Abstract
Background and Objectives
The hematological side effects associated with mycophenolic acid (MPA) are relatively common and have severe consequences. The majority of literature data have not shown clear consistency in the MPA exposure-neutropenia relationship. We hypothesized that (i) adult de novo kidney transplant recipients who develop neutropenia have relatively higher dose-normalized MPA exposure than patients without neutropenia, and (ii) the observed neutropenia may be explained by polymorphisms in metabolism and/or transporter genes responsible for MPA disposition.
Methods
Adult kidney transplant recipients on steady-state tacrolimus and MPA, not receiving a corticosteroid, and with stable renal function were recruited for investigation at three periods post-transplant (1, 3, and 12 months; n = 21, 17, and 13, respectively). Clinical variables (age, weight, MPA daily dose, albumin, serum creatinine, absolute neutrophil count), tacrolimus and MPA concentrations (for exposure calculation), and genotypes (UGT2B7 G211T, UGT2B7 C802T, UGT1A9 T-275A, UGT1A9 T98C, MRP2 C-24T, MRP2 G1249A, OATP1B1 A388G, OATP1B1 C463A) were characterized.
Results
A significant inverse association between dose-normalized MPA exposure (a surrogate marker for apparent MPA clearance) and absolute neutrophil count in all three study periods (r2 ~ 0.3–0.7) was observed. No associations between characterized single nucleotide polymorphisms and MPA exposure or absolute neutrophil count were established. However, significant alterations in the minor allele frequencies of UGT2B7*2 C802T, UGT1A9 T275A, and MRP2 G1249A were evident.
Conclusion
These findings support the clinical strategy for conducting MPA therapeutic drug monitoring in adult kidney transplant patients on steroid-free immunosuppressant therapy. The novel population genomic analysis data warrant further epidemiological investigations in a larger study sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We have provided evidence on the association between absolute neutrophil count and dose-normalized mycophenolic acid exposure (a marker of apparent MPA clearance) in adult kidney transplant patients on steroid-free antirejection regimens. These findings are clinically relevant and provide further support for conducting mycophenolic acid therapeutic drug monitoring. |
Significantly altered minor allele frequencies of UGT2B7*2, UGT1A9 T275A, and MRP2 G1249A were observed in the study population, which could be associated with the risk of renal disease. |
1 Introduction
Mycophenolic acid (MPA) is widely used as a first-line antirejection drug in adult kidney transplant recipients in combination with a calcineurin inhibitor (e.g., tacrolimus or cyclosporine) and corticosteroids [1, 2]. Due to unwanted side effects associated with corticosteroids, however, steroid-sparing regimens have been utilized in various transplant centers to improve cardiovascular outcomes for patients with minimal immunological risks [3, 4]. The hematological side effects (e.g., leukopenia, neutropenia) associated with MPA can be relatively frequent [1, 2] and have severe consequences. A neutropenic patient is more likely to develop infections, which may increase the risk of graft rejection, and would require careful evaluation (e.g., sometimes with-holding MPA) of their pharmacotherapy [1, 2]. Despite the robust relationship between MPA exposure and efficacy using established therapeutic targets in kidney transplant recipients [5], data correlating MPA exposure to neutropenia are still relatively scarce [6], especially in patients on steroid-free regimens. Moreover, the majority of the studies have characterized leukopenia rather than neutropenia, and the collective literature has shown no clear consistency in the exposure-neutropenia relationship [7]. To fill this important knowledge gap, this article provides further evidence demonstrating a correlation between dose-normalized MPA exposure and neutropenia in kidney transplant patients on steroid-free anti-rejection regimens.
Mycophenolic acid exhibits extremely large pharmacokinetic variability in humans [7]. It is highly protein bound and undergoes extensive hepatic conjugation in the formation of the inactive MPA-glucuronide and the putatively pharmacologically active MPA-acyl-glucuronide [7]. The two glucuronidation reactions are primarily mediated by UGT (UDP-glucuronosyltransferase) 1A9 and UGT2B7, respectively, and polymorphic forms of these enzymes have been characterized [8]. Although associations between polymorphic UGT1A9 or UGT2B7 alleles and altered MPA pharmacokinetics have been reported [8], relatively little data are available establishing associations with pharmacodynamic changes (i.e., toxicity) that have proven more difficult to characterize [8]. The conjugated metabolites of MPA can be eliminated renally or further subjected to enterohepatic recirculation (EHR), the latter an important process responsible for the recycling of MPA into the systematic circulation. EHR is mediated by multidrug resistance-associated protein 2 (MRP2 or ABCC2) and organic anion-transporting polypeptide (OATP) enzymes [7]. Similar to MPA metabolism, the EHR of MPA can be influenced by polymorphic forms of MRP2 and OATP transporters in human subjects [8]. Despite the emerging pharmacogenomics data related to MPA metabolism, little is known of the effects of common genetic polymorphisms in UGT1A9, UGT2B7, MRP2, and OATP1B1 on the neutropenic adverse effects of MPA in renal transplant patients on steroid-free regimens.
