relation for whole-organism metabolic rate as a function of body mass. The “allometric 3/4 power model” fits for unicells, poikilotherms and homeotherms, uncorrected for temperature, are also shown. From Gillooly et al. [28], with permission
Allometric size modelling is used with increasing frequency in paediatric PK population analyses. The model building procedure can be accomplished by either fixing the allometric exponent (PWR) [9, 20, 50] or by estimating it as a parameter of a nested model [53, 67]. Estimation of an allometric exponent from typical human data is fraught with problems because of the relatively narrow weight ranges and the estimation error involved in such a nonlinear parameter. It is safer to rely on theory and observation from much broader data and use the “1/4 power” allometric models. Allometric scaling is a mechanistic approach that has a strong theoretical and empirical basis [5, 49, 50, 65, 66]. Fixing the power exponent allows investigators to delineate secondary covariate effects from the effect of size. Allometric scaling also allows the direct comparison of paediatric estimates with adults when a weight standard of 70 kg is used. For example, a clearance of 0.2 L/h/kg, when standardised to a 70-kg person (14 L/h/70 kg), is reduced in a 1.5-kg intrauterine growth retarded term neonate (5.4 L/h/70 kg) when compared to a 4-kg term neonate (6.9 L/h/70 kg). Clearance is not the same in the three individuals, although the linear per kilogram model would suggest that this is so. The emergence of a relationship between clearance and age can be seen in Fig. 3a, where clearance is scaled allometrically, compared to Fig. 3b, where the linear per kilogram model was used.
Data from a study investigating paracetamol clearance. a Individual predicted paracetamol clearances (CL) from the NONMEM post hoc step, expressed using the allometric 3/4 power model, are plotted against postnatal age. The trend line shows an increase in clearance with age. b Individual predicted paracetamol clearances (CL) from the NONMEM post hoc step, expressed using the linear per kilogram model, are plotted against postnatal age. There is no obvious relationship between age and clearance
Allometric scaling is commonly used to predict paediatric dosing based on adult dose (Table 1). Estimates are commonly taken from the surface area model but fail in infancy because age, which is commonly used to describe clearance maturation, is unaccounted for.
Effects of age
Clearance
Once size is standardised, the effects of other covariates, such as age [9], temperature [3] and renal function [17], can be investigated. Age is used to describe the maturation of clearance. The quantitative models used to describe this maturation process vary depending on the span of the ages under investigation. A linear model is commonly used for a population sample limited to a small defined age band. An exponential model may describe the gradual increase of clearance in premature neonates [1], while a first-order process has been used to describe clearance maturation from birth to adolescence [9].
The maturation of clearance begins before birth, suggesting that postmenstrual age (PMA) would be a better predictor of drug elimination than postnatal age (PNA). The foetus is capable of metabolising morphine (hepatic enzyme uridine 5′-diphosphate glucuronosyl transferase-2B7, UGT2B7) from 15 weeks gestation [46, 47]. The neonate can use sulphate conjugation as an alternative route for substrates, such as morphine or acetaminophen, before glucuronidation matures. There are distinct patterns associated with isoform-specific developmental expression of the cytochrome P450 (CYP) enzymes. CYP2D6 has been detected in premature neonates as young as 25 weeks PMA [2]. Although some CYPs appear to be switched on by birth, while in others birth is necessary but not sufficient for the onset of expression [31, 35, 39], there are no direct demonstrations that clearance changes as a consequence of being born. Maturation of clearance in neonates may be described by both PMA and PNA, but PMA is a more physiologically appropriate covariate to explain the time course of changes in clearance.
An example of a linear model investigating age-related changes for clearance is:
where F PMA is the factor for PMA and is centred on 40 weeks PMA (full term gestation), PMA is the postmenstrual age in weeks and SLPCL is a slope parameter describing changes of clearance with PMA.
