Abstract
Background and Objectives
In the USA the benefit-risk profile of fluoroquinolones for treating patients with acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections (uUTIs) is considered unfavorable. However, the number of fluoroquinolone products in the EU indicated and prescribed for these infections is uncertain. The objective of this study was to provide data on indications for fluoroquinolones in Europe and examine the prevalence of prescribing in France, Germany and the UK.
Methods
Descriptive analysis of indications for systemic fluoroquinolone antibiotics across the European Economic Area (EEA) and descriptive analysis of systemic fluoroquinolone antibiotic prescribing in France, Germany and UK electronic health records (2000–2015).
Results
All EEA countries had fluoroquinolone products indicated for acute sinusitis, acute bronchitis, or uUTIs, with differences in the number of products between countries for: acute sinusitis (19.5–51.9%), acute bronchitis (22.2–73.4%), and uUTIs (52.0–89.1%). The prevalence of fluoroquinolone prescribing for the treatment of respiratory tract infections (RTIs) appeared to fall with time in all countries and for UTI in France and UK only. Changes were greatest in the UK. In France, Germany, and the UK, respectively: acute sinusitis accounted for 29.5, 20.6, and 40.7% of all oral fluoroquinolone prescriptions for upper RTIs; acute bronchitis accounted for 63.0, 83.0, and 89.9% of all fluoroquinolone prescriptions for lower RTIs; uUTIs accounted for 83.3, 89.9, and 32.2% of all fluoroquinolone prescriptions for UTIs.
Conclusion
Large numbers of fluoroquinolone products in Europe are listed for the treatment of milder infections such as acute bronchitis, acute sinusitis and uUTIs. Among the countries assessed, fluoroquinolones were commonly prescribed for these conditions and potentially should lead to a review of therapeutic guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Milder infections such as acute sinusitis, acute bronchitis and uncomplicated UTI are common indications for systemic fluoroquinolone therapy in Europe. |
Although fluoroquinolone prescribing has fallen in France, Germany and the UK, trends appear to vary by country and by indication. |
1 Introduction
Fluoroquinolone antibiotics inhibit type II topoisomerases required for bacterial DNA replication, transcription and repair [1]. They are widely prescribed treatments for a broad range of infections including those affecting the urinary, respiratory, and gastrointestinal systems. Due to their broad spectrum of activity they are effective against a wide range of infections. However, as with most medicines, fluoroquinolones have the potential to cause adverse reactions, which may be persistent in some patients. Adverse reactions from systemic fluoroquinolone exposure may involve tendons, muscles, joints, and nerves, and may include reactions such as tendon rupture, peripheral neuropathy, and neuropsychiatric reactions [2,3,4,5,6,7,8]. Furthermore, there have been increasing concerns over antimicrobial resistance resulting from antibiotic prescribing, with resistance rates displaying wide variations in Europe depending on the bacterial species, antimicrobial group, and geographical region [9]. The benefit-risk profile for the use of systemic fluoroquinolone antibiotics may differ for less severe infections such as those with and without complicated infections, for example cystitis and pyelonephritis.
These concerns have resulted in the European Medicines Agency (EMA) reviewing the persistence of side effects known to occur with fluoroquinolone antibiotics [10]. Data are therefore required on patterns of fluoroquinolone use, particularly for these less severe indications. A US Food and Drug Administration (FDA) safety review using evidence from prescribing data, published literature, and the FDA’s Adverse Events Reporting System previously concluded that fluoroquinolone antibiotics may be associated with potentially long-term serious side effects when administered via systemic routes of administration (i.e., tablets, capsules, and intravenous formulations) [11]. In May 2016, the FDA advised that because of the potential for long-term serious adverse reactions, the benefit-risk profile of fluoroquinolones for treating patients with acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections (uUTIs) was unfavorable [12]. It was decided by the FDA that fluoroquinolones should not be prescribed for these indications unless no other alternatives are available and that the product labels and medication guides for all fluoroquinolone antibacterial drugs be updated in the US to reflect new safety information. Off-label use of systemic fluoroquinolone antibiotics may also occur, the extent of which may vary between country.
