Abstract
Background and Objective
Evidence indicates that vitamin K antagonists (