Alternative models (exponential, first-order, variable slope sigmoidal) can approximate the linear model over a narrow age range (e.g. premature neonates) (Fig. 4a). The maturation of clearance must develop before birth and extrapolation of a linear function does not allow this. An exponential function allows for a gradual increase in clearance at earlier postmenstrual ages (Fig. 4b). This exponential function was used by Kimura et al. [36] in their study of vancomycin pharmacokinetics in neonates:
a The four models used to investigate the relationship between age and clearance maturation are similar in the age range of the population studied. The boxed area is shown in more detail in Fig. 5b. b The first-order and linear models intercept the x axis at fixed times during the second trimester. This seems unlikely, as organogenesis is occurring during the second trimester of pregnancy. The exponential and variable slope sigmoidal model (Hill equation) allow for gradual clearance maturation that starts early in foetal life. c, d Individual predicted paracetamol clearances (CL) from the NONMEM post hoc step, expressed using the allometric 34 power model, are plotted against postmenstrual age: (c) a first-order model is used to describe CL maturation with age; (d) a variable slope sigmoid model is used to describe CL maturation with age
An exponential model may be a more robust empirical model than the linear model because the prediction is always positive. This exponential function, in common with the linear model, predicts increasing clearance with age. This model is empirical and does not extrapolate to adult values, although, physiologically, clearance must rise and plateau at some age. A first-order model, common in biological systems, has been previously used to investigate the clearance maturation of other drugs during infancy [9, 17]. A “mature” clearance can be estimated (Fig. 4c) at a plateau by:
where βcl is a parameter estimating the fractional CLstd at 40 weeks PMA and Tcl describes the maturation half-life of the age-related changes of CL. This model has been used to investigate paracetamol age-related clearance and consequent dosing predictions [8]. Clearance was low (0.74 L/h/70 kg) at 28 weeks PMA, but reached 86% of adult values (i.e. plateau 12.5 L/h/70 kg) by 60 weeks. A mean steady state target concentration above 10 mg/L at trough can be achieved by a maintenance oral dose of 25 mg/kg/day in 30-week-gestation premature neonates, rising to 90 mg/kg/day at 6 months. The dose required in infancy to achieve a set target concentration is greater than in older children because clearance is nonlinearly related to weight [8].
The first-order model has been successfully used to compare clearance maturation rates for different clearance enzyme processes. The maturation half-time for morphine (uridine 5′-diphosphate UGT2B7) of 12 weeks [17] is quite different from the rapid clearance maturation of 5 days described for the opioid, alfentanil (CYP3A4) [5]. This model has also been used to investigate the pharmacogenetics of tramadol in premature neonates [2]. Maturational clearance of tramadol was almost completed by 44 weeks PMA. CYP2D6 activity, measured by metabolite formation, was observed as early as 25 weeks PMA, but the impact of CYP2D6 polymorphism on the variability in the pharmacokinetics, metabolism and pharmacodynamics of tramadol could not be established.
This first-order model suffers because it assumes zero clearance at the x intercept (Fig. 4b,c). A variable slope sigmoidal model (Hill equation) allows the gradual maturation of clearance in early life and a “mature” clearance to be achieved at a later age (Fig. 4b,d):
where EMATCL 50 is the PMA at which clearance is 50% that of the mature value and HillCL is the Hill coefficient for clearance. A variable slope model has been used to investigate vancomycin clearance and covariate effects in premature neonates [4]. Size explained 49%, postmenstrual age 18% and renal function 14% of the clearance variability. The use of a variable slope sigmoidal model to describe the relationship between clearance and postmenstrual age predicted a reasonable adult clearance of 3.79 (95% CI: 2.76-4.98) L/h/70 kg from premature neonatal data [4]. Scaling adult data to predict infant clearance is unreliable in infancy because maturation is unaccountable; scaling neonatal data may predict adult clearance when size and age are included as covariates.
Plasma esterases are responsible for the hydrolysis of propacetamol to paracetamol. This rate of hydrolysis has been investigated [6]. The rate was the same in all age groups when standardised for size (physiological time) [5]. The rate in real time (chronological time) is fastest in the youngest patients. Remifentanil is also cleared by plasma esterases and its clearance is similarly fastest in the newborn period when expressed as per kilogram [34, 51].
These models for the effect of age on clearance may prove useful in teasing out information concerning possible temporal switches that may “turn up” clearance at birth. It is postulated that birth itself may have an effect on the ontogeny of clearance. The disappearance of the placenta as “external” clearance may also play a role. The placenta has enzyme capacity to clear drugs (e.g. cytochrome P450 and conjugation) and the interpretation of hepatic maturation remains unravelled in the foetus.