Recently, our group developed and validated the first optimal limited sampling strategy (LSS) in adult renal transplant recipients using MPA area under the concentration–time curve (AUC) on a tacrolimus-based steroid-free regimen [9]. Using this specific LSS, we hypothesized that adult de novo kidney transplant recipients who develop neutropenia will have relatively higher MPA exposure than patients without neutropenia. More specifically, we have used dose-normalized MPA exposure (AUC/dose) in our analyses because normalized exposure (i) allows the characterization of the pharmacologically relevant “apparent MPA clearance” (CL/F) and (ii) controls for the large variabilities in oral bioavailability (F, which was not characterized in our study) known to be associated with MPA [10]. We also proposed that MPA-associated neutropenia may be explained by polymorphisms in metabolism and/or transporter genes responsible for MPA disposition. Our primary objective was to determine associations between absolute neutrophil count (ANC) and dose-normalized MPA exposure using regression modeling. Our secondary objectives were to examine the effects of single nucleotide polymorphisms (SNP) in select UGT, MRP2, and OATP enzymes on MPA-associated neutropenia while determining the relative frequencies of these SNPs in our study population in comparison to the general population without kidney disease.
2 Patients and Methods
Blood vacutainers containing ethylenediaminetetraacetic acid (6 ml) were obtained from BD Diagnostics (Franklin Lakes, NJ, USA). DNA was isolated using QIAmp Blood Maxi Kit (Qiagen, Toronto, ON, Canada). Primers were purchased from Integrated DNA Technologies (Coralville, IA, USA). PCR Reagent was ReadyMix Taq PCR reaction mix with MgCl2 and the DNA ladder was Sigma-Aldrich 20 bp low-range marker (Sigma-Aldrich, St Louis, MO, USA). Restriction enzymes were purchased from New England BioLabs (Whitby, ON, Canada). Gel staining was done with SYBR Safe DNA gel stain (Invitrogen, Carlsbad, CA, USA). All other reagents were from VWR (Edmonton, AB, Canada).
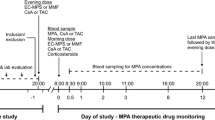
Adult (> 18 years old) de novo kidney transplant recipients were recruited from the solid organ transplant clinic at Vancouver General Hospital (Vancouver, BC, Canada) if they were on steady-state tacrolimus and MPA, not receiving a corticosteroid, and had stable renal function as determined by estimated glomerular filtration rates (GFR) (> 40 ml/min/1.73 m2) on two preceding clinic visits. All included subjects received mycophenolate mofetil (CellCept formulation) and immediate-release tacrolimus (generic formulation). Exclusion criteria included an organ rejection episode in the preceding month, underlying gastrointestinal disease, or cytomegalovirus (CMV)-seronegative recipients receiving allograft from a CMV-seropositive donor (CMV mismatch). Operational approval from the Vancouver Coastal Health Research Institute and Clinical Research Ethics Board at the University of British Columbia were obtained prior to the study. Recruited patients were studied prospectively at three different time periods post-transplant (corresponding to periods where neutropenic episodes are mostly likely to take place in our institution) according to the following schedules: Day 20–40 (period 1): collection of four plasma samples (5 ml each, at 0, 1, 2, and 4 h) for the determination of (i) MPA and tacrolimus AUCs using LSS [9], (ii) blood biochemistries (e.g., serum creatinine (SrCr), albumin, ANC), and (iii) genotyping. The same procedures (except for genotyping) were repeated at month 5–7 (period 2) and month 11–13 (period 3). Additional patient demographic data (age, weight, MPA daily dose, albumin, SrCr) were also collected as part of routine care at each patient visit. Twenty-one subjects were recruited for the first study period (a convenience sample size as no prior data were available), of which 17 subjects completed period 2, and 13 subjects completed period 3. Plasma MPA and whole blood tacrolimus concentrations from an overnight fast were determined by validated clinical assays at Vancouver General Hospital Clinical Laboratory as part of routine care [11]. Patient genotyping was conducted using restriction fragment length polymorphism analysis (see Online Supplementary Material 1). Polymorphism frequencies in the study cohort were compared to published population frequencies adjusted by ethnicity (e.g., the expected allele frequencies in Caucasians were multiplied by the percentage of Caucasians in the study population and added to the frequencies for other ethnicities each multiplied by their percentage in our sample cohort) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26].
Descriptive statistics on baseline patient demographic data were summarized using SigmaStat (Version 3.5). For the primary objective, we first conducted linear regression on log-transformed data (except for gender, which was coded into 1 = female, and 2 = male) in order to determine discrete clinical predictors for dose-normalized MPA AUC. Subsequently, significant predictors of dose-normalized MPA AUC were further incorporated into multiple regression models (as independent variables) in order to obtain the best clinical predictor(s). To complement these regression models, we also sub-categorized subjects based on their ANC (cut-off at 4500 neutrophil count (cells/mm3), which represents the median value in the normal range) and compared the MPA AUC values in the two divided groups. Multiple regression models were constructed using stepwise (backward/forward) analyses. The final sample sizes obtained for each clinical variable are indicated in Table 1. For the secondary objectives (genomic analyses), we compared MPA AUC or ANC between subjects carrying SNPs of UGT1A9, UGT2B7, MRP2, or OTAP1B1 and wild-type carriers. Statistical significance was established using Student’s t test or the Wilcoxon rank-sum test (for non-parametric data) for comparisons between two groups. For comparison of SNP frequencies between our study population and literature data, Chi squared or Fisher’s exact test (two-tailed) was conducted in Prism 5.0 (Graph Pad Prism, San Diego, CA, USA). Statistical significance was deemed a priori at p < 0.05.
