Abstract
Background
We previously reported that incretin-based drugs, such as dipeptidyl peptidase-4 (DPP-4) inhibitors or glucagon-like peptide-1 (GLP-1) analogs, improved glycemic control and liver inflammation in non-alcoholic fatty liver disease (NAFLD) patients with type 2 diabetes mellitus (T2DM). However, the effect on alanine aminotransferase (ALT) normalization was still limited.
Aims
The aim of this study is to elucidate the effectiveness of sodium-glucose co-transporter 2 (SGLT-2) inhibitors as second-line treatments for NAFLD patients with T2DM who do not respond to incretin-based therapy.
Methods
We retrospectively enrolled 130 consecutive Japanese NAFLD patients with T2DM who were treated with GLP-1 analogs or DPP-4 inhibitors. Among them, 70 patients (53.8 %) had normal ALT levels. Of the remaining 60 patients (46.2 %) who did not have normal ALT levels, 24 (40.0 %) were enrolled in our study and were administered SGLT-2 inhibitors in addition to GLP-1 analogs or DPP-4 inhibitors. We compared changes in laboratory data including ALT levels and body weight at the end of the follow-up.
Results
Thirteen patients were administered a combination of SGLT-2 inhibitors with DPP-4 inhibitors, and the remaining 11 patients were administered a combination of SGLT-2 inhibitors with GLP-1 analogs. The median dosing period was 320 days. At the end of the follow-up, body weight (from 84.8 to 81.7 kg, p < 0.01) and glycosylated hemoglobin levels (from 8.4 to 7.6 %, p < 0.01) decreased significantly. Serum ALT levels also decreased significantly (from 62 to 38 IU/L, p < 0.01) with an improvement in the FIB-4 index (from 1.75 to 1.39, p = 0.04). Finally, 14 patients (58.3 %) achieved normalization of serum ALT levels.
Conclusions
Administration of SGLT-2 inhibitors led to not only good glycemic control, but also to a reduction in body weight, normalization of ALT levels, and a reduction in the FIB-4 index even in patients who did not respond to incretin-based therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ipragliflozin led not only to good control of type 2 diabetes mellitus but also to reduced liver inflammation, ameliorated liver fibrosis, and a reduction in body weight. |
Body weight reduction was an especially favorable outcome of ipragliflozin treatment in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus. Moreover, ipragliflozin is administered orally, which is highly preferable over glucagon-like peptide-1 analogs that need to be injected. |
1 Introduction
Non-alcoholic fatty liver disease (NAFLD) is reported to be the most common liver disease; it is not only increasing in Western countries but also in Japan owing to the rising prevalence of obesity [1, 2]. NAFLD shows a progression from simple steatosis to steatohepatitis and finally to cirrhosis. It is reported that approximately 3 % of patients who have NAFLD develop cirrhosis [3]. The main pathological condition in NAFLD patients is insulin resistance. Thus, there is a clear association between NAFLD and metabolic syndrome, which induces type 2 diabetes mellitus (T2DM), obesity, hypertension, and dyslipidemia [4]. Improvement of insulin resistance and sensitivity has a therapeutic effect on NAFLD because the accumulation of triglycerides in hepatocytes is considered to be the first step in the current two-hit theory of the pathophysiological development of NAFLD [5]. Several studies indicate that improving insulin resistance and sensitivity improves the extent of fatty liver disease and might prevent the second step in hepatocyte injury caused by oxidative stress [6–8].
Sodium-glucose co-transporter 2 (SGLT-2) inhibitors are the newest class of oral diabetes agents available on the market [9–11]. This class of drug targets the highest capacity subtype of sodium-coupled transporters related to the reabsorption of filtered glucose in the proximal tubules of kidneys. As a result, excess glucose is discharged from the body, which can lead to a reduction in glycosylated hemoglobin (HbA1c) levels of more than 1 %. Although SGLT-2 normally transports 90 % of the filtered glucose, its inhibition results in egestion of only 30–50 % glucose; therefore, the risk of hypoglycemia is low. An important advantage of SGLT-2 inhibitors is their insulin independence, which allows this class of drugs to be effective at any stage of T2DM progression. Furthermore, an additional benefit is that patients have reported a greater than 2-kg reduction in body weight, as observed in a case from Japan [12]. It is for these reasons that SGLT-2 inhibitors are favorable for the treatment of NAFLD patients with T2DM.
