Abstract
West syndrome (WS), also known as infantile spasms, occurs in infancy with a peak between 4 and 7 months. Spasms, neurodevelopmental regression and hypsarrhythmia on electroencephalogram (EEG) basically define WS. The International League Against Epilepsy commission classifies the aetiologies of WS into genetic, structural, metabolic and unknown. Early diagnosis and a shorter lag time to treatment are essential for the overall outcome of WS patients. These goals are feasible with the addition of brain magnetic resonance imaging (MRI) and genetic and metabolic testing. The present work analysed the medical literature on WS and reports the principal therapeutic protocols of its management. Adrenocorticotropic hormone (ACTH), vigabatrin (VGB) and corticosteroids are the first-line treatments for WS. There is no unique therapeutic protocol for ACTH, but most of the evidence suggests that low doses are as effective as high doses for short-term treatment, which is generally 2 weeks followed by dose tapering. VGB is generally administered at doses from 50 to 150 mg/kg/day, but its related retinal toxicity, which occurs in 21–34% of infants, is most frequently observed when treatment periods last longer than 6 months. Among corticosteroids, a treatment of 14 days of oral prednisolone (40–60 mg/day) has been considered effective and well tolerated. Considering that an early diagnosis and a shorter lag time to treatment are essential for successful outcomes in these patients, further studies on efficacy of the different therapeutic approaches with evaluation of final outcome after cessation of therapy are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
West syndrome (WS) is characterized by the combination of spasms in clusters, hypsarrhythmia on electroencephalogram (EEG) and psychomotor regression. |
Therapy should be quickly initiated as soon as possible after the identification of spasms in infants to rapidly control seizures and improve long-term outcomes. |
Adrenocorticotropic hormone (ACTH), vigabatrin (VGB) and corticosteroids are the primary recommended drugs in these patients. |
1 Introduction
West syndrome (WS) is characterized by the combination of spasms in clusters, hypsarrhythmia on electroencephalogram (EEG) and psychomotor regression, which must not inevitably exist before the onset of spasms [1]. The general practitioner William James West from Tunbridge first described this disorder in 1841 and reported the clinical condition of his 4-month-old son, who exhibited “slight bobbings of the head forward”, which increased in frequency and intensity over time. Doctor West’s description of his son’s spasms is considered a classic portrayal. The letter describes spasms that often begin during the first year of life, occur in series, and may arise in infants who are otherwise seemingly healthy in association with intellectual and motor deterioration [2]. Approach to WS is not clearly standardized. The present work analysed the medical literature in depth, reports principal therapeutic solutions related to a specific cause, and considers the possible adverse effects and implications in the lives of WS patients.
2 West Syndrome (WS): From Epidemiology to Diagnosis
WS is also known as infantile spasms (IS), and it is an age-related disorder that generally occurs during the first year of life with a peak between 4 and 7 months. Some cases emerge up to 14 years of age, as evidenced in the International League Against Epilepsy (ILAE), which demonstrates that this condition is not limited to the first year of infancy. The incidence of WS is estimated at 2–5/10,000 newborns, with a preference for males [3,4,5].
WS spasms are the main clinical feature of this condition. Spasms are sudden and brief contractions of the muscles of the neck, trunk and limbs that occur in a variable time frame, from a fraction of a second to 1–2 s. Spasms generally present in clusters and range from several to hundreds during 1 day. Spasms often occur during awakening or while falling asleep. Jerks are generally flexor but may also be extensor or mixed. Spasms are sometimes preceded by crying or shouting, and they are occasionally associated with other clinical events, such as cyanosis, pallor, deviation of the eyes and modification of respiratory or heart rate [3, 6,7,8].
The typical EEG pattern of WS is hypsarrhythmia, which was defined by Gibbs as a chaotic and disorganized basal activity with high amplitude slow waves and focal or multifocal spikes that are asynchronous and non-rhythmic [9]. Hypsarrhythmia generally occurs in non-rapid eye movement (NREM) sleep, and it disappears during the awake state, rapid eye movement (REM) sleep and spasm clusters. EEG evaluation is certainly important for WS diagnosis even if hypsarrhythmia may be absent in some cases. Caraballo et al. [10] analysed 16 patients with spasm clusters and noticed focal spikes on seven EEGs, bilateral spikes or spikes and waves in two multifocal spikes in five EEGs, and normal findings in two EEGs. A normal EEG excludes the diagnosis of WS [11]. The neurodevelopmental delay plays an important role in the management of WS. This delay is considered a strict criterion for the diagnosis of WS and an essential end-point for a good outcome. Patients generally show a relevant psychomotor retardation, but some milder forms maintain certain cognitive profiles. The developmental delay may be present before, during or after the onset of spasms [7].