3 Results
3.1 Subject Characteristics and Genotyping Data
The average age of the study population (n = 21, n = 10 females) was 56 ± 11 years (mean ± SD) at recruitment. Patient demographic and biochemistry data in each of the study periods are presented in Table 1. Patient ethnicity and genotype data are summarized in Table 2. Because we had decided to characterize each period individually a priori, no attempts to compare the values (in Table 1) between the three different periods were carried out. Overall, no apparent differences were observed for weight, SrCr, GFR, and albumin values between the different study periods (Table 1). However, a trend toward decreasing tacrolimus doses in relation to post-transplant time was observed, which is consistent with our practice guidelines. On the other hand, MPA TDM was not routinely conducted in our institution, and the dose differences of mycophenolate mofetil (MMF, prodrug of MPA) observed between the different periods were based on adjustments tailored to clinical presentation (e.g., adverse effects). Furthermore, the bioavailability of MPA is known to exhibit wide variability and is not linearly associated with MPA dose [10]. Based on these findings, it was decided to normalize MPA (to better characterize CL/F) and TAC AUCs to their respective doses for subsequent statistical analyses. Relatively large variabilities in ANC values were also observed, precluding the establishment of trends between the study periods (Table 1). Significant correlations between MPA AUCs and individual MPA concentrations or between tacrolimus AUC and tacrolimus concentrations were observed in our dataset (r2 = 0.3–0.8, p < 0.05; i.e., the expected observations; data not shown), supporting the validity of our data.
3.2 Linear Regression Analyses to Determine the Associations between Clinical Covariates and ANC
Linear regression analyses were conducted to assess the strength and significance of the associations between each specific clinical co-variate and ANC in each study period (Table 3). Our results indicated that “sex” was significantly associated with ANC in period 1 (r2 = 0.222) and period 2 (r2 = 0.336), whereas “weight” was a significant co-variate in period 1 (r2 = 0.210). The only co-variate consistently (r2 ~ 0.3–0.7) associated with ANC across all three study periods was MPA AUC/dose (hence, MPA CL/F) (Table 3). An inverse association between MPA AUC/dose and ANC was observed (Figs. 1, 2, 3), providing the initial piece of evidence supporting the hypothesis that higher dose-normalized MPA exposure is associated with reduced ANC. In contrast to dose-normalized MPA AUC, tacrolimus AUC was not significantly associated with ANC in any study period (data not shown).
3.3 Multiple Regression Analyses to Determine the Associations between Clinical Covariates and ANC
To further assess the associations between clinical co-variates and ANC, significant co-variates identified from single regression analyses (“sex,” “weight,” and “MPA AUC/dose”) were further incorporated into multiple regression models (using “ANC” as dependent variable and “sex,” “weight,” and “MPA AUC/dose” as independent variables) for each study period. Forward selection and backward elimination multiple regression modeling indicated that “MPA AUC/dose” was the only co-covariate significantly predicting “ANC” in each study period (Table 4). This observation is consistent with the data obtained from single linear regression modeling, providing another piece of evidence supporting our primary hypothesis. Moreover, results from multiple regression modeling further emphasized the associations between “MPA AUC/dose,” but not “sex” or “weight,” and “ANC.” The strengths of the associations (r2 values ~ 0.3–0.7 across the three study periods) observed for MPA AUC/dose suggested that fluctuations in MPA exposure contributed to about 30–70% of the variabilities in ANC in our study cohort.
3.4 Categorical Analyses of the Effects of ANC (> or < 4.5 × 103 cells/mm3) on Dose-Normalized MPA Exposure
As a complementary approach to regression analyses (Tables 3 and 4), subjects in period 1 (n = 21) were further sub-categorized based on ANC (< or > 4.5 × 103 cells/mm3, the median value in the normal range) in order to compare the MPA AUC/dose values between these two groups. Consistent with the findings obtained from regression analyses, subjects with ANC < 4.5 × 103 cells/mm3 (n = 9; mean ± SD, 3.69 ± 0.61 × 103 cells/mm3) exhibited significantly higher MPA AUC/dose values (53.4 ± 14.5 vs. 41.2 ± 11.5 mg h/L/g, respectively) than subjects with ANC > 4.5 × 103 cells/mm3 (n = 12; mean ± SD 5.62 ± 1.06 × 103 cells/mm3) (Fig. 4). These findings further support an inverse relationship between MPA exposure and ANC. This post hoc analysis was conducted for period 1 only due to insufficient sample sizes in other periods.