The aim of this study was to provide data on potential indications for systemic fluoroquinolone products authorized in Europe, with an emphasis on acute sinusitis, acute bronchitis, and uUTIs, and to examine the prevalence of fluoroquinolone prescribing for these conditions in France, Germany, and the UK.
2 Methods
2.1 Data Sources
2.1.1 Article 57 Database
The EMA Article 57 database contains information on all medicinal products for human use in Europe, which marketing authorization holders are required to submit, and is based upon the content of their Summary of Product Characteristics (SPCs) [13]. The Article 57 database is considered around 97% complete as determined through comparison with national databases in a study conducted by the EMA. Products are recorded per country and contain descriptions of indications for use for that product in that country.
2.1.2 Databases
A full description of each database is contained in the Online Supplementary Material. In brief the IMS® Disease Analyzer France and Germany (versions June 2016) databases contain anonymized electronic primary-care medical record data on diagnoses (coded using the International Classification of Disease (ICD) coding system) and prescriptions from a representative panel of general practitioners (GPs) from each country commencing from 1997 and 1992, respectively. Data are representative in terms of age, gender, and geographical distribution. The UK THIN database (version May 2016) contains electronic primary-care medical records extracted from general practices covering approximately 6% of the UK population. Data are representative of the UK population in terms of age, deprivation, and geographical distribution. Diagnoses, symptoms, and other relevant health information are recorded using the Read Code clinical classification system, a hierarchical classification system.
2.2 Populations
Patients were included from the IMS® Disease Analyzer France and Germany cohort if they had a consultation date between the start and end of the study period (1 January 2000 and 31 December 2015, respectively) with cohort start determined by the date of the first recording; all patients with a consultation during the year were used as population denominator. For the UK database, cohort entry was defined as the latest of the following dates: start of the study period (1 January 2000) or date of registration with a general practice. Cohort exit was defined by the earliest of the following dates: deregistration from the general practice; death; date of last electronic data collection; end of the study period (31 December 2015).
2.3 Exposures
In the IMS® Disease Analyzer France and Germany databases, prescriptions for incident fluoroquinolones were identified by EphMRA ATC code J01G1 (non-topical fluoroquinolone administration) and by gemscript codes in the UK. Only incident fluoroquinolone prescription episodes were evaluated, defined as a prescription for a systemic fluoroquinolone with no prescription in the previous 30 days. In the UK, incident fluoroquinolones were predominantly prescribed second line, whilst in France and Germany there was a higher proportion of first-line use. Please refer to the Online Supplementary Material for further methodological details.
2.4 Fluoroquinolone Indications
Indications were identified from the Article 57 database by searching disease descriptions for each fluoroquinolone product. Clinical indications were identified in the electronic medical record databases by identifying ICD10 and Read codes recorded in people prescribed a systemic fluoroquinolone antibiotic. Disease descriptions and codes were first broadly categorized into the following pre-specified groups based upon a scoping exercise: upper respiratory tract infections (URTIs); lower respiratory tract infections (LRTIs); urinary tract infections (UTIs); ear infections; gastrointestinal infections (including hepatobiliary infections); genital infections (excluding testicular and prostatic infections considered separately); testicular infections; prostatic infections; skin and soft tissue infections; bone infections; and other indications. Codes that could not be readily grouped into one of the above categories were considered as unclassified. Codes were screened by two reviewers, with a third reviewer used to reach consensus if needed. For upper respiratory tract infections (URTIs), lower respiratory tract infections (LRTIs,) and urinary tract infections (UTIs), indications were then categorized into acute sinusitis, acute bronchitis, and uUTIs as described in the Online Supplementary Material.
2.5 Analysis
A descriptive analysis of indications for treatment with systemic fluoroquinolone products in the Article 57 database was undertaken for each country in the EEA. The prevalence of incident systemic fluoroquinolone prescribing in France, Germany, and the UK was calculated for each indication category as a percentage of the total number of incident fluoroquinolone prescriptions identified with a systemic route of administration. The descriptive analysis of indications recorded in the Article 57 database was then compared to clinical indications through routine electronic prescribing for France, Germany, and the UK with yearly trends in the prevalence of fluoroquinolone prescribing plotted. In a post hoc analysis, Joinpoint regression using the grid search method and permutation testing for model selection was used to determine at what point statistically significant changes in trends occurred in each country for uUTIs, acute bronchitis, and acute sinusitis [14]. All analyses contained within this study are based on “own calculation” (i.e., analysis was conducted by the study authors and not by the database providers). The study was registered in the EU register of post-authorization studies (number EUPAS20889).