The pathophysiology of WS is not completely known because of the complexity of this syndrome. The most common possible mechanisms are an increased excitability and a loss of inhibition, which are observed in animal models. Increased corticotropin-releasing hormone (CRH) and N-methyl-d-aspartic acid (NMDA) characterize the increased neuronal excitability [12, 13]. The loss of interneurons due to the expression of the aristaless-related homeobox, X-linked (ARX) gene underlies the loss of inhibition [14]. Infantile spasms are observed in many diseases. One study of 207 infants with this clinical condition demonstrated that only 127 (61%) of these infants had a verified aetiology. The primary causes were hypoxic-ischaemic encephalopathy (HIE) (10%), chromosomal aberrations (8%), malformations (8%), stroke (8%), tuberous sclerosis complex (TSC) (7%) and periventricular leukomalacia or haemorrhage (5%). Sixty-eight (33%) of the remaining patients had no identified cause, and 12 (6%) patients were not fully investigated [15]. The larger availability of genetic and metabolic tests increased the number of patients with a recognized aetiology. Wirrell et al. [16] performed genetic studies and found a causal aberration in 23.5% of 112 patients, without a definite cause after the initial evaluation, followed by magnetic resonance imaging (MRI) of the brain. Metabolic tests were performed in most of these 251 patients, and pathology was confirmed in only five cases (4.5%). Recent studies identified many genes that were associated with infantile spasms, such as ARX, TSC1 and 2, cyclin-dependent kinase-like 5 (CDKL5) [17, 18], sodium channel protein type 1 subunit alpha (SCN1A), sodium channel protein type 2 subunit alpha (SCN2A), huntingtin interacting protein 1 (HIP1), gamma-aminobutyric acid receptor subunit beta 3 (GABRB3) [19, 20] and membrane-associated guanylate kinase inverted 2 (MAGI2) [21]. Some chromosomal abnormalities may also cause spasms, including trisomy 21 (Down’s syndrome), deletion 1p36 syndrome, deletion 7q11.23 (Williams syndrome plus), maternal duplication 15q11q13 (duplication 15 q syndrome), tetrasomy 12p (Pallister–Killian syndrome) and deletion 17p13 (Miller–Dieker syndrome) [20, 22]. Metabolic disorders are also implicated in infantile spasms, such as pyridoxine dependency, biotidinidase deficiency, mitochondrial diseases, phenylketonuria, non-ketotic hyperglycaemia, organic acidaemias, Menkes syndrome, developmental delay-epilepsy-neonatal diabetes (DEND) syndrome and progressive encephalopathy with oedema, hypsarrhythmia and optic atrophy (PHEO) syndrome [1, 23].
The characteristics of infantile spasms are different from the typical seizure spasms, and these spasms may be unrecognized initially. A recent multicentre retrospective study by Auvin et al. [24] demonstrated the difficulty in correctly and promptly identifying infantile spasms. These authors enrolled 83 patients with available data on diagnostic delay and demonstrated that most of the consulted practitioners (83%) could not explain the clinical picture in these cases. Suggested diagnoses included behavioural disorders or organic diseases, such as gastroesophageal reflux (7%), constipation (7%) or colitis (3%). However, Nelson [25] suggested a comprehensive flow-chart to establish the diagnosis of WS, including history and physical evaluation followed by video-EEG, which may be prolonged for 24 h or repeated after 1–2 weeks if hypsarrhythmia or other electrical abnormalities are not found. If there is no EEG evidence of spasms after this time, then different conditions should be considered. An MRI of the brain should be performed to detect potential structural malformations, ischaemic lesions or tubers. Patients with normal results may undergo genetic (single-nucleotide polymorphism (SNP), array comparative genomic hybridization (aCGH) and typical epilepsy genes panel) and metabolic tests to clarify their condition.
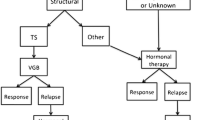
Infantile spasms were initially classified as idiopathic, cryptogenic and symptomatic based on clinical assessment. The first and second groups indicate patients with normal and abnormal neurological development, respectively, prior to the onset of symptoms and no identified cause. The last group includes infants with impaired development and a recognized aetiology [26]. Some cases are synthetically divided into ‘cryptogenic’ and ‘symptomatic’. The ILAE Commission on Classification and Terminology substituted genetic, structural, metabolic and unknown aetiologies for ‘cryptogenic’ and ‘symptomatic’ in 2010 [8]. One crucial aspect of WS management is therapy, which must abolish spasms and normalize the EEG pattern to reverse regular neurodevelopmental delay.
3 Therapeutic Strategies in West Syndrome (WS)
Therapy should be forcefully initiated as soon as possible after the identification of spasms in infants to rapidly control seizures and improve long-term outcomes. Adrenocorticotropic hormone (ACTH), corticosteroids and vigabatrin (VGB) are the primary recommended drugs in these patients. Numerous studies have evaluated the effects of these drugs using different dose regimens, focusing on drug efficacy and possible adverse effects, but many controversies remain.
3.1 Adrenocorticotropic Hormone (ACTH)
ACTH was first noted as being effective in 1950, and it exhibited activity in four of six patients aged 4.5–16 years with different seizure types who were resistant to other treatments [27]. Sorel and Dusacy-Bauloye [28] first reported the exceptional and peculiar utility of ACTH in infants with spasms and typical EEG patterns in 1958. Various hypotheses were formulated for the mechanism of action, and ACTH produces neurosteroids via different mechanisms to promote an anticonvulsant result [29]. Different forms of ACTH exist, including ‘natural’ (bovine or porcine origin) and ‘synthetic’ (obtained from isolation of the first 24 amino acids in the ACTH peptide). The first formulation is not available in Europe, and the second formulation, which exhibits prolonged action, is not available in the USA [26]. Baram et al. [30] enrolled 29 patients who received a high dose of natural ACTH (150 IU/m2/day, given twice daily), followed by a gradual reduction (30 IU/m2 in the morning for 3 days, 15 IU/m2 in the morning for 3 days, 10 IU/m2 in the morning for 3 days and 10 IU/m2 every other morning for 6 days) or prednisone (2 mg/kg/day in two divided doses) for 2 weeks. They demonstrated that 13 of the 15 (87%) infants treated with ACTH and only four of the 14 (29%) infants treated with prednisone exhibited a clinical and EEG response, which supported the superiority of the first drug. Mytinger et al. [31] performed a recent retrospective study to characterize another therapeutic approach. This work examined patients with infantile spasms from January 2009 to September 2013 using two different high-dose natural ACTH protocols. The first protocol was 12 weeks: 75 IU/m2 twice daily the first week, 75 IU/m2 daily the second week and 75 IU/m2 on alternate days the third and fourth weeks, with a slow complete reduction during the remaining 8 weeks. The second protocol was 4 weeks, with 2 weeks at 75 IU/m2 twice daily and a quick taper during the other 2 weeks. A clinical response was interpreted as a complete absence of symptomatology for a period of 28 days, and a relapse was considered the return of spasms. They identified 87 patients, but only 39 patients were evaluated. A total of 21 ACTH-responders were found, with 11 of the 25 patients assigned to the long protocol and 10 of the 14 patients assigned to the short protocol. No significant difference was observed between the two groups. There were also no differences in drug efficacy between patients with known or unknown aetiology. Only one patient in the long protocol group (with Down’s syndrome) suffered a serious adverse effect, and this patient died of sepsis. A complete clinical remission was noted within 2 weeks after the initial dose of ACTH in 96% of cases, and 100% of patients within 3 weeks. Therefore, the authors concluded that a change in therapy should be considered after this period.