Dose-normalized MPA AUC values in subjects with ANC < 4.5 × 103 cells/mm3 (n = 9) versus ANC > 4.5 × 103 cells/mm3 (n = 12) in de novo kidney transplant recipients 20–40 days (period 1) post kidney transplant. *p < 0.05 based on Student’s t-test (data satisfied both normality and constant variance tests). AUC area under the concentration–time curve, MPA mycophenolic acid
3.5 Effects of SNPs in UGT2B7, UGT1A9, MRP2, and OATP1B1 on Dose-Normalized MPA Exposure and ANC
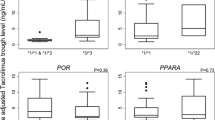
This secondary analysis examined the effects of SNPs in various common genes associated with MPA metabolism (UGT2B7 G211T, UGT2B7 C802T, UGT1A9 T-275A, UGT1A9 T98C, MRP2 C-24T, MRP2 G1249A, OATP1B1 A388G, OATP1B1 C463A) on MPA exposure and ANC in the first study period. No subjects carrying polymorphic alleles were identified for UGT1A9*3 T98C and MRP2 G1249A in our study (Table 5). With respect to the other SNPs, the majority of subjects were heterozygous carriers. Due to the exploratory nature of this secondary analysis and the relatively small study cohort, not all SNPs had sufficient sample size for further statistical analyses. Despite a trend toward increased dose-normalized MPA exposure in heterozygotes of UGT1A9 (T-275A), no associations between dose-normalized MPA AUC or ANC and any of the SNPs identified were evident in our study cohort (Table 5). This post hoc analysis was conducted in period 1 only, due to insufficient sample sizes in other periods.
3.6 Frequencies of SNPs in UGT2B7, UGT1A9, MRP2, and OATP1B1 in the Study Population Compared to Population Controls
This secondary analysis examined the minor allele frequencies of the characterized SNPs in the study cohort in comparison to the frequencies found in population controls. Because the ethnicities of our study subjects were known, the expected minor allele frequencies for each ethnicity in the general population (drawn from the literature) could be compared for the tested SNPs (Table 6) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Where possible, the Indian, Chinese, Korean, and Caucasian minor allele frequencies were extracted. If multiple reports were available in the literature, average values were calculated for these ethnicities for the specific SNP. For patients whose ethnicity was recorded as “Asian Oriental,” Chinese, Japanese, or Korean minor allele frequencies were utilized for statistical analysis. Because there are no reports of minor allele frequencies in the literature for people of Indian descent for UGT2B7*2, UGT1A9 T-275A, UGT1A9*3, and MRP2 G1249A, the average Asian minor allele frequencies were utilized in our calculations. Based on these analyses, UGT2B7*2 C802T (frequency decreased vs. control), UGT1A9 T-275A (increased), and MRP2 G1249A (decreased) exhibited significantly different minor allele frequencies in our study cohort compared to population frequencies in the controls (Table 6).
4 Discussion
Neutropenia is commonly observed in kidney transplant patients in the early de novo period, and may be associated with significant co-morbidities [27, 28]. MMF usage has been associated with neutropenia, or the more commonly characterized leukopenia, in kidney transplant recipients [7, 29]. Hematologic toxicities (including neutropenia) also appear to be the primary reason for MMF dose reduction during the first year post kidney transplant in adult patients [30], which may result in increased graft rejection. However, data on the associations between neutropenia, or leukopenia, and MPA exposure have proven inconsistent in various studies in kidney transplant [7, 29]. Moreover, the majority of studies that have examined the relationship between MPA exposure and hematological side effects have characterized “leukopenia,” which may have completely different etiologies/mechanisms to “neutropenia” [28]. Likewise, to our knowledge, no studies examining the relationship between neutropenia and MPA exposure have yet been conducted in strictly “steroid-free” kidney transplant patients. Given the known induction effects of corticosteroids on MPA metabolism [31] or leukocytosis [32] (potentially affecting the nature of neutropenia, e.g., Vitko et al. [33]) and the lack of available correlation data in adult kidney transplant recipients on steroid-free regimens, our primary finding of a significant inverse association between dose-normalized MPA exposure and ANC in this specific population is novel and of clinical relevance, especially in the context of the increasing utilization of steroid-free regimens today [34].
Our findings of a significant inverse association between dose-normalized MPA exposure and ANC were based on multiple complementary approaches that included single regression modeling (Table 3, Figs. 1, 2, 3), multiple regression modeling (Table 4), and categorical analysis (Fig. 4). Despite the relatively small sample size, the strength and significance of the identified associations found in all three study periods supported the robustness of our observation. Based on r2 values, dose-normalized MPA AUC correlated more strongly with ANC in study period 2 (r2 = 0.697) or 3 (r2 = 0.682) than period 1 (r2 = 0.295), suggesting additional clinical factors (not characterized in this study) may also contribute to reduced ANC early after transplant. The discrepancy in the strength of associations in period 1 (vs. 2 or 3) is unlikely attributed to renal function or protein binding because SrCr and albumin concentrations were comparable across periods (Table 1). On the other hand, a trend toward a lower dose-normalized MPA exposure was observed in period 1, which might suggest that higher dose-normalized MPA exposure (i.e. ≥ 60 mg h/L/g) as evident in periods 2 and 3 may result in stronger associations, or MPA becoming the primary driver, of lower ANC. Additional investigations with a larger sample size and other clinical factors are needed to address this specific observation.
We have strategically utilized dose-normalized MPA exposure (AUC/dose) because it allowed the characterization of the pharmacologically relevant “apparent MPA clearance” (CL/F) and acted as a control for the large variabilities in MPA’s bioavailability, which was not characterized in our oral study [10]. When the same analyses were conducted with un-normalized MPA AUC, the significant association with ANC was still evident in study period 1 (r2 = 0.231, p < 0.05) but not in periods 2 and 3 (data not shown). These discrepancies may be secondary to the relatively smaller samples in periods 2 and 3 in the context of un-controlled MPA variability (i.e., without dose-normalization). A larger sample size in future studies should be able to confirm this hypothesis. Nevertheless, our observation of a significant association (based on period 1 data) between ANC and AUC, irrespective of dose-normalization, would still support our overall conclusion.