3 Results
3.1 Listed Indications for Treatment with Fluoroquinolones in Europe
The Article 57 database contained a total of 2694 fluoroquinolone product entries across 30 EEA countries, of which 2353 had a systemic route of administration (1762 (74.9%) oral; 591 (25.1%) intravenous). The most frequently recorded fluoroquinolone active substances were ciprofloxacin (45.3%), levofloxacin (26.4%), moxifloxacin (12.4%), ofloxacin (9.0%), and norfloxacin (3.6%) (Supplementary Table 2).
These 2694 product entries contained a sum total of 36,830 indication descriptions (mean 13.7 per product). All EEA countries had one or more fluoroquinolone products indicated for the treatment of acute sinusitis, acute bronchitis, and uUTIs that varied according to the type of active substance, with norfloxacin licenced for uUTIs only (Online Supplementary Table 3). This ranged from: 19.5–51.9% for acute sinusitis; 22.2–73.4% for acute bronchitis; and 52.0–89.1% for uUTIs (Table 1).
3.2 Prevalence of Fluoroquinolone Prescribing in France, Germany, and the UK
The most frequently prescribed fluoroquinolone in France was norfloxacin (26.9%), with ciprofloxacin more commonly prescribed in Germany (51.0%) and in the UK (89.4%) (Supplementary Table 4). Clinical indications were identified in 70.1, 83.5, and 62.1% of incident fluoroquinolone prescriptions from the French, German, and UK databases, respectively. The most frequent indication for fluoroquinolone treatment in France, Germany, and the UK was for UTIs (58.7, 43.4, and 33.5%, respectively). Figure 1 shows the proportion of incident fluoroquinolone prescriptions for the treatment of LRTIs, URTIs, and UTIs along with the subset of milder infections of acute bronchitis, acute sinusitis, and uUTIs in France, Germany, and the UK. Fluoroquinolones were prescribed for the treatment of acute bronchitis, acute sinusitis, and uUTIs in: 10.0, 4.7, and 48.9% of instances, respectively, in France; in 29.9, 3.4, and 39.0% of instances, respectively, in Germany; and in 3.7, 1.7, and 19.9% of instances, respectively, in the UK. Figure 2 shows the proportion of incident fluoroquinolone prescriptions for the treatment of the remaining indication categories in France, Germany, and the UK. The proportion of incident fluoroquinolone prescriptions for different indications varied by substance within each country, with levofloxacin and moxifloxacin mainly being used for the treatment of URTIs and LRTIs, whilst norfloxacin and ofloxacin were mainly used for the treatment of UTIs (Supplementary Tables 5, 6, and 7, Supplementary Fig. 1).
Proportion of total fluoroquinolone prescribing for the treatment of a LRTIs, b URTIs, and c UTIs and for the milder infections of acute bronchitis, acute sinusitis, and uncomplicated UTIs in France, Germany, and the UK. LRTI lower respiratory tract infection, URTI upper respiratory tract infection, UTI urinary tract infection, uUTI uncomplicated UTI
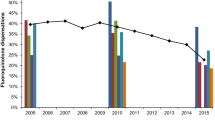
3.3 Trends in Systemic Fluoroquinolone Prescribing in France, Germany, and the UK
Trends in systemic fluoroquinolone prescribing are shown in Fig. 3. There were rising trends in fluoroquinolone prescribing until 2007 in France and the UK, and 2009 in Germany. Fluoroquinolone prescribing then fell in France and the UK, particularly for the treatment of LRTIs, URTIs, and UTIs, whilst in Germany changes were smaller and for UTIs remained relatively stable. In absolute terms the numbers of fluoroquinolone prescriptions for indications other than for RTIs and UTIs were small. Similar trends in fluoroquinolone prescribing for the treatment of acute bronchitis, acute sinusitis, and uUTIs are shown in Fig. 4. Of the three indications, uUTI was the most common indication for fluoroquinolone prescribing in all three countries. In France there were significant changes in trend in 2006 and 2014 for uUTIs, and in 2004 and 2009 for acute bronchitis and acute sinusitis. In Germany, there were significant changes in trend in 2005 and 2010 for uUTIs, 2005 for acute bronchitis, and in 2005 and 2013 for acute sinusitis. In the UK, there were significant changes in trend in 2007 and 2011 for uUTIs, and in 2007 and 2012 for acute bronchitis and acute sinusitis (Supplementary Table 8).