Renal function
The clearance maturation of drugs almost exclusively cleared by renal elimination using PMA as a predictor predicts the time course of development of the glomerular filtration rate (GFR). Estimates for amikacin clearance [1] mirror GFR estimates in premature neonates [57, 59]. GFR matures during infancy and approaches an adult rate (6 L/h/70 kg) by 6 months postnatal age [11, 14]. Difficulties arise in determining the renal function in children, although a number of formulae have been published that allow the estimation of GFR from clinical characteristics [45] (e.g. Schwartz et al. [54, 55]).
Creatinine concentration decreases with age in the newborn. Consequently, vancomycin clearance estimates have been made using simply an inverse relationship to creatinine concentration in premature neonates [29]. Creatinine concentration in the first few days of life reflects maternal concentrations more than neonatal renal function and subsequent concentrations are influenced by tubular reabsorption [30]. Attempts to use the Cockcroft and Gault models [22] to predict the creatinine production rate (CPR) fail. Creatinine clearance (CLcr) can be predicted using postmenstrual age (PMA) as a covariate to predict the creatinine production rate (CPR) with a scaling constant (Kage) for age. This is based on assuming a CPR of 516 μmol/h in a 70-kg, 40-year-old male [22]:
The renal function (RF) can then be standardised to an adult creatinine clearance of 6 L/h/70 kg to enable the comparison of neonatal with adult data [17, 43]. The increase of CPR with age in neonates is assumed to be a consequence of increasing muscle bulk with age, as opposed to the decrease in muscle bulk that occurs with age in adults.
The population approach has been used to directly estimate GFR in patients with renal disease in children aged 0.8–18 years. The use of the squared length compensates for the nonlinear relationship between muscle mass and age-related changes of serum creatinine [40]:
Cole et al. [23] used a population approach to investigate CLcr in 50 paediatric cancer patients of similar age range (0.8–19.8 years) and reported a linear model with good predictive properties:
Volume of distribution
The total body water content constitutes 85% of the body weight in the preterm neonate and 75% in term neonates. This decreases to approximately 60% at 5 months and remains relatively constant from this age onwards [27]. The major component contributing to this reduction in body water is the decrease in extracellular fluid (ECF). ECF constitutes 45% of the body weight at birth and 26% at 1 year. There is a further ECF reduction during childhood until adulthood, where it contributes 18%. The percentage of body weight contributed by fat is 3% in a 1.5-kg premature neonate and 12% in a term neonate; this proportion doubles by 4–5 months of age. “Baby fat” is lost when the infant starts walking and protein mass increases (20% term neonate, 50% adult). Albumin, globulin, lipoprotein and glycoprotein concentrations change over the first year, affecting drug binding [16]. Relative body proportions change dramatically over the first few years of life and may affect the volumes of the distribution of drugs.
Volume of distribution (V) changes and their relation to age that occur as a consequence of body composition changes can be described using quantitative functions similar to those used for clearance maturation. Clarification of these changes has debunked myths such as respiratory drive sensitivity in neonates given morphine that is attributable to the leaky blood–brain barrier [64]. Morphine volume of distribution is reduced in neonates and increases exponentially with a maturation half-life of 26 days from 83 l per 70 kg at birth [17]. Neonates given a similar dose as adults (mg/kg) achieve higher serum concentrations because of the reduced V. Respiratory depression is the same in children from 2 to 570 days of age at the same morphine concentration [41]. Pethidine causes less respiratory depression in neonates and it was thought that pethidine, unlike morphine, is lipid soluble and, therefore, crossed the immature or mature BBB equally. However the lesser respiratory depression in neonates given an equivalent mg/kg adult dose is probably consequent upon an increased pethidine V and reduced serum concentration.