Incretin-based therapies such as dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) analogs are already well known as popular strategies for the treatment of T2DM, first, because they generally do not causes hypoglycemia, and second, because they possess weight-neutral or weight-loss properties [13, 14]. These synergic effects seem to be favorable for the treatment of NAFLD patients with T2DM. We previously reported that the administration of GLP-1 analogs led not only to good control of T2DM but also to a decrease in liver inflammation, amelioration of liver fibrosis, and body weight reduction in these patients [15].
However, the effect of GLP-1 analogs on alanine aminotransferase (ALT) normalization is still limited [15]. Furthermore, all GLP-1 analogs need to be injected, and the invasiveness associated with this form of administration is a major barrier to patient compliance, even though the benefits of this drug include decreased liver inflammation and weight reduction. Based on the findings from these studies, we conducted a retrospective cohort study to elucidate the effectiveness of ipragliflozin, a SGLT-2 inhibitor, as a second-line treatment for NAFLD patients with T2DM who did not respond to incretin-based therapy.
2 Methods
2.1 Patients
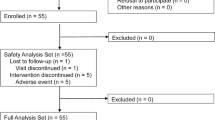
Between January 1, 2010 and June 30, 2015, 130 patients who were clinically diagnosed with NAFLD with T2DM visited the outpatient clinic in the Department of Diabetes and Metabolism or Department of Gastroenterology, Mitsui Memorial Hospital. A diabetologist assigned patients to treatment with either GLP-1 analogs or DDP-4 inhibitors (both incretin-based drugs). Seventy patients (53.8 %) achieved normal ALT levels, while the remaining 60 patients (46.2 %) did not. Of these 60 patients, verbal informed consent was obtained from 24 of these patients (40.0 %) to receive SGLT-2 inhibitors in addition to incretin-based drugs. Changes in laboratory data including ALT levels and body weight at the end of follow-up were monitored. Patients were also divided into two groups based on the achievement or non-achievement of ALT normalization, and the features of the two groups were compared.
All patients were negative for hepatitis B and C viral infection and presence of anti-mitochondrial and anti-nuclear antibodies. Furthermore, none of the patients had been previously diagnosed with hemochromatosis or Wilson’s disease. Clinical diagnosis of NAFLD was based on the following criteria: evidence of fatty liver on ultra-sonography, alcohol consumption of <20 g ethanol per day, and continuous elevation of ALT ≥40 IU/L for more than 6 months.
Diagnosis of T2DM was based on medical history or the 75-g oral glucose tolerance test. Dyslipidemia was defined as a blood total cholesterol level >220 mg/dL, triglyceride level >150 mg/dL, or a history of taking oral drugs for dyslipidemia. Hypertension was defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or a history of taking oral drugs for hypertension. Body mass index (BMI) was routinely assessed at the beginning of treatment and calculated as body weight (in kg) divided twice by body height (in meters).
The evaluation of liver fibrosis was based on the FIB-4 index [16]. The FIB-4 index was calculated from blood test results obtained 1 month prior to administration of ipragliflozin using the following formula: FIB-4 index = (age [years] × aspartate aminotransferase [IU/L])/(platelet count [109 /L] × (ALT [IU/L])1/2). Using a lower cutoff value of 1.30, a FIB-4 score < 1.30 has a negative predictive value of approximately 90 % for advanced fibrosis (Ishak fibrosis score 4–6, which includes early bridging fibrosis to cirrhosis). In contrast, a FIB-4 score >2.67 has ~95 % specificity and a positive predictive value of ~65 % for advanced fibrosis. Finally, this study was conducted according to the STROBE statement [17].