One important topic in ACTH treatment was the possibility of establishing low-dose regimens that would be as effective as high doses but with only minor side effects. Ito et al. [32] performed a retrospective multicentre study of 138 WS infants who were resistant to other therapies and received low doses of synthetic ACTH (0.005–0.032 mg/kg/day) for 1–5 weeks. A total of 106 patients (77%) exhibited a complete clinical result, 23 patients (17%) exhibited a normal result with a 50% reduction in seizure frequency, and nine patients (7%) exhibited a poor result. ACTH was more effective in patients with symptomatic causes than in patients with a cryptogenic disease, and only 22% of patients reported no side effects. Only 47% of infants exhibited mild adverse effects (e.g. endocrine and sleep disorders), 23% exhibited moderate adverse effects (e.g. renal or liver dysfunction), and 7% exhibited severe adverse effects (e.g. hypertension, infections and intracranial haemorrhage) that required therapy cessation. Kondo et al. [33] enrolled WS patients with symptomatic and cryptogenic aetiologies and examined two low-dose regimens for 2 weeks that were prolonged up to 4 weeks because of enduring clinical and EEG disorders. One group (18 patients) received synthetic ACTH (0.015 mg/kg/dose), the other 16 patients received synthetic ACTH (0.01 mg/kg/dose) and a clinical response was observed in 94% of cases. There were no significant differences in adverse events, including irritability (31 patients), infections (three patients), and hypertension (four patients), between the two groups. These authors indicated that 0.01 mg/kg was effective in cryptogenic forms of WS and that 0.015 mg/kg was effective in symptomatic patients. Other investigators [34] demonstrated that a gradual increase in ACTH doses based on an insufficient response reduced the incidence of adverse events. They included 31 infants who initially received synthetic 0.005 mg/kg/day ACTH once daily for 2–3 weeks and a 1- to 2-week taper, followed by 0.025 mg/kg/day for 2 weeks and a 2-week taper if abnormalities were persistent. Eight patients required the additional treatment, but only two patients experienced a cessation of spasms. None of the patients exhibited hypertension, but a temporary elevation of liver enzymes, body weight gain and mild brain atrophy confirmed using computed tomography (CT) were observed.
Other studies compared low- and high-dose ACTH regimens to investigate variations in the spasm abolition, hypsarrhythmia resolution and adverse events. Yanagaki et al. [35] included 25 WS patients (16 with symptomatic and nine with cryptogenic causes) in a prospective randomized controlled study and administered high-dose (0.025 mg/kg/day in 13 infants) and low-dose (0.005 mg/kg/day in 12 infants) synthetic ACTH for 8 weeks. They found no significant differences between the two treatments in clinical improvement or side effects. A total of 75% of patients in the symptomatic group exhibited a good clinical response in both therapeutic protocols. Good results were achieved in 75% of patients in the cryptogenic group with the low-dose regimen and in 100% of infants with the high-dose regimen. Adverse events were irritability, body weight gain, increased liver enzymes and hypokalaemia, and more cases of brain volume modifications were noted in the high-dose group. Other authors demonstrated the same results [36, 37]. One study compared high-dose (150 IU/m2/day for 3 weeks, 80 IU/m2/day for 2 weeks, 50 IU/m2/day on alternate days for 1 week and complete reduction in 3 weeks) and low-dose (20–30 IU/day for 2–6 weeks) natural ACTH regimens. Treatments were similarly effective in 13 of 26 (50%) patients in the first group and in 14 of 24 (58%) patients in the second group.
Adverse events included irritability, infection, oral thrush and hypokalaemia, with a higher incidence of hypertension in patients who followed the high-dose protocol [36]. Another retrospective study divided 135 infants into three synthetic ACTH dosage groups and demonstrated no significant differences in the cessation of spasms or EEG improvement. These findings were observed in 63 of the 72 patients (88%) who received 0.02 mg/kg/day, 11 of the 13 patients (85%) who received 0.015 mg/kg/day and 39 of the 50 patients (78%) who received 0.0125 mg/kg. However, significant variations were found in treatment suspension due to adverse events in the third group of infants compared to the other groups. Overall, 57 children (42.2%) reported side effects (e.g. respiratory infections, hypertension and arrhythmia), and 23 of these children (17%) ceased the treatment [37]. Consequently, the authors concluded that a low-dose ACTH regimen should be preferred to a high-dose regimen because of the similar efficacy and improved tolerability [35,36,37] (Table 1).
3.2 Vigabatrin (VGB)
VGB has been largely used in Europe for treatment of infantile spasms since 1989. VGB irreversibly inhibits gamma-aminobutyric acid-transaminase (GABA-T), which increases GABA concentrations and suppresses seizure activity. The US Food and Drug Administration (FDA) approved VGB for infantile spasms in 2009 [38]. The basis for this approval was clearly documented [39, 40] and established the relevance of this drug. Appleton et al. [39] performed a multicentre, randomized, double-blind, placebo-controlled study of 40 infants with a recent diagnosis of infantile spasms who exhibited typical clinical and EEG features and excluded infants who used other antiepileptic drugs (AEDs) within the previous 2 months. Patients presented cryptogenic (30%) and symptomatic (70%) causes, but none presented tuberous sclerosis. Patients were randomized to VGB or placebo for 5 days after a baseline period of 2–3 days. VGB was administered at 50 mg/kg/day and increased up to 150 mg/kg/day if spasm control was not achieved. The authors also proposed a continuation of treatment, in an open-label period of 24 weeks, to both groups. This treatment was followed by 36 of 40 infants but completed only by 29 of these patients. At the end, the highest dosage was received by 11 of 20 patients in the VGB group and by 19 of 20 patients in the placebo group. A total cessation of spasms was observed in seven infants (35%) in the VGB group and in two infants (10%) in the placebo group. However, clinical improvement was achieved in 77.9% of the patients in the first group and 25.9% of the patients in the second group. After 24 weeks, 15 of the 40 patients achieved a complete abolition of spasms, and nine of these patients became spasm-free within 12 days of VGB treatment. Physicians assessed psychomotor development at baseline and at the end of the study using the Denver test. They noted that only three patients exhibited a normal finding initially, but seven patients achieved good results after treatment. The authors highlighted that all of these patients were spasm-free. A total of 12 patients (60%) in the VGB group, six patients (30%) in the placebo group, and 24 patients (66.7%) during the open-label phase experienced adverse events. Eight patients reported drowsiness, which was the most common side effect (Table 2).