Overall, our novel observation of a significant association between dose-normalized MPA exposure and ANC in steroid-free subjects is consistent with other clinical studies that have examined a similar relationship between hematological effects and MPA concentrations, but in adult kidney transplant recipients on steroid-based regimens. For example, Mourad et al. [35] found an association between elevated MPA concentration taken at 30 h post-dose and the presence of adverse events, including hematologic effects, in the early post-transplant period. However, Mourad et al. did not characterize neutropenia as a specific end-point or find MPA exposure to be a significant predictor of adverse events. Similarly, Atcheson et al. [36] examined the pharmacokinetics of MPA 5 days post-transplant and reported higher free MPA (but not total) values in patients exhibiting the composite adverse events of leukopenia/thrombocytopenia/infectious disease. A notable difference is that these studies [35, 36] enrolled subjects on cyclosporine (vs. only tacrolimus in our study), which is known to affect the EHR, hence the exposure, of MPA [2]. Moreover, Kuypers et al. reported a higher incidence of leukopenia in patients with MPA exposure > 60 mg/L/h (up to 5 years post-transplant) [6] and, in a separate study, higher exposure of MPA at 3 and 12 months post-transplant in subjects characterized to be leukopenic [37]. However, neither study specifically characterized neutropenia or established regression models to determine the strength of the relationship. Because their categorical analysis [6] was based on the conventional MPA AUC target range established in subjects on cyclosporine [5], and they included subjects on tacrolimus only, the clinical validity of their analysis may be questioned. Overall, we have provided, to our knowledge, the first evidence on the association between neutropenia and dose-normalized MPA AUC (hence, MPA CL/F), specifically in the steroid-free patient population. The strengths of the associations identified in our study cohort (r2 ~ 0.3–0.7) indicated that dose-normalized MPA exposure is a significant contributor (i.e., 30–70%) to the variability in ANC in this patient group. These findings are clinically relevant and provide further support for conducting MPA therapeutic drug monitoring [38]. Future studies in larger samples are needed to determine the therapeutic thresholds (i.e., AUC cut-offs that may result in neutropenia), preferably using receiver operating characteristic analyses which can provide an assessment on the predictive values of MPA exposure on neutropenia, and whether LSS-mediated MPA AUC determination can mitigate neutropenic side effects.
In contrast to MPA, no association between dose-normalized tacrolimus exposure and ANC was observed in our study population (Table 3). This is consistent with the literature in patients taking steroid-based immunotherapy regimens that neutropenic episodes have more often been associated with MPA (and not tacrolimus) in adult kidney transplant recipients [28, 37]. On the other hand, case reports of probable tacrolimus-associated neutropenia are available in three adult kidney transplant recipients on steroid-based therapies within 3 months of transplant [39]. Elevated trough tacrolimus concentrations (> 8 ng/ml) at 12 months post-transplant have also been documented in a relatively large cohort of adult kidney transplant recipients, although no AUC or ANC data were available [40]. Due to the limited number of studies characterizing tacrolimus-associated neutropenia, it was difficult to discern factors leading to these discrepancies. Overall, our observation is, to our knowledge, the first supporting a lack of association between dose-normalized tacrolimus exposure and ANC specifically in the steroid-free adult kidney transplant population. Taken in the context of a significant association with MPA, these negative findings with tacrolimus are also clinically relevant as they allow clinicians to focus on MPA (rather than tacrolimus) while managing neutropenic episodes. Further mechanistic examinations of neutropenia can also be directed specifically toward MPA.
As a mechanistic approach to further characterize MPA-associated neutropenia in kidney transplant recipients, we conducted a post hoc genomic analysis in attempts to draw associations between SNPs in common genes responsible for MPA disposition and ANC (Table 5). Our initial control analyses did not find significant effects of genotype on dose-normalized MPA exposure. Our finding of a trend toward an increase of dose-normalized MPA AUC in heterozygotes of UGT1A9 (T-275A) are in contrast to that reported by Kuypers et al. [41], who have illustrated a reduction in MPA exposure in carriers of the same polymorphic allele compared to controls. The discrepancies might be attributed to the study population (i.e., strictly Caucasian vs. a significant Asian population), the nature of immunotherapy (steroid vs. steroid-free), or dose effects (2 g vs. < 2 g) in Kuypers et al. [41] as compared to our work, respectively. In general, there is a lack of consensus in the literature describing the effects of the studied SNPs on MPA exposure [41, 42]. Furthermore, our secondary post hoc analyses indicating a lack of association between gene markers and ANC (Table 5) are very likely due to the small sample sizes, and, therefore, the low frequency of SNP carriers identified in our study. As such, further studies in larger patient samples are needed to rule out any potentially false-negative findings. Because no subjects were carrying polymorphic forms of UGT1A9 (T98C) or MRP2 (G1239A) (Table 5), we could not assess the effects of these polymorphic alleles. Because the literature data describing the effects of the characterized SNPs on MPA pharmacodynamics (e.g., neutropenia) have also proven inconsistent [8, 43], further characterizations on the pharmacogenomics of MPA, with respect to MPA metabolism and MPA-associated neutropenia, are warranted.