2.2 Treatment and Follow-up
All patients were treated in our outpatient clinic and had poorly controlled T2DM (HbA1c >6.5 %) with the use of incretin-based therapies. The administration of incretin-based drugs (either GLP-1 analogs or DDP-4 inhibitors) was decided by outpatient diabetologists. Ipragliflozin was administered orally once daily at a dose of 25 mg up to 50 mg if necessary. The beginning of follow-up was defined as the administration date of ipragliflozin, and the end of follow-up was June 30, 2015. The follow-up consisted of a monthly or bi-monthly physical examination including body weight measurement and blood tests. Patients who had to switch to intensive therapies, such as insulin injection owing to an exacerbation of diabetes, were treated as intention to treat. Even when other oral glucose-lowering agents were added to the treatment protocol, follow-up was still considered valid provided ipragliflozin was continued. Patients who discontinued ipragliflozin treatment owing to an improvement in their diabetes symptoms were also treated as intention to treat. Patients were evaluated by outpatient clinicians to monitor exacerbation or improvement of diabetes.
2.3 Statistical Analyses
Data are expressed as medians and ranges (25–75th percentiles) unless otherwise indicated. Changes in parameters after the administration of ipragliflozin were compared by the paired t test. Patients were divided into two groups based on achievement or non-achievement of ALT normalization, and the features between the two groups were compared. Continuous variables between the two groups were compared by the unpaired t test. Categorical variables were compared by the Chi square test. There were no missing data. Data processing and analysis were performed using StatView version 5.0 (SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Patient Profile
Baseline characteristics are shown in Table 1. The median age of all patients was 54.2 years, and 19 patients (79.2 %) were male. The median dosing period was 320 days. All patients were already taking incretin-based medications: 11 patients (45.8 %) were taking GLP-1 analogs, and the remaining 13 patients (54.2 %) were taking DDP-4 inhibitors. Sulfonylurea agents and metformin were additionally administered in 10 (41.7 %) and 13 patients (54.2 %), respectively. Comorbidity with dyslipidemia was present in 19 patients (79.2 %), and hypertension was present in nine patients (37.5 %). Twelve patients were smokers.
3.2 Changes in Parameters After the Administration of Ipragliflozin
Table 2 shows the changes in parameters after administration of ipragliflozin. There were significant reductions in both body weight (from 84.8 to 81.7 kg) and BMI (from 30.1 to 27.6 kg/m2) (both p < 0.01). The control of diabetes, as measured by clinical indicators, also improved markedly: HbA1c decreased from 8.4 to 7.6 % (p = 0.01), and high-density lipoprotein-cholesterol increased from 42 to 44 mg/dL (p = 0.01). Additionally, there was a significant reduction in liver inflammation and improvement in the liver fibrosis score: aspartate aminotransferase decreased from 37 to 28 IU/L, ALT decreased from 62 to 38 IU/L, γ-GTP decreased from 75 to 60 IU/L, and the FIB-4 index decreased from 1.75 to 1.39 (all p < 0.05).
3.3 Comparison of Patients Who Did vs. Did Not Achieve Normalization of Serum ALT Levels
During the follow-up period, 14 patients (58.3 %) achieved normalization of serum ALT levels (Group A), while 10 patients (41.7 %) did not (Group B). The differences in baseline characteristics between the two groups are shown in Table 3. There were no significant differences in the use of other diabetes medications between the two groups. The administration period of ipragliflozin was significantly longer in Group A (p = 0.05). Although the median body weight was significantly higher in Group A (p = 0.04), differences in BMI were not statistically significant. Differences in the extent of diabetes and dyslipidemia were not significantly different between the two groups. As expected, baseline ALT level and γ-GTP level were significantly lower in Group A (both p < 0.05). Platelet counts were significantly higher in Group A, which is consistent with a higher number of patients with advanced liver fibrosis belonging to Group B. However, differences in the FIB-4 index were not statistically significant.
Table 4 indicated each change about body weight, ALT level, and FIB-4 index between group A and group B. There were no significant differences about reduction rate of body weight, ALT level, and FIB-4 index between the two groups.