Integrating priors
Estimates from prior analyses of data (e.g. adult, paediatric or animal work) can be incorporated into paediatric studies when these values cannot be estimated from the available current data. The use of 0.75 as the power parameter to scale clearance is based on prior knowledge from animal [38, 49] and fractal geometry [65, 66]. Paediatric studies investigating metabolite data can determine inter-compartment rate constants, but knowledge of inter-compartment CL estimates may be of greater practical use. The amount of drug that is metabolised down different clearance pathways is unknown. This may be calculated from a complete urine collection, but observed data such as parent and metabolite concentrations only do not allow the estimation of the metabolite volume of distribution. Adult priors for metabolite V have been used in paediatric studies [17, 60].
Pharmacodynamics
Pharmacokinetics (PK) is what the body does to the drug, while pharmacodynamics (PD) is what the drug does to the body. Variability in PD response may be even greater than that in PK. The precise boundary between PK and PD is ill defined and often requires a link describing the movement of drug from the plasma to the biophase and its target. It is postulated that PD factors such as the number, affinity and type of receptors or the availability of natural ligands alter the drug–receptor interaction and are altered in childhood, but there are few data concerning PD differences between adults and children. Bronchodilators are less effective in infants because of the immaturity of bronchial smooth muscle fibres. Cyclosporin [42], warfarin [35] and benzodiazepine [21] are all thought to have altered PD in children, but further examples are rare. Pharmacodynamics may be further complicated by the development of functional tolerance and the additive, synergistic or antagonistic effect of drug metabolites or isomers.
Observed effects may not be directly related to serum concentration. There may be a delay due to the transfer of the drug to the effect site (tubocurarine), a lag time (frusemide), physiological response (antipyresis), active metabolite (propacetamol) or elimination of physiological substances (warfarin). A plasma concentration effect plot can form a hysteresis loop because of this delay in effect.
Hull et al. [33] and Sheiner et al. [56] introduced the effect compartment concept for neuromuscular blocking drugs. The effect compartment concentration is not the same as the blood or serum concentration and is not a real measurable concentration. A single first-order parameter (Teq, T1/2ke0) describes the equilibration half-time. It is assumed that the concentration in the central compartment is the same as that in the effect compartment at equilibration, but that a time delay exists before the drug reaches the effect compartment. The concentration in the effect compartment is used to describe the concentration–effect relationship.
Measures of response are not always as straightforward as neuromuscular blockade monitoring in adults and can be more difficult to quantify in children. Analgesic effect measures, for example, vary between ages and even within one age [61–63]. Covariate effects may be subtle and unaccounted for in many studies. The placebo effect contributed 50% of analgesia attributed to paracetamol a few hours after tonsillectomy [10]. Similarly, Brown et al. [19], in a study of paracetamol antipyresis, noted the confounding effect of the initial temperature and normal temperature fluctuations on antipyretic efficacy assessment. A circadian night rhythm effect was noted in an investigation of propofol sedation after major craniofacial surgery [48].
The relation between drug concentration and the magnitude of effect may be described by a variable slope sigmoid curve (Fig. 5) according to the following equation:
where Emax is the maximum effect change, Ce is the concentration in the effect compartment, EC 50 is the concentration producing 50% effect and N is the Hill coefficient defining the steepness of the concentration–response curve. At low concentrations, the nonlinear relationship may approach linearity. A variable slope sigmoid model is commonly used to describe the concentration–response relationship. Ordinal data may be described using logistic regression [7].
An understanding of the paracetamol analgesic effect has come from population modelling. PD estimates for an Emax model, in which the greatest possible pain relief (VAS 0–10) equates to an Emax of 10, were Emax 5.17 (64%) and EC 50 9.98 (107%) mg/l. The equilibration half-time (Teq) of the analgesic effect compartment was 53 (217%) min [10]. These estimates confirm paracetamol as a mild analgesic and that there is a delay after the peak serum concentration before maximal pain relief becomes apparent. An effect site concentration of 10 mg/L resulted in a pain reduction of 2.6 pain units. An increased effect site concentration of 40 mg/L reduced pain by 4.2 mg/L. Higher concentrations are associated with a decrease in the incremental pain relief and the maximum pain reduction can only be 5.17 pain units. Increasing the dose will not maximise benefit but will increase propensity to side effects such as hepatotoxicity.