Trends in fluoroquinolone prescribing for acute bronchitis, acute sinusitis and uncomplicated UTI in France, Germany, and the UK. Medium grey diamond = uncomplicated UTI. Dark grey square = acute bronchitis. Light grey triangle = acute sinusitis. UTI urinary tract infection. Analyses based upon own calculation
Comparisons between listed indications and those in clinical practice demonstrated that apart from ciprofloxacin, all other fluoroquinolone active substances were prescribed to treat infections in a greater number of indication categories than listed, suggesting off-label use, although in absolute terms some of this usage was small.
4 Discussion
In light of recent decisions in the USA on the use of fluoroquinolone antibiotics for the treatment of acute sinusitis, acute bronchitis, and uUTIs, we performed a descriptive analysis of listed indications for treatment with systemic fluoroquinolone products across the EEA and examined the prevalence of fluoroquinolone prescribing in France, Germany, and the UK. This study found that fluoroquinolone products across the EEA are frequently indicated for the treatment of acute sinusitis, acute bronchitis, and uUTIs, the extent of which appeared to vary by country and by fluoroquinolone active substance. Fluoroquinolones were also prescribed for these milder infections in France, Germany, and the UK, with some evidence of off-label use to treat infections in other systems. Although the prevalence of fluoroquinolone prescribing has fallen over recent years, variation in changes occurred by country and by indication, with trends in fluoroquinolone prescribing for the treatment of UTIs in Germany remaining fairly stable.
Although a large percentage of fluoroquinolone products across the EEA had a listed indication related to the recent FDA safety communication, it is perhaps not the number of products but how fluoroquinolones are prescribed in clinical practice that is more important. This study shows that compared with France and Germany, fluoroquinolone prescribing has fallen more steeply in the UK since 2008, which may be related to antibiotic stewardship interventions and changes in antibiotic guidelines in response to tackling Clostridium difficile infection [15, 16]. This has important implications in reducing antibiotic resistance, which may result in treatment failure and delays in clinical recovery after treatment with antibiotics; however, high-quality trials for clinician-targeted interventions to influence antibiotic prescribing behavior for RTIs in primary care may still be required to provide robust evidence of their comparative effectiveness [17, 18].
Overall, the most common indication for fluoroquinolones was for the treatment of UTIs. However, at substance level levofloxacin and moxifloxacin were more commonly used to treat LRTIs, with norfloxacin being the most commonly prescribed fluoroquinolone in France compared to ciprofloxacin in the UK and Germany. This is also important because the benefit-risk for fluoroquinolone antibiotics will not only depend upon the severity of infections but also on the comparable safety of different fluoroquinolone active substances. Fluoroquinolones where prescribed for the treatment of URTIs in France and Germany more often than in the UK, where prescribing for the treatment of gastrointestinal infections was more common. In relative terms, acute bronchitis appeared to account for 66.9 and 53.6% of all fluoroquinolones prescribed for the treatment of LRTIs in Germany and France, respectively, compared to only 7.6% in the UK. Similarly, uUTIs appeared to account for 89.9 and 83.3% of all fluoroquinolone prescribing for the treatment of UTIs in Germany and France, respectively, compared to 59.5% in the UK.