We also analyzed the effect of ipragliflozin between patients who were treated with GLP-1 analogs and DDP-4 inhibitors because there was different effects on body weight change. There were 11 patients who were treated with GLP-1 analogs and 13 patients who were treated with DDP-4 inhibitors. There was no significant difference about mean body weight reduction (kg) between the two groups (−3.5 kg in the GLP-1 group and −2.8 kg in the DPP-4 inhibitors group, p = 0.68).
4 Discussion
NAFLD is a disease that is characterized histologically by hepatic steatosis, lobular inflammation, and hepatocellular ballooning [18]; it has been reported that at least 3 % of patients develop liver cirrhosis [3]. This disease is considered to be common owing to an increase in comorbidities including fatty liver disease, obesity, and T2DM [19]. Multiple pharmacologic interventions have been investigated with variable success. In particular, trials of glucose-lowering agents such as metformin and pioglitazone have provided promising results [20, 21]. In this study, drastic improvement of serum ALT levels and a reduction in body weight were observed, suggesting that ipragliflozin treatment not only improved the diabetic status but also reduced liver inflammation in T2DM patients with NAFLD who did not respond to incretin-based therapies.
We previously demonstrated a reduction in liver inflammation and improvement of diabetes in NAFLD patients with T2DM after treatment with GLP-1 analogs and DPP-4 inhibitors [15]. Administration of GLP-1 analogs led not only to good control of T2DM but also a reduction in liver inflammation, amelioration of liver fibrosis, and a reduction in body weight. Body weight reduction was considered to be the most favorable outcome because weight reduction is generally recommended as an initial step in the management of NAFLD [22], and its effectiveness was confirmed in a randomized controlled trial [8].
Because only 70 out of 130 patients (53.8 %) achieved normalization of serum ALT levels with incretin-based therapy, compared with 60 patients (46.2 %) who did not, the effect of incretin-based therapy on ALT normalization seems to be limited. Finally, of the 60 patients who did not achieve normalization of serum ALT levels with incretin-based therapy, 24 were treated with ipragliflozin, among whom 14 patients (58.3 %) achieved normalization of serum ALT levels. This result indicates that even in NAFLD patients with T2DM who do not respond to incretin-based therapies, it is still possible to reduce liver inflammation (as indicated by normalization of serum ALT levels) with SGLT-2 inhibitors such as ipragliflozin. Therefore, SGLT-2 inhibitor agents may have the potential to be first-line treatments for NAFLD patients with T2DM. However, further prospective studies are needed to elucidate the true effect of these agents.
In a randomized controlled trial, NAFLD patients treated with pioglitazone showed a reduction in liver inflammation but no change in the extent of liver fibrosis [6]. These results indicate that a reduction in liver inflammation does not always correlate with an improvement in the degree of liver fibrosis. Because this current study was based on outpatient clinic care, liver biopsies were not performed in our study population. Non-invasive measurements of liver stiffness, such as transient elastography, were also not available at our institute. Thus, we applied the FIB-4 index to evaluate the degree of liver fibrosis [16]. In this study, the FIB-4 index significantly improved after administration of ipragliflozin. Although the effect of ipragliflozin treatment on liver fibrosis is unclear, it has been reported that SGLT-2 inhibitors have a prophylactic effect on hepatic steatosis and fibrosis in a mice model, whereby fibrosis was induced by a choline-deficient, l-amino acid-defined diet [23]. Therefore, further clinical studies are needed.
Obesity is considered one of the most important risk factors for NAFLD [22]. Weight reduction via lifestyle intervention is generally recommended as an initial step in the management of NAFLD [22], and its effectiveness was confirmed in a randomized controlled trial [8]. However, lifestyle intervention depends on a patient’s individual efforts and is sometimes difficult to achieve [24]. In this current study, body weight dramatically changed after the administration of ipragliflozin, even in patients who already had not responded to incretin-based therapies. Although the first step in the management of NAFLD is lifestyle intervention, ipragliflozin may support body weight reduction via excretion of urinary sugar and may lead to improvement in NAFLD.