Conclusions
Growth and development are two major aspects of children not seen in adults. Problems attributable to covariate co-linearity can be approached using size as the first covariate. Size standardisation is achieved using allometric scaling, a mechanistic approach that has a strong theoretical and empirical basis. Age is used to describe the maturation of clearance. The quantitative models (linear, exponential, first-order, variable slope sigmoidal) used to describe this maturation process vary depending on the span of the ages under investigation. Postmenstrual age (PMA) is a more physiologically appropriate covariate to explain the time course of changes in clearance and studies using PMA have improved the understanding of clearance maturation. It is anticipated that this exploration of covariates will assist in improving the understanding cellular clearance pathways, the role of the placenta and differences attributable to pharmacogenetic polymorphisms. Covariate investigation in children is improving the understanding of developmental aspects of drug disposition and effects in the paediatric population, ultimately leading to more effective use of medications.
Abbreviations
- BMR:
-
Basal metabolic rate
- BSA:
-
Body surface area
- CL:
-
Clearance
- CLcr:
-
Creatinine clearance
- CPR:
-
Creatinine production rate
- GFR:
-
Glomerular filtration rate ka; absorption rate constant
- ln:
-
Natural logarithm
- NONMEM:
-
Nonlinear mixed effects model
- PD:
-
Pharmacodynamics
- PK:
-
Pharmacokinetics
- PMA:
-
Postmenstrual age
- PNA:
-
Postnatal age
- PWR:
-
Allometric power exponent
- Tabs:
-
Absorption half time
- TDM:
-
Therapeutic drug monitoring
- V:
-
Volume of distribution
References
Allegaert K, Anderson BJ, Cossey V, Holford NH (2006) Limited predictability of amikacin clearance in extreme premature neonates at birth. Br J Clin Pharmacol 61(1):39–48
Allegaert K, Anderson BJ, Verbesselt R, Debeer A, de Hoon J, Devlieger H, van den Anker JN, Tibboel D (2005) Tramadol disposition in the very young: an attempt to assess in vivo cytochrome P-450 2D6 activity. Br J Anaesth 95(2):231–239
Anderson BJ (2005) Phenytoin elimination in a child during hypothermia for traumatic brain injury. Paediat Perinat Drug Ther 6(3):133–138
Anderson BJ, Allegaert K, van den Anker JN, Cossey V, Holford NHG (2006) Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Brit J Clin Pharmacol (in press)
Anderson BJ, Meakin GH (2002) Scaling for size: some implications for paediatric anaesthesia dosing. Paediatr Anaesth 12(3):205–219
Anderson BJ, Pons G, Autret-Leca E, Allegaert K, Boccard E (2005) Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: a population analysis. Paediatr Anaesth 15(4):282–292
Anderson BJ, Ralph CJ, Stewart AW, Barber C, Holford NH (2000) The dose–effect relationship for morphine and vomiting after day-stay tonsillectomy in children. Anaesth Intensive Care 28(2):155–160
Anderson BJ, van Lingen RA, Hansen TG, Lin YC, Holford NH (2002) Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology 96(6):1336–1345
Anderson BJ, Woollard GA, Holford NH (2000) A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br J Clin Pharmacol 50(2):125–134
Anderson BJ, Woollard GA, Holford NH (2001) Acetaminophen analgesia in children: placebo effect and pain resolution after tonsillectomy. Eur J Clin Pharmacol 57(8):559–569
Arant BS Jr (1978) Developmental patterns of renal functional maturation compared in the human neonate. J Pediatr 92(5):705–712
Beal S, Sheiner L (1980) The NONMEM system. Am Stat 34:118–119
Beal SL (1991) Computing initial estimates with mixed effects models: a general method of moments. Biometrika 78(1):217–220
Bergstein JM (2000) Introduction to glomerular diseases. In: Behrman RE, Kliegman RM, Jenson HB (ed) Nelson textbook of pediatrics, 16th edn. WB Saunders, Philadelphia, Pennsylvania, pp 1574–1575
Bonate PL (1999) The effect of collinearity on parameter estimates in nonlinear mixed effect models. Pharm Res 16(5):709–717