Elterman et al. [40] compared the clinical effects of two VGB doses in 221 children with a recent diagnosis of infantile spasms in a large randomized single-blind study. Investigators compared a high-dose (100–148 mg/kg/day) and low-dose (18–36 mg/kg/day) VGB treatment for 14–21 days. Additional drugs were permitted in cases with different seizure types, but no dose adjustments were performed in the first 21 days. The study also included a follow-up phase of up to 3 years, but only 17.2% of patients were evaluated at the end of this period. The authors initially imposed strict criteria that were difficult to follow, and the final end point was spasm cessation, which was defined as 7 consecutive days without clinical manifestations occurring at any time during the study and confirmed using video-EEG within 9 days since the cessation of symptoms. Spasm abolition was achieved in 68.2% of patients in the high-dose group (a total of 107) and 51.8% of patients in the low-dose group (a total of 114) using the revised parameters. A clinical response was obtained within 1 week in the first group compared to the second group, with average results observed in 6 and 13 weeks, respectively. They demonstrated that 21.1% (8/38) of patients with tuberous sclerosis were spasm-free, but only 7.9% (10/26) of patients with other symptomatic causes and 12.3% (7/57) of infants with cryptogenic aetiologies achieved this result. Adverse events included sedation (16.7%), somnolence (13.5%) and irritability (9.9%).
Vigevano et al. [41] first demonstrated the clinical efficacy of VGB in 1997 in 42 infants with infantile spasms aged 2–9 months. Physicians used 100–150 mg/kg/day VGB or 10 IU/day synthetic ACTH as first-line treatment. Twenty-three patients, seven with a cryptogenic cause and 16 with a symptomatic cause, received VGB, and ACTH was given to 19 infants, eight with a cryptogenic cause and 11 with a symptomatic cause. The investigators could modify the therapy or switch to the other drug if there was no response or if the patient experienced severe side effects during the first 20 days. There were no significant differences in the spasm cessation rate during this phase of the study, with 47.8% (11 infants) in the VGB group and 73.6% (14 infants) in the ACTH group achieving cessation. Seven of the 11 patients responded within 3 days to VGB, and all patients exhibited a complete response within 14 days. ACTH exhibited a better reaction in children with perinatal hypoxic/ischaemic injuries, and VGB was more effective in infants with cerebral malformations and tuberous sclerosis. Both treatments produced good results on cryptogenic infantile spasms. Changes in therapy produced clinical control in two of the five patients who switched to VGB and in 11 of the 12 patients who switched to ACTH. Relapses were reported after 3 months in six ACTH patients and one VGB patient. Adverse events occurred in three infants (13%) in the VGB group and seven infants (36.8%) in the ACTH group. Spasm cessation was inferior in the VGB group, but this study stressed the importance of VGB and its use as a possible first-line drug, particularly in patients with cerebral malformations and tuberous sclerosis. This finding was clearly demonstrated in a multicentre prospective work by Chiron et al. [42] who compared the efficacy of VGB and hydrocortisone in 22 patients with infantile spasms associated with tuberous sclerosis. Eleven infants were randomized to 150 mg/kg/day VGB, and the remaining 11 were treated with ACTH (15 mg/kg/day) for 1 month. Patients who exhibited no clinical response were switched to the other drug for 2 months. Children with spasm abolition continued VGB or gradually reduced hydrocortisone. All children in the VGB group were spasm-free at the end of the first month compared to only five children in the hydrocortisone group. The remaining six patients without a good response and the one patient in this group who experienced a serious side effect were treated with VGB and achieved complete symptom resolution. Relapse occurred in one VGB patient who received 75 mg/kg/day. However, this patient became spasm-free in 1 day when he switched to 100 mg/kg/day without any difficulty. Clinical results were achieved faster in patients treated with VGB, with a mean of 3.5 days. Clinical results appeared after 13 days in the hydrocortisone group. Adverse events were observed in 14 patients (five on VGB and nine on hydrocortisone). The most common side effects in the VGB group were drowsiness and hyperexcitability. The other group of patients experienced more hyperexcitability, sleep disorders and weight changes. In addition to the adverse events observed in the above-mentioned studies, the most severe side effects associated with VGB were visual field defects (VFDs). Several hypotheses were formulated, but the real mechanism of VGB-induced retinal damage is not known [43]. Many authors suggested that VFDs were caused by bilateral concentric constriction affecting more extensively the nasal field than the temporal field [44,45,46]. The daily dose, cumulative dose and duration of treatment were implicated in VGB-related vision loss [47, 48]. Maguire et al. [49] reported that VFDs occurred in 52% of adults and in 34% of children. Therapy should be suspended if there is no clinical response after 2–4 weeks [43], and minimizing VGB treatment for 6 months or less reduces the prevalence of retinal damage [50]. Gaily et al. [51] noted that 1 of 16 patients (6%) aged 6–12 years who were treated with VGB for 12–24 months exhibited VFDs, but two recent studies [50, 52] reported vision loss rates of 21 and 34%, respectively. Patients treated with VGB also reported MRI abnormalities with an incidence of 22–32%, mostly new-onset and reversible T2-weighted hyperintensities and restricted diffusion in thalami, globus pallidus, dentate nuclei, brainstem and corpus callosum [53, 54].