Because this current study was based on a retrospective cohort, there are many limitations. A major limitation is that the study cohort consisted of patients treated with a combination of various anti-diabetic agents such as GLP-1 analogs, DPP-4 inhibitors, metformin, sulfonylurea agents, and insulin. Metformin has been reported to have some effects on liver steatosis and inflammation. Because we also reported an effect of GLP-1 analogs and DPP-4 inhibitors on NAFLD improvement in patients with T2DM [15, 20], this study may not reflect the true outcome of ipragliflozin in NAFLD patients with T2DM.
In conclusion, we have demonstrated a reduction in liver inflammation and an improvement in diabetes in NAFLD patients with T2DM treated with ipragliflozin. An elevation in the liver fibrosis score can lead to future liver cirrhosis. Similarly, body weight gain can exacerbate liver inflammation and other metabolic disorders. Ipragliflozin led not only to good control of T2DM but also to reduced liver inflammation, ameliorated liver fibrosis, and a reduction in body weight. Body weight reduction was an especially favorable outcome of ipragliflozin treatment in NAFLD patients with T2DM. Moreover, ipragliflozin is administered orally, which is highly preferable over GLP-1 analogs that need to be injected.
5 Conclusion
Administration of SGLT-2 inhibitors led to not only good glycemic control, but also to a reduction in body weight, normalization of ALT levels, and a reduction in the FIB-4 index even in patients who did not respond to incretin-based therapy.
References
Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7.
Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–58.
Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19.
Angelico F, Del Ben M, Conti R, et al. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90:1578–82.
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5.
Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85.
Dixon JB, Bhathal PS, Hughes NR, et al. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–54.
Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–9.
Veltkamp SA, Kadokura T, Krauwinkel WJ, et al. Effect of ipragliflozin (ASP1941), a novel selective sodium-dependent glucose co-transporter 2 inhibitor, on urinary glucose excretion in healthy subjects. Clin Drug Investig. 2011;31:839–51.
Fonseca VA, Ferrannini E, Wilding JP, et al. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complicat. 2013;27:268–73.
Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther. 2013;139:51–9.
Mori H, Okada Y, Kawaguchi M. A case of diabetes mellitus with NASH successfully treated using integrated therapy including ipragliflozin. J Japan Diabetes Soc. 2015;58:286–92.
Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268–78.
Iwasaki T, Yoneda M, Inamori M, et al. Sitagliptin as a novel treatment agent for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology. 2011;58:2103–5.
Ohki T, Isogawa A, Iwamoto M, et al. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. Sci World J. 2012;2012:496453.
Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25.
von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–8.
Ludwig J, Viggiano TR, McGill DB, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8.
Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95.
Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90.
Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307.
Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–50.
Hayashizaki-Someya Y, Kurosaki E, Takasu T, et al. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline-deficient l-amino acid-defined diet in rats. Eur J Pharmacol. 2015;754:19–24.
Wang RT, Koretz RL, Yee HF Jr. Is weight reduction an effective therapy for nonalcoholic fatty liver? A systematic review. Am J Med. 2003;115:554–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Contributions
The first author collected all the data and wrote this article. Dr. A. Isogawa, N. Toda, and Dr. K. Tagawa are outpatient clinic doctors who contributed to this study.
Funding
This study received no funding.
Informed consent
Oral informed consent was obtained from all patients when they began treatment but was not required for the present analyses because this was a retrospective study.
Conflict of interest
Takamasa Ohki has received speaking fees from Otsuka Pharmaceutical Co., Ltd. The other authors have nothing to disclose.
Ethical approval
Formal approval was not required for this type of study.
Rights and permissions
About this article
Cite this article
Ohki, T., Isogawa, A., Toda, N. et al. Effectiveness of Ipragliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor, as a Second-line Treatment for Non-Alcoholic Fatty Liver Disease Patients with Type 2 Diabetes Mellitus Who Do Not Respond to Incretin-Based Therapies Including Glucagon-like Peptide-1 Analogs and Dipeptidyl Peptidase-4 Inhibitors. Clin Drug Investig 36, 313–319 (2016). https://doi.org/10.1007/s40261-016-0383-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0383-1