Booker PD, Taylor C, Saba G (1996) Perioperative changes in alpha 1-acid glycoprotein concentrations in infants undergoing major surgery. Br J Anaesth 76(3):365–368
Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH (2004) Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth 92(2):208–217
Boxenbaum H, DiLea C (1995) First-time-in-human dose selection: allometric thoughts and perspectives. J Clin Pharmacol 35(10):957–966
Brown RD, Kearns GL, Wilson JT (1998) Integrated pharmacokinetic–pharmacodynamic model for acetaminophen, ibuprofen, and placebo antipyresis in children. J Pharmacokinet Biopharm 26(5):559–579
Capparelli EV, Englund JA, Connor JD, Spector SA, McKinney RE, Palumbo P, Baker CJ (2003) Population pharmacokinetics and pharmacodynamics of zidovudine in HIV-infected infants and children. J Clin Pharmacol 43(2):133–140
Chugani DC, Muzik O, Juhasz C, Janisse JJ, Ager J, Chugani HT (2001) Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol 49(5):618–626
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Cole M, Price L, Parry A, Keir MJ, Pearson AD, Boddy AV, Veal GJ (2004) Estimation of glomerular filtration rate in paediatric cancer patients using 51CR-EDTA population pharmacokinetics. Br J Cancer 90(1):60–64
Crawford JD, Terry ME, Rourke GM (1950) Simplification of drug dosage calculation by application of the surface area principle. Pediatrics 5(5):783–790
Dawson WT (1940) Relations between age and weight and dosages of drugs. Ann Intern Med 13:1594–1613
Du Bois D, Du Bois EF (1916) Clinical calorimetry: tenth paper. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871
Friis-Hansen B (1961) Body water compartments in children: changes during growth and related changes in body composition. Pediatrics 28:169–181
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293(5538):2248–2251
Grimsley C, Thomson AH (1999) Pharmacokinetics and dose requirements of vancomycin in neonates. Arch Dis Child Fetal Neonatal Ed 81(3):F221–F227
Guignard JP, Drukker A (1999) Why do newborn infants have a high plasma creatinine? Pediatrics 103(4):e49
Hines RN, McCarver DG (2002) The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther 300(2):355–360
Holford NHG (1996) A size standard for pharmacokinetics. Clin Pharmacokinet 30(5):329–332
Hull CJ, Van Beem HB, McLeod K, Sibbald A, Watson MJ (1978) A pharmacodynamic model for pancuronium. Br J Anaesth 50(11):1113–1123
Kan RE, Hughes SC, Rosen MA, Kessin C, Preston PG, Lobo EP (1998) Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology 88(6):1467–1474
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 349(12):1157–1167
Kimura T, Sunakawa K, Matsuura N, Kubo H, Shimada S, Yago K (2004) Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob Agents Chemother 48(4):1159–1167
Kleiber M (1961) The fire of life: an introduction to animal energetics. Wiley, New York
Knibbe CA, Zuideveld KP, Aarts LP, Kuks PF, Danhof M (2005) Allometric relationships between the pharmacokinetics of propofol in rats, children and adults. Br J Clin Pharmacol 59(6):705–711
Koukouritaki SB, Manro JR, Marsh SA, Stevens JC, Rettie AE, McCarver DG, Hines RN (2004) Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther 308(3):965–974, Epub 2003 Nov 21
Leger F, Bouissou F, Coulais Y, Tafani M, Chatelut E (2002) Estimation of glomerular filtration rate in children. Pediatr Nephrol 17(11):903–907
Lynn AM, Nespeca MK, Opheim KE, Slattery JT (1993) Respiratory effects of intravenous morphine infusions in neonates, infants, and children after cardiac surgery. Anesth Analg 77(4):695–701
Marshall JD, Kearns GL (1999) Developmental pharmacodynamics of cyclosporine. Clin Pharmacol Ther 66(1):66–75
Matthews I, Kirkpatrick C, Holford N (2004) Quantitative justification for target concentration intervention—parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br J Clin Pharmacol 58(1):8–19
Mitchell D, Strydon NB, van Graun CH, van der Walt WH (1971) Human surface area: comparison of the Du Bois formula with direct photometric measurement. Pfugers Arch 325(2):188–190