3.3 Other Drugs and Recent Advances in Therapy
An evidence-based guideline update in 2012 reported two important concepts [20]. One concept is that the evidence is insufficient to recommend the use of oral corticosteroids, such as prednisolone, dexamethasone and methylprednisolone, as being as effective as ACTH for the short-term treatment of infantile spasms. The second concept concerns the adoption of other drugs (e.g. felbamate, sodium valproate, zonisamide, topiramate, pyridoxine, nitrazepam, levetiracetam and the ketogenic diet) for infantile spasms.
Two recent studies from 2004 to 2005 confirmed this information and demonstrated new therapeutic options for WS [55, 56]. The United Kingdom Infantile Spasms Study was a multicentre, randomized, controlled trial that involved 150 hospitals in the UK. A total of 107 patients aged 2–12 months with clinical and EEG patterns of infantile spasms were enrolled. Patients received VGB (100–150 mg/kg/day, 52 patients), oral prednisolone (40–60 mg/day, 30 patients) or intramuscular tetracosactide (0.5–0.75 mg, equivalent to 40–60 IU, 25 patients) on alternate days. The first evaluation on days 13 and 14 revealed spasm cessation in 28 of 52 infants (53.8%) in the VGB group and in 40 of 55 infants (72.7%) in the hormonal treatment group [21 of 30 prednisolone patients (70%) and 19 of 25 tetracosactide patients (76%)]. Adverse events were experienced in both groups (53.8% in the VGB group vs. 54.5% in the hormonal treatment group), and EEGs improved in patients with hormonal treatments. The low abolition of spasm rate in the VGB group was likely due to the exclusion of patients with infantile spasms that arose from tuberous sclerosis. The study was also prolonged to assess whether early seizure control was associated with improved developmental and epilepsy outcomes at 12–14 months. A total of 101 patients continued in the follow-up period, and their developmental status was measured using Vineland Adaptive Behavior Scales (VABS). Overall, the clinical response was similar at 14 months: 41 of 55 infants (74.5%) in the hormonal treatment group and 39 of 51 patients (76.5%) in the VGB group. VABS scores were also equivalent between the two groups (78.56 in the hormonal treatment group and 77.5 in the VGB group). Better results were observed in infants with a cryptogenic aetiology who were on hormonal therapy [55, 56].
Knupp et al. [57] created a multicentre database in 2012 that included patients with a recent diagnosis of WS between 2 months and 2 years of age. The available data were obtained from June 2012 and July 2014. Clinical information, EEG and MRI evaluations were collected at baseline and after 3 months. Development was also assessed. Drugs proposed were ACTH, prednisolone, VGB and other non-standard therapies. A total of 232 infants were included, and the first treatment choice was associated with the aetiologies. VGB was preferred in infants with structural causes (tuberous sclerosis). ACTH was administered in 42% of cases, oral corticosteroids in 23%, VGB in 20% and non-standard therapies in 14%. Patients treated with ACTH exhibited mild or no problems compared to infants treated with non-conventional drugs, who experienced unfavourable results. Overall, spasm cessation and hypsarrhythmia resolution were observed in 91 of the 198 patients (46%) who followed standard therapies compared to three of the 32 infants who followed other treatments. These findings were obtained in 53 of the 97 (55%) patients who received ACTH, 21 of the 54 patients (39%) who received OCS, 17 of the 47 patients (36%) who received VGB, and three of the 32 patients (9%) who received other drugs. Five of the eight infants with tuberous sclerosis (a total of 11) treated with VGB exhibited a clinical response, but patients treated with ACTH (one infant) and non-standard therapies (two infants) exhibited no results. Children with unknown aetiology and normal development achieved a better resolution rate (20/34, 59%) compared to children with genetic (40/106, 38%) or structural causes (34/90, 38%). Therefore, the authors concluded that infants who received standard therapies, particularly ACTH, had a greater likelihood of obtaining a clinical response [57].
A recent multicentre open-label randomized trial, the International Collaborative Infantile Spasms Study (ICISS), compared the combination of hormonal treatment and VGB to hormonal treatment alone and found spasm resolution for 4 weeks. Between March 2007 and May 2014 a total of 377 infants aged 2–14 months with clinical and EEG characteristics of infantile spasms were included. They were randomized to the two therapeutic groups. Moreover, an additional randomisation of type of hormonal therapy used was proposed with parental approval. The following drugs were administered: prednisolone (10 mg four times daily, increased up to 20 mg three times daily if spasms persisted), tetracosactide (0.5 mg–40 IU intramuscularly, increased up to 0.75 mg every other day if spasms persisted) and VGB (50–100 mg/kg, increased up to 150 mg/kg if spasms continued). Hormonal therapies were administered orally for 2 weeks, and gradually reduced until day 29. VGB was used for 3 months and reduced over 4 weeks. A total of 186 participants were assigned to the hormonal treatment with VGB, and 191 participants were assigned to hormonal therapy alone. Spasm cessation was achieved in 133 of 186 patients (72%) in the first group and in 108 of 191 patients (57%) in the second group. A statistically relevant reduction in response was observed in patients who had a lead time to treatment of greater than 2 months. These results were obtained previously in combined therapy patients (average value of 2 days) compared to the other patients (approximately 4 days). Improved EEG patterns (evaluated initially in 227 of the 374 infants) were observed in 123 of 185 patients (66%) in the first group and in 104 of 189 patients (55%) in the second group. Regarding the causes underlying the spasms in infants with unidentified aetiology, the early clinical response rate was 85% in patients treated with combined therapy and 60% in patients treated with hormonal treatment alone. A total of 228 patients experienced adverse events (111 in the hormonal treatment group and 117 in the other group), and a serious reaction occurred in 33 of these patients (16 in the hormonal therapy group and 17 in the combined therapy group). Infections were the most common side effect, occurring in five patients in the hormonal alone group and in four infants in the combined therapy group. Movement disorders were also observed in 16 infants (two in the hormonal group and 14 in the combined treatment group), which were likely related to the underlying diseases. Therefore, the authors concluded that combined therapy was associated with early and effective clinical and EEG results [58].