In contrast to the overall negative results from our categorical analyses attempting to draw associations between SNPs and MPA-related neutropenia, our subsequent post hoc population pharmacogenomic frequency analyses revealed potentially hypothesis-generating findings. Of the eight SNPs tested, five showed population minor allele frequencies as expected, whereas two polymorphisms were expressed at lower frequencies (UGT2B7*2 and MRP2 G1249A) and one (UGT1A9 T275A) was expressed at higher frequency in our study compared to population controls [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] (Table 6). Because UGT2B7*2 and MRP2 G1249A have usually been associated with reduced enzyme/transporter activities, and UGT1A9 T275A protein can be associated with higher catalytic activities (with discrepancies reported in literature) [8, 43], our novel observation of significantly altered minor allele frequencies could result in the formulation of the following general hypothesis: that altered allele frequencies of UGT2B7*2, UGT1A9 T275A, and MRP2 G1249A as observed in our population of adult kidney transplant recipients may be associated with the risk of renal disease. Because the polymorphic alleles were detected in positive controls as well as patient samples, these results are unlikely to be artefactual. Moreover, the large reduction in minor allele frequency (e.g., from 37% to 10% for UGT2B7*2) and the high levels of statistical significance (indicating a high signal-to-noise ratio) suggest that these findings are likely biologically relevant. This hypothesis would require further testing in a larger cohort.
Our study has the following limitations: (i) the observational, open-label nature with small sample size (which is sufficient for our primary end-point that established robust associations between ANC and dose-normalized MPA-AUC, but insufficient for some secondary genomic analyses); (ii) the very few “neutropenic” episodes (i.e., ANC < 1.5 × 103 cells/mm3) actually characterized, thereby potentially limiting the extrapolation of our findings to truly neutropenic patients; (iii) the high frequency of Asian subjects (with equal proportion of Caucasians) in the cohort that is fairly unique to the Vancouver (Canada) population, and hence reducing the generalizability of findings; (iv) the limited duration of follow-up (only up to 1 year post-transplant) and therefore the lack of information on more stable, chronic patients that remain on steroid-free regimens; and (v) the lack of MPA metabolite or additional SNP (e.g., UGT1A8) data, which can be potentially associated (with inconsistencies reported in literature) with the neutropenic side effects of MPA [8, 43].
5 Conclusion
Our significant and novel finding on the inverse association between dose-normalized MPA AUC, a surrogate marker for CL/F, and ANC in adult kidney transplant patients on steroid-free anti-rejection regimens is of clinical relevance because (i) it supports the clinical strategy to focus on MPA while managing a neutropenic episode and (ii) it provides further support for conducting MPA drug monitoring in this specific population.
References
Kiang TK, Ensom MH. Anti-rejection drugs. In: Murphy JE, editor. Clinical pharmacokinetics. 6th ed. American Society of Health-System Pharmacists: Bethesda; 2017. p. 205–20.
Kiang TK, Ensom MH. Immunosuppressants. In: Beringer PE, editor. Basic clinical pharmacokinetics. 6th ed. South Holland: Wolters Kluwer; 2017. p. 320–58.
Lemieux I, Houde I, Pascot A, Lachance JG, Noel R, Radeau T, Despres JP, Bergeron J. Effects of prednisone withdrawal on the new metabolic triad in cyclosporine-treated kidney transplant patients. Kidney Int. 2002;62(5):1839–47.
Andrade-Sierra J, Rojas-Campos E, Cardona-Munoz E, Evangelista-Carrillo LA, Puentes-Camacho A, Lugo-Lopez O, Gomez B, Valdespino C, Cerrillos I, Medina-Perez M, Jalomo B, Nieves JJ, Sandoval M, Ramos-Solano F, Monteon-Ramos F, Cueto-Manzano AM. Early steroid withdrawal in a renal transplant cohort treated with tacrolimus, mycophenolate mofetil and basiliximab. Nefrologia. 2014;34(2):216–22.
van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, Hene RJ, Verpooten GA, Navarro MT, Hale MD, Nicholls AJ. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation. 1999;68(2):261–6.
Kuypers DR, de Jonge H, Naesens M, de Loor H, Halewijck E, Dekens M, Vanrenterghem Y. Current target ranges of mycophenolic acid exposure and drug-related adverse events: a 5-year, open-label, prospective, clinical follow-up study in renal allograft recipients. Clin Ther. 2008;30(4):673–83.
Staatz CE, Tett SE. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol. 2014;88(7):1351–89.
Barraclough KA, Lee KJ, Staatz CE. Pharmacogenetic influences on mycophenolate therapy. Pharmacogenomics. 2010;11(3):369–90.
Poulin E, Greanya ED, Partovi N, Shapiro RJ, Al-Khatib M, Ensom MH. Development and validation of limited sampling strategies for tacrolimus and mycophenolate in steroid-free renal transplant regimens. Ther Drug Monit. 2011;33(1):50–5.
de Winter BC, Mathot RA, Sombogaard F, Vulto AG, van Gelder T. Nonlinear relationship between mycophenolate mofetil dose and mycophenolic acid exposure: implications for therapeutic drug monitoring. Clin J Am Soc Nephrol. 2011;6(3):656–63.