Variation in prescribing is important and there are several potential factors that may influence the use of fluoroquinolone antibiotics, including clinical guidelines, local drug formularies, and differences in prescribing culture. For example, a study of recommendations for empiric first-choice antibiotic treatment of uUTIs in six European countries found considerable differences in recommendation, and most countries offered more than one first-choice antibiotic, with nitrofurantoin being listed as an option in five countries and fluoroquinolones in one country only [19]. In our study, fluoroquinolones were more often prescribed as first-line therapy in France and Germany than in the UK. Antimicrobial resistance rates display wide variations in Europe depending on the bacterial species, antimicrobial group and geographical region [9]. In general, lower resistance rates have been reported by northern European countries compared to southern European countries that are considered likely related to variations in antimicrobial use, infection prevention and control practices, and dissimilarities in diagnostic and healthcare utilization patterns in the countries. Antimicrobial resistance can result from antimicrobial use, but may also drive the choice of different antibiotics, which may explain some of the differences observed in our study. However, we analyzed data from France, Germany, and the UK only because we had access to these databases. Although differences in the first-choice antibiotic may depend upon different factors such as the local patterns of bacterial resistance, they are perhaps less likely to be a factor in deciding between different fluoroquinolones. In a qualitative study exploring reasons to prescribe fluoroquinolone antibiotics, interviews were conducted among GPs from high and average fluoroquinolone prescribing practices in the UK [20]. This study found that prescribing fluoroquinolone antibiotics, as opposed to narrow-spectrum antibiotics, depended upon several clinical considerations, perceptions of patient expectations, and organizational influences including prioritizing patient needs and considering long-term consequences. Understanding variation in the choice of fluoroquinolone antibiotic prescribed is also important because they may have different properties, and it cannot be assumed that all fluoroquinolones are equally effective or equally safe.
Fluoroquinolones may cause serious adverse reactions including peripheral neuropathy, tendinopathies, and psychiatric reactions [2,3,4,5,6,7]. Factors to consider when assessing their benefit-risk may include overall effectiveness, the frequency and severity of infections treated with fluoroquinolones and the frequency and severity of adverse effects. Bacterial infections such as acute sinusitis, acute bronchitis, and uUTIs are common and can be relatively mild. There is also the potential for misdiagnosis of viral causes for sinusitis and bronchitis. Part of assessing benefit-risk may also depend upon whether these drugs are used first or second line. In this regard, it was recently noted in an editorial that antibiotic stewardship programs may help to reduce inappropriate use of fluoroquinolone prescribing and indeed variation, and by improving the level of knowledge of antibiotics among community prescribers may be required, particularly with increasing antibiotic resistance in health-care settings [21].
This study has several limitations. First, the definitions of acute bronchitis, acute sinusitis, and uUTI used in this study were based on definitions that included unspecified conditions (bronchitis and UTI), which potentially could introduce some misclassification. However, codes for sinusitis and UTI are commonly recorded in primary-care records and it is likely many will be for acute and uncomplicated infections, respectively, based upon our primary-care experience. Second, identifying clinical indications in the electronic medical records relied upon screening of codes using a look-back period because clinical indications were not routinely linked to the prescription. However, we carefully tailored our look-back periods based upon the frequency of codes and coding practices observed within each database. Despite this, we cannot confirm the indication with either the patient or the health-care professional that would ideally be required to undergo proper validation. Lastly, we used only broad high-level indication categories rather than disease-specific indications as these were considered too extensive to analyze and report, particularly when our focus specifically related to sinusitis, bronchitis, and uUTIs. In this regard, significant variation in prescribing is likely to exist within indication categories, the extent of which is uncertain.
5 Conclusion
In conclusion, many fluoroquinolone products with a systemic route of administration across the EEA appear to have an indication for the treatment of milder infections including acute sinusitis, acute bronchitis, or uUTI. In clinical practice, fluoroquinolones are not infrequently prescribed for these indications, which may have implications for clinical guidelines and antibiotic stewardship interventions.
References
Hooper DC. Mechanisms of action of antimicrobials: focus on fluoroquinolones. Clin Infect Dis. 2001;32(Suppl 1):S9–15.
Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open. 2015;5(11):e010077.
Sode J, Obel N, Hallas J, Lassen A. Use of fluroquinolone and risk of Achilles tendon rupture: a population-based cohort study. Eur J Clin Pharmacol. 2007;63:499–503.
Arabyat RM, Raisch DW, McKoy JM, Bennett CL. Fluoroquinolone-associated tendon-rupture: a summary of reports in the Food and Drug Administration’s adverse event reporting system. Expert Opin Drug Saf. 2015;14(11):1653–60.
Ali AK. Peripheral neuropathy and Guillain-Barré syndrome risks associated with exposure to systemic fluoroquinolones: a pharmacovigilance analysis. Ann Epidemiol. 2014;24(4):279–85.