However, two prospective clinical trials showed superiority of corticosteroid therapy on ACTH [59, 60] and, due to the high cost of ACTH, in some countries corticosteroid therapy is still considered a first-line treatment. In one of these studies, children (aged 2 months–2 years) with previously untreated WS, were randomized to receive 40–60 IU every other day of intramuscular ACTH or 40–60 mg/day of oral prednisolone for 14 days. Improvement of hypsarrhythmia was assessed blindly using a hypsarrhythmia severity scale before and after completion of therapy. Adverse effects were assessed on day 14 using a symptom diary. The hypsarrhythmia severity score significantly improved with hormonal therapy for 2 weeks [(10.45 ± 2.65 vs. 3.45 ± 2.67); p < 0.01]. When individual treatment arms were compared using mean differences in the improvement of scores, improvement in the prednisolone arm (7.95 ± 2.76) was significantly greater than that in the ACTH arm (6.00 ± 2.61; p < 0.01). Both forms of therapy were tolerated well. Frequent crying, irritability, weight gain, increased appetite and abdominal distension were more common (but not statistically significant) with prednisolone. The same group of authors in another single-centre, single-blind, parallel-group clinical trial randomized newly diagnosed infants with WS to receive 14 days of oral prednisolone (40–60 mg/day) or a synthetically prepared intramuscular long-acting ACTH [40–60 IU/every other day (0.5–0.75 mg)] [60]. By day 14, cessation of infantile spasms occurred in 28/48 (58.3%) infants on prednisolone compared with only 18/49 (36.7%) infants given ACTH (p = 0.03) and electroclinical remission in 21 on prednisolone compared with nine on ACTH (p = 0.007). Sustained spasm control for 28 consecutive days following electroclinical remission occurred in 15 children on prednisolone compared with six on ACTH (p = 0.008). The total number of days required for spasm cessation was significantly less in those treated with prednisolone (3.85 days ± 2.4) compared with ACTH (8.65 days ± 3.7; p = 0.001). Among patients who did not achieve remission, there was a non-significant trend toward greater quantitative reduction of spasms with prednisolone than with adrenocorticotropic hormone (p = 0.079). These were the first ever documentation of a superior therapeutic role of oral steroids in WS [59, 60] (Table 3).
4 Prognosis
Focusing on the developmental outcome, in WS patients it is important to consider many aspects. The underlying aetiology and the time from seizure onset to start of treatment are essential [3]. Better developmental outcomes are noticed in patients without an identified aetiology and even better in those treated with hormonal therapy rather than vigabatrin [61]. In particular, cryptogenic patients may have a good or near-good outcome up to 54.3% of the time [62], while only 12.5% of symptomatic patients have a normal outcome [63]. About the relevance of the lag time to treatment there are important evidences. In a retrospective study, including only patients with a cryptogenic cause, Kivity et al. [64] proposed a long-term treatment with high-dose tetracosactide followed by oral corticosteroids for about 9 months. They showed that a normal cognitive outcome resulted in all patients treated within 1 month of the onset of infantile spasms but only in 40% of those treated later. Favourable cognitive outcome with shorter interval to treatment (< 3 weeks [65], < 4 weeks [66]) was also appraised in recent studies collecting patients with both cryptogenic and symptomatic spasms.
5 Conclusions
WS is a peculiar and age-related epileptic disorder with possible disastrous consequences and with a diagnostic and therapeutic approach that is not clearly standardized. The objective of WS management is the cessation of spasms and the achievement of a good developmental outcome. Physicians, parents and caregivers must be alert in infants with movements compatible with spasms. Videos and the internet provide valuable support to assess these findings. A comprehensive clinical assessment, EEG evaluation, brain MRI and genetic and metabolic tests help achieve an early diagnosis.
The underlying aetiology is crucial for the therapeutic choice. ACTH, VGB and corticosteroids are the first-line treatments in WS patients. There is no unique therapeutic protocol for ACTH, but the evidence suggests that low doses are as effective as high doses for short-term treatment (generally 2 weeks, followed by drug tapering) [20]. VGB is generally administered from 50 to 150 mg/kg/day, and its related retinal toxicity, which occurs in 21–34% of infants, may be associated with treatment periods longer than 6 months [50, 52]. Among corticosteroids, a treatment of 14 days of oral prednisolone (40–60 mg/day) has been considered effective and well tolerated [59, 60]. Because an early diagnosis and a shorter lag time to treatment are essential for successful outcomes in these patients, further studies on efficacy of the different therapeutic approaches with evaluation of final outcome after cessation of therapy are needed.
References
Pavone P, Striano P, Falsaperla R, Pavone L, Ruggieri M. Infantile spasms syndrome, West syndrome and related phenotypes: what we know in 2013. Brain Dev. 2014;36:739–51.
West WJ. On a peculiar form of infantile convulsions. Lancet. 1841;1:724–5.
Wilmshurst JM, Ibekwe RC, O’ Callaghan FJK. Epileptic spasms—175 years on: trying to teach and old dog new tricks. Seizure. 2017;44:81–6.
Rantala H, Putkonen T. Occurence, outcome and prognostic factors of infantile spasms and Lennox–Gastaut syndrome. Epilepsia. 1999;40:286–9.
Commision and Classification and Terminology of the International. League against epilepsy. Workshop on infantile spasms. Epilepsia. 1992;33:195.
Wong M, Trevathan E. Infantile spasms. Pediatr Neurol. 2001;24:89–98.
Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the west Delphi group. Epilepsia. 2004;45:1416–28.
Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology 2005–2009. Epilepsia. 2010;51:676–85.