Paap CM, Nahata MC (1995) Prospective evaluation of ten methods for estimating creatinine clearance in children with varying degrees of renal dysfunction. J Clin Pharm Ther 20(2):67–73
Pacifici GM, Franchi M, Giuliani L, Rane A (1989) Development of the glucuronyltransferase and sulphotransferase towards 2-naphthol in human fetus. Dev Pharmacol Ther 14(2):108–114
Pacifici GM, Sawe J, Kager L, Rane A (1982) Morphine glucuronidation in human fetal and adult liver. Eur J Clin Pharmacol 22(6):553–558
Peeters MY, Prins SA, Knibbe CA, Dejongh J, van Schaik RH, van Dijk M, van der Heiden IP, Tibboel D, Danhof M (2006) Propofol pharmacokinetics and pharmacodynamics for depth of sedation in nonventilated infants after major craniofacial surgery. Anesthesiology 104(3):466–474
Peters HP (1983) Physiological correlates of size. In: Beck E, Birks HJB, Conner EF (eds) The ecological implications of body size. Cambridge University Press, Cambridge, UK, pp 48–53
Rajagopalan P, Gastonguay MR (2003) Population pharmacokinetics of ciprofloxacin in pediatric patients. J Clin Pharmacol 43(7):698–710
Ross AK, Davis PJ, Dear Gd GL, Ginsberg B, McGowan FX, Stiller RD, Henson LG, Huffman C, Muir KT (2001) Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg 93(6):1393–1401
Schmidt-Nielsen K (1984) Scaling: why is animal size so important? Cambridge University Press, Cambridge, UK
Schuttler J, Ihmsen H (2000) Population pharmacokinetics of propofol: a multicenter study. Anesthesiology 92(3):727–738
Schwartz GJ, Feld LG, Langford DJ (1984) A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104(6):849–854
Schwartz GJ, Gauthier B (1985) A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106(3):522–526
Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J (1979) Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to D-tubocurarine. Clin Pharmacol Ther 25(3):358–371
Sonntag J, Prankel B, Waltz S (1996) Serum creatinine concentration, urinary creatinine excretion and creatinine clearance during the first 9 weeks in preterm infants with a birth weight below 1500 g. Eur J Pediatr 155(9):815–819
Tenenbein M (2000) Why young children are resistant to acetaminophen poisoning. J Pediatr 137(6):891–892
van der Heijden AJ, Grose WF, Ambagtsheer JJ, Provoost AP, Wolff ED, Sauer PJ (1988) Glomerular filtration rate in the preterm infant: the relation to gestational and postnatal age. Eur J Pediatr 148(1):24–28
van der Marel CD, Anderson BJ, Romsing J, Jacqz-Aigrain E, Tibboel D (2004) Diclofenac and metabolite pharmacokinetics in children. Paediatr Anaesth 14(6):443–451
van Dijk M, Koot HM, Saad HH, Tibboel D, Passchier J (2002) Observational visual analog scale in pediatric pain assessment: useful tool or good riddance? Clin J Pain 18(5):310–316
van Dijk M, Peters JW, Bouwmeester NJ, Tibboel D (2002) Are postoperative pain instruments useful for specific groups of vulnerable infants? Clin Perinatol 29(3):469–491
van Dijk M, Simons SH, Tibboel D (2004) Pain assessment in neonates. Paed Perinatal Drug Ther 6(2):97–103
Way WL, Costley EC, Way EL (1965) Respiratory sensitivity of the newborn infant to meperidine and morphine. Clin Pharmacol Ther 6:454–461
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276(5309):122–126
West GB, Brown JH, Enquist BJ (1999) The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284(5420):1677–1679
Yukawa E, Satou M, Nonaka T, Yukawa M, Ohdo S, Higuchi S, Kuroda T, Goto Y (2002) Pharmacoepidemiologic investigation of clonazepam relative clearance by mixed-effect modeling using routine clinical pharmacokinetic data in Japanese patients. J Clin Pharmacol 42(1):81–88
Acknowledgement
The clinical research of K. Allegaert is supported by the Fund for Scientific Research, Flanders (Belgium) by a Clinical Doctoral Grant (A 6/5–KV–G 1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, B.J., Allegaert, K. & Holford, N.H.G. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 165, 819–829 (2006). https://doi.org/10.1007/s00431-006-0189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-006-0189-x