Hedenmalm K, Spigset O. Peripheral sensory disturbances related to treatment with fluoroquinolones. J Antimicrob Chemother. 1996;37(4):831–7.
Etminan M, Brophy JM, Samii A. Oral fluoroquinolone use and risk of peripheral neuropathy: a pharmacoepidemiologic study. Neurology. 2014;83(14):1261–3.
Joint Formulary Committee. British National Formulary Section 5.1.12 Quinolones (online) London: BMJ Group and Pharmaceutical Press. http://www.medicinescomplete.com. Accessed 17 Jan 2017.
European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2016 Report. 2017. https://ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2016. Accessed 20 Jul 2018.
European Medicines Agency. Quinolone and fluoroquinolone article 31 referral. 2017. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Quinolones_and_fluoroquinolones_containing_medicinal_products/human_referral_prac_000065.jsp&mid=WC0b01ac05805c516f. Accessed 1 Sept 2017.
FDA joint meeting of the antimicrobial drugs advisory committee (formerly known as the anti-infective drugs advisory committee) and drug safety and risk management advisory committee meeting announcement. 2015. http://www.fda.gov/AdvisoryCommittees/Calendar/ucm465275.htm. Accessed 17 Jan 2017.
FDA drug safety communication (online). 2016. http://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed 17 Jan 2017.
European Medicines Agency. Detailed guidance on the electronic submission of information on medicinal products for human use by marketing authorisation holders to the European Medicines Agency in accordance with Article 57(2) of Regulation (EC) No. 726/2004. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2012/03/WC500123681.pdf. Accessed 17 Jan 2017.
Joinpoint Trend Analysis Software. https://surveillance.cancer.gov/joinpoint/. Accessed 30 May 2017.
Public Health England. Clostridium difficile: guidance, data and analysis. https://www.gov.uk/government/collections/clostridium-difficile-guidance-data-and-analysis. Accessed 1 Aug 2017.
Hernandez-Santiago V, Marwick CA, Patton A, Davey PG, Donnan PT, Guthrie B. Time series analysis of the impact of an intervention in Tayside, Scotland to reduce primary care broad-spectrum antimicrobial use. J Antimicrob Chemother. 2015;70:2397–404.
van Hecke O, Wang K, Lee JJ, Roberts NW, Butler CC. Implications of antibiotic resistance for patients’ recovery from common infections in the community: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:371–82.
Tonkin-Crine SK, Tan PS, van Hecke O, Wang K, Roberts NW, McCullough A, Hansen MP, Butler CC, Del Mar CB. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: an overview of systematic reviews. Cochrane Database Syst Rev. 2017;9:CD012252.
McQuiston Haslund J, Rosborg Dinesen M, Sternhagen Nielsen AB, Llor C, Bjerrum L. Different recommendations for empiric first-choice antibiotic treatment of uncomplicated urinary tract infections in Europe. Scand J Prim Health Care. 2013;31:235–40.
Wood F, Simpson S, Butler CC. Socially responsible antibiotic choices in primary care: a qualitative study of GPs’ decisions to prescribe broad-spectrum and fluroquinolone antibiotics. Fam Pract. 2007;24:427–34.
Tillotson GS. FDA and the safe and appropriate antibiotic use of fluoroquinolones. Lancet Infect Dis. 2016;16(3):e11–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study.
Conflict of interest
Daniel Morales has no conflicts of interest. Jim Slattery has no conflicts of interest. Luis Pinheiro has no conflicts of interest. Xavier Kurz has no conflicts of interest. Karin Hedenmalm has no conflicts of interest.
Ethical approval
Approval to conduct the studies using anonymized data was granted by the Scientific Review Committees of The Health Improvement Network (THIN) and IMS® (17THIN032).
Disclaimer
The views expressed in this article are the personal views of the author(s) and may not be not be understood or quoted as reflecting the views of the EMA or one of its committees or working parties.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morales, D.R., Slattery, J., Pinheiro, L. et al. Indications for Systemic Fluoroquinolone Therapy in Europe and Prevalence of Primary-Care Prescribing in France, Germany and the UK: Descriptive Population-Based Study. Clin Drug Investig 38, 927–933 (2018). https://doi.org/10.1007/s40261-018-0684-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-018-0684-7