Gibbs EL, Fleming MM, Gibbs FA. Diagnosis and prognosis of hypsarrhythmia and infantile spasms. Pediatrics. 1954;13:66–73.
Caraballo RH, Ruggieri V, Gonzalez G, Cerosimo R, Gamboni B, Rey A, et al. Infantile spasms without hypsarrhythmia: a study of 16 cases. Seizure. 2011;20:197–202.
Kossof EH. Infantile spasms. Neurologist. 2010;2:69–75.
Baram TZ. Models for infantile spasms: an arduous journey to the Holy Grail. Ann Neurol. 2007;61:89–91.
Velisek L, Jehle K, Asche S, Veliskova J. Model of infantile spasms induced by N-methyl-d-aspartic acid in prenatally impaired brain. Ann Neurol. 2007;61:109–19.
Marsh E, Fulp C, Gomez E, Nasrallah I, Minarcik J, Sudi J, et al. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132:1563–76.
Osborne JP, Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CRC, et al. The underlying etiology of infantile spasms (West syndrome): information from the United Kingdom Infantile Spams Study (UKISS) on contemporary causes and their classification. Epilepsia. 2010;51:2168–74.
Wirrell EC, Shellhaas RA, Joshi C, Keator C, Kumar S, Mitchell WG, et al. How should children with West syndrome be efficiently and accurately investigated? Results from the National Infantile Spasms Consortium. Epilepsia. 2015;56:617–25.
Kato M, Das S, Petras K, Sawaishi Y, Dobyns WB. Polyalanine expansion of ARX associated with cryptogenic West syndrome. Neurology. 2003;61:267–76.
Wallerstein R, Sugalski R, Cohn L, Jawetz R, Friez M. Expansion of the ARX spectrum. Clin Neurol Neurosurg. 2008;110:631–4.
Shields WD. Infantile spasms: little seizures. BIG consequences. Epilepsy Curr. 2006;6:63–9.
Go CY, Mackay MT, Weiss SK, Stephens D, Adams-Webber T, Ashwal S, et al. Evidenced-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78:1974–80.
Marshall CR, Young EJ, Pani AM, Freckmann ML, Lacassie Y, Howald C, et al. Infantile spasms is associated with deletion of the MAGI2 gene on chromosome 7q11.23-q21.11. Am J Hum Genet. 2008;83:106–11.
Sanmaneechai O, Sogawa Y, Silver W, Ballaban-Gil K, Moshè SL, Shinnar S. Treatment outcomes of West syndrome in infants with Down syndrome. Pediatr Neurol. 2013;48:42–7.
Alrifai MT, AlShaya MA, Abulaban A, Alfadhel M. Hereditary neurometabolic causes of infantile spasms in 80 children presenting to a tertiary care center. Pediatr Neurol. 2014;51:390–7.
Auvin S, Hartman AL, Desnous B, Moreau AC, Alberti C, Delanoe C, et al. Diagnosis delay in West syndrome: misdiagnosis and consequences. Eur J Pediatr. 2012;171:1695–701.
Nelson GR. Management of infantile spasms. Transl Pediatr. 2015;4(4):260–70.
Shumiloff NA, Man Lam W, Manasco KB. Adrenocorticotropic hormone for the treatment of West syndrome in children. Ann Pharmacother. 2013;47:744–54.
Klein R, Livingston S. The effect of adrenocorticotrophic hormone in epilepsy. J Pediatr. 1950;37:733–42.
Sorel L, Dusaucy-Bauloye A. A propos de cas d’hypsarythmia de Gibbs: son traitment spectulaire per l’ACTH. Acta Neurol Belg. 1958;58:130–41.
Stafstrom CE, Arnason BG, Baram TZ, Catania A, Cortez MA, Glauser TA, et al. Treatment of infantile spasms: emerging insights from clinical and basic science perspectives. J Child Neurol. 2011;26:1411–21.
Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97:375–9.
Mytinger JR, Weber A, Heyer GL. The response to ACTH is determined early in the treatment of infantile spasms. Epileptic Disord. 2015;17:52–7.
Ito M, Aiba H, Hashimoto K, Kuroki S, Tomiwa K, Okuno T, et al. Low-dose ACTH therapy for West syndrome: initial effects and long-term outcome. Neurology. 2002;58:110–4.
Kondo Y, Okumura A, Watanabe K, Negoro T, Kato T, Kubota T, et al. Comparison of two low dose ACTH therapies for West syndrome: their efficacy and side effect. Brain Dev. 2005;27:326–30.
Oguni H, Yanagaki S, Hayashi K, Imai K, Funatsuka M, Kishi T, et al. Extremely low-dose ACTH step-up protocol for west syndrome: maximum therapeutic effect with minimal side effects. Brain Dev. 2006;28:8–13.
Yanagaki S, Oguni H, Hayashi K, Imai K, Funatuka M, Tanaka T, et al. A comparative study of high-dose and low-dose ACTH therapy for West syndrome. Brain Dev. 1999;21:461–7.
Hrachovy RA, Frost JD Jr, Glaze DG. High-dose, long-duration versus low-dose, short duration corticotropin therapy for infantile spasms. J Pediatr. 1994;124:803–6.
Hamano SI, Yamashita S, Tanaka M, Yoshinari S, Minamitani M, Eto Y. Therapeutic efficacy and adverse effects of adrenocorticotropic hormone therapy in West syndrome: differences in dosage of adrenocorticotropic hormone, onset of age, and cause. J Pediatr. 2006;148:485–8.
Faulkner MA, Tolman JA. Safety and efficacy of vigabatrin for the treatment of infantile spasms. J Cent Nerv Syst Dis. 2011;3:199–207.
Appleton RE, Peters AC, Mumford JP, Shaw DE. Randomised, placebo-controlled study of vigabatrin as first-line treatment of infantile spasms. Epilepsia. 1999;40:1627–33.
Elterman RD, Shields WD, Bittman RM, Torri SA, Sagar SM, Collins SD. Vigabatrin for the treatment of infantile spasms: final report of randomized trial. J Child Neurol. 2010;25:1340–7.