Vancouver General Hospital Department of Pathology and Laboratory Medicine. Standard operating procedure: immunosuppressants. SOP. 2007;14768C:1–13.
Kim JY, Cheong HS, Park BL, Kim LH, Namgoong S, Kim JO, Kim HD, Kim YH, Chung MW, Han SY, Shin HD. Comprehensive variant screening of the UGT gene family. Yonsei Med J. 2014;55(1):232–9.
Johnson LA, Oetting WS, Basu S, Prausa S, Matas A, Jacobson PA. Pharmacogenetic effect of the UGT polymorphisms on mycophenolate is modified by calcineurin inhibitors. Eur J Clin Pharmacol. 2008;64(11):1047–56.
Zakerska O, Skrzypczak-Zielinska M, Mikstacki A, Tamowicz B, Malengowska B, Szalata M, Slomski R. Genotype and allele frequencies of polymorphic UGT1A9 in the Polish population. Eur J Drug Metab Pharmacokinet. 2013;38(3):217–21.
van Schaik RH, van Agteren M, de Fijter JW, Hartmann A, Schmidt J, Budde K, Kuypers D, Le Meur Y, van der Werf M, Mamelok R, van Gelder T. UGT1A9 -275T > A/-2152C > T polymorphisms correlate with low MPA exposure and acute rejection in MMF/tacrolimus-treated kidney transplant patients. Clin Pharmacol Ther. 2009;86(3):319–27.
Levesque E, Delage R, Benoit-Biancamano MO, Caron P, Bernard O, Couture F, Guillemette C. The impact of UGT1A8, UGT1A9, and UGT2B7 genetic polymorphisms on the pharmacokinetic profile of mycophenolic acid after a single oral dose in healthy volunteers. Clin Pharmacol Ther. 2007;81(3):392–400.
Edavana VK, Penney RB, Yao-Borengasser A, Starlard-Davenport A, Dhakal IB, Kadlubar S. Effect of MRP2 and MRP3 polymorphisms on anastrozole glucuronidation and MRP2 and MRP3 gene expression in normal liver samples. Int J Cancer Res Mol Mech. 2015. https://doi.org/10.16966/2381-3318.112.
Ho WF, Koo SH, Yee JY, Lee JD. Genetic variations of the ABCC2 gene in the Chinese, Malay, and Indian populations of Singapore. Drug Metab Pharmacokinet. 2008;23(5):385–91.
Peng KW, Bacon J, Zheng M, Guo Y, Wang MZ. Ethnic variability in the expression of hepatic drug transporters: absolute quantification by an optimized targeted quantitative proteomic approach. Drug Metab Dispos. 2015;43(7):1045–55.
Zhang X, Pu Z, Ge J, Shen J, Yuan X, Xie H. Association of CYP2D6*10, OATP1B1 A388G, and OATP1B1 T521C polymorphisms and overall survival of breast cancer patients after tamoxifen therapy. Med Sci Monit. 2015;21:563–9.
Giacomini KM, Balimane PV, Cho SK, Eadon M, Edeki T, Hillgren KM, Huang SM, Sugiyama Y, Weitz D, Wen Y, Xia CQ, Yee SW, Zimdahl H, Niemi M. International Transporter Consortium. International Transporter Consortium commentary on clinically important transporter polymorphisms. Clin Pharmacol Ther. 2013;94(1):23–6.
Zhai X, Wang H, Zhu X, Miao H, Qian X, Li J, Gao Y, Lu F, Wu Y. Gene polymorphisms of ABC transporters are associated with clinical outcomes in children with acute lymphoblastic leukemia. Arch Med Sci. 2012;8(4):659–71.
Olson KC, Dellinger RW, Zhong Q, Sun D, Amin S, Spratt TE, Lazarus P. Functional characterization of low-prevalence missense polymorphisms in the UDP-glucuronosyltransferase 1A9 gene. Drug Metab Dispos. 2009;37(10):1999–2007.
Deng XY, Wang CX, Wang XD, Bi HC, Chen X, Li JL, Huang M. Genetic polymorphisms of UGT1A8, UGT1A9, UGT2B7 and ABCC2 in Chinese renal transplant recipients and a comparison with other ethnic populations. Pharmazie. 2013;68(4):240–4.
Ramesh K, Hemanth Kumar AK, Kannan T, Vijayalakshmi R, Sudha V, Manohar Nesakumar S, Bharathiraja T, Lavanya J, Swaminathan S, Ramachandran G. SLCO1B1 gene polymorphisms do not influence plasma rifampicin concentrations in a South Indian population. Int J Tuberc Lung Dis. 2016;20(9):1231–5.
Lu XF, Zhou Y, Bi KS, Chen XH. Mixed effects of OATP1B1, BCRP and NTCP polymorphisms on the population pharmacokinetics of pravastatin in healthy volunteers. Xenobiotica. 2016;46(9):841–9.
Hurst FP, Belur P, Nee R, Agodoa LY, Patel P, Abbott KC, Jindal RM. Poor outcomes associated with neutropenia after kidney transplantation: analysis of United States Renal Data System. Transplantation. 2011;92(1):36–40.