Vigevano F, Cilio MR. Vigabatrin versus ACTH as first-line treatment for infantile spasms: a randomized, prospective study. Epilepsia. 1997;38:1270–4.
Chiron C, Dumas C, Jambaque I, Mumford J, Dulac O. Randomized trial comparing vigabatrin and hydrocortisone in infantile spasms due to tuberous sclerosis. Epilepsy Res. 1997;26:389–95.
Pesaturo KA, Spooner LM, Belliveau P. Vigabatrin for infantile spasms. Pharmacotherapy. 2011;31:298–311.
Willmore LJ, Abelson MB, Ben-Menachem E, Pellock JM, Shields WD. Vigabatrin: 2008 update. Epilepsia. 2009;50:163–73.
Lerner JT, Salomon N, Sankar R. Clinical profile of vigabatrin as monotherapy for treatment of infantile spasms. Neuropsychiatr Dis Treat. 2010;6:731–40.
Agrawal S, Mayer DL, Hansen RM, Fulton AB. Visual fields in young children treated with vigabatrin. Optom Vis Sci. 2009;86:767–73.
Conway M, Cubbidge RP, Hosking SL. Visual field severity indices demonstrate dose-dependent visual lossrom vigabatrin therapy. Epilepsia. 2008;49:108–16.
Durbin S, Mirabella G, Buncic JR, Westall CA. Reduced grating acuity associated with retinal toxicity in children with infantile spasms on vigabatrin therapy. Invest Ophthalmol Vis Sci. 2009;50:4011–6.
Maguire MJ, Hemming K, Wild JM, Hutton JL, Marson AG. Prevalence of visual field loss following exposure to vigabatrin therapy: a systematic review. Epilepsia. 2010;51:2423–31.
Westall CA, Wright T, Cortese F, Kumarappah A, Snead OC, Buncic JR. Vigabatrin retinal toxicity in children with infantile spasms: an observational cohort study. Neurology. 2014;83:2262–8.
Gaily E, Jonsson H, Lappi M. Visual field defects at school-age in children treated with vigabatrin in infancy. Epilepsia. 2009;50:206–16.
Riikonen R, Rener-Primec Z, Carmant L, Dorofeeva M, Hollody K, Szabo I, et al. Does Vigabatrin treatment for infantile spasms cause visual field defects? An international multicentre study. Dev Med Child Neurol. 2015;57:60–7.
Pearl PL, Vezina LG, Saneta RP, McCarter R, Molloy-Wells E, Heffron A, et al. Cerebral MRI abnormalities associated with vigabatrin therapy. Epilepsia. 2009;50:184–94.
Wheless JW, Carmant L, Bebin M, Conry JA, Chiron C, Elterman RD. Magnetic resonance imaging abnormalities associated with vigabatrin in patients with epilepsy. Epilepsia. 2009;50:195–205.
Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, et al. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364:1773–8.
Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4:712–7.
Knupp KG, Coryell J, Nickels KC, Ryan N, Leister E, Loddenkemper T, et al. Response to treatment in a prospective National Infantile Spasms cohort. Ann Neurol. 2016;79:475–84.
O’Callaghan FJK, Edwards SW, Alber FD, Hancock E, Johnson AL, Kennedy CR, et al. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomised, multicentre, open-label trial. Lancet Neurol. 2017;16:33–42.
Wanigasinghe J, Arambepola C, Ranganathan SS, Sumanasena S, Muhandiram EC. The efficacy of moderate-to-high dose oral prednisolone versus low-to-moderate dose intramuscular corticotropin for improvement of hypsarrhythmia in West syndrome: a randomized, single-blind, parallel clinical trial. Pediatr Neurol. 2014;51:24–30.
Wanigasinghe J, Arambepola C, Ranganathan SS, Sumanasena S, Attanapola G. Randomized, single-blind, parallel clinical trial on efficacy of oral prednisolone versus intramuscular corticotropin on immediate and continued spasm control in West syndrome. Pediatr Neurol. 2015;53:193–9.
Darke K, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Lux AL, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP, Trial steering committee on behalf of participating investigators. Developmental and epilepsy outcomes at age 4 years in the UKISS trial comparing hormonal treatments to vigabatrin for infantile spasms: a multi-centre randomised trial. Arch Dis Child. 2010;95:382–6.
Karvelas G, Lortie A, Scantlebury MH, Duy PT, Cossette P, Carmant L. A retrospective study on aetiology based outcome of infantile spasms. Seizure. 2009;18:197–201.
Widjaja E, Go C, McCoy B, Snead OC. Neurodevelopmental outcome of infantile spasms: a systematic review and meta-analysis. Epilepsy Res. 2015;109:155–62.
Kivity S, Lerman P, Ariel B, Danziger Y, Mimouni M, Shinnar S. Long-term cognitive outcomes of a cohort of children with cryptogenic infantile spasms treated with high-dose adrenocorticotropic hormone. Epilepsia. 2004;45:255–62.
Primec Z, Stare J, Neubauer D. The risk of lower outcome in infantile spasms increases after three weeks of hypsarrhythmia duration. Epilepsia. 2006;47:2202–5.
Cohen-Sadan S, Kramer U, Ben-Zeev B, Lahat E, Sahar E, Nevo Y, Eidlitz T, Zeharia A, Kivity S, Goldberg-Stern H. Multicenter long-term follow-up of children with idiopathic West syndrome: aCTH versus vigabatrin. Eur J Neurol. 2009;16:482–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflicts of interest to declare.
Funding
This review was supported by an unrestricted grant from the World Association of Infectious Diseases and Immunological Disorders (WAidid).
Rights and permissions
About this article
Cite this article
D’Alonzo, R., Rigante, D., Mencaroni, E. et al. West Syndrome: A Review and Guide for Paediatricians. Clin Drug Investig 38, 113–124 (2018). https://doi.org/10.1007/s40261-017-0595-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-017-0595-z