Zafrani L, Truffaut L, Kreis H, Etienne D, Rafat C, Lechaton S, Anglicheau D, Zuber J, Ciroldi M, Thervet E, Snanoudj R, Mamzer MF, Martinez F, Timsit MO, Bergougnoux L, Legendre C. Incidence, risk factors and clinical consequences of neutropenia following kidney transplantation: a retrospective study. Am J Transplant. 2009;9(8):1816–25.
Le Meur Y, Borrows R, Pescovitz MD, Budde K, Grinyo J, Bloom R, Gaston R, Walker RG, Kuypers D, van Gelder T, Kiberd B. Therapeutic drug monitoring of mycophenolates in kidney transplantation: report of the Transplantation Society consensus meeting. Transplant Rev (Orlando). 2011;25(2):58–64.
Vanhove T, Kuypers D, Claes KJ, Evenepoel P, Meijers B, Naesens M, Vanrenterghem Y, Cornelis T, Bammens B. Reasons for dose reduction of mycophenolate mofetil during the first year after renal transplantation and its impact on graft outcome. Transpl Int. 2013;26(8):813–21.
Lam S, Partovi N, Ting LS, Ensom MH. Corticosteroid interactions with cyclosporine, tacrolimus, mycophenolate, and sirolimus: fact or fiction? Ann Pharmacother. 2008;42(7):1037–47.
Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71(5):773–8.
Vitko S, Klinger M, Salmela K, Wlodarczyk Z, Tyden G, Senatorski G, Ostrowski M, Fauchald P, Kokot F, Stefoni S, Perner F, Claesson K, Castagneto M, Heemann U, Carmellini M, Squifflet JP, Weber M, Segoloni G, Backman L, Sperschneider H, Kramer BK. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation. 2005;80(12):1734–41.
Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, Grinyo J, FREEDOM Study Group. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8(2):307–16.
Mourad M, Malaise J, Chaib Eddour D, De Meyer M, Konig J, Schepers R, Squifflet JP, Wallemacq P. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem. 2001;47(1):88–94.
Atcheson BA, Taylor PJ, Mudge DW, Johnson DW, Hawley CM, Campbell SB, Isbel NM, Pillans PI, Tett SE. Mycophenolic acid pharmacokinetics and related outcomes early after renal transplant. Br J Clin Pharmacol. 2005;59(3):271–80.
Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther. 2004;75(5):434–47.
Kiang TK, Ensom MH. Therapeutic drug monitoring of mycophenolate in adult solid organ transplant patients: an update. Expert Opin Drug Metab Toxicol. 2016;12(5):545–53.
De Rycke A, Dierickx D, Kuypers DR. Tacrolimus-induced neutropenia in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6(3):690–4.
Shihab FS, Olyaei A, Wiland A, McCague K, Norman DJ. Tacrolimus exposure in the real world: an analysis from the Mycophenolic acid Observational REnal transplant study. Clin Transplant. 2014;28(7):768–75.
Kuypers DR, Naesens M, Vermeire S, Vanrenterghem Y. The impact of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T on early mycophenolic acid dose-interval exposure in de novo renal allograft recipients. Clin Pharmacol Ther. 2005;78(4):351–61.
Woillard JB, Picard N, Thierry A, Touchard G, Marquet P, DOMINOS Study Group. Associations between polymorphisms in target, metabolism, or transport proteins of mycophenolate sodium and therapeutic or adverse effects in kidney transplant patients. Pharmacogenet Genomics. 2014;24(5):256–62.
Picard N, Bergan S, Marquet P, van Gelder T, Wallemacq P, Hesselink DA, Haufroid V. Pharmacogenetic biomarkers predictive of the pharmacokinetics and pharmacodynamics of immunosuppressive drugs. Ther Drug Monit. 2016;38(Suppl 1):S57–69.
Acknowledgements
We sincerely thank Trana Hussaini for assistance in patient recruitment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by an unrestricted educational grant from Hoffmann-La Roche Limited (principal investigator: Mary H.H. Ensom) and the 2015 Vancouver Coastal Health Research Institute Team Grant Award (principal investigator: Tony K.L. Kiang). No editorial assistance was received for the preparation of this article.
Conflicts of interest
Nilufar Partovi, R. Jean Shapiro, Jacob M. Berman, and Abby Collier report no conflicts of interest related to the publication of this article. Tony K.L. Kiang and Mary H.H. Ensom held grant supports as detailed above (which did not pose a conflict of interest with this article), and do not report any other conflicts of interest.
Ethics Approval
All procedures in this study were in accordance with the 1964 Helsinki Declaration (and its amendments). Ethics and operation approval were obtained from the University of British Columbia Clinical Research Ethics Board and Vancouver Coastal Health Research Institute, respectively.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Data Source
Original data can be made available upon request to the corresponding author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiang, T.K.L., Partovi, N., Shapiro, R.J. et al. Regression and Genomic Analyses on the Association Between Dose-Normalized Mycophenolic Acid Exposure and Absolute Neutrophil Count in Steroid-Free, De Novo Kidney Transplant Recipients. Clin Drug Investig 38, 1011–1022 (2018). https://doi.org/10.1007/s40261-018-0694-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-018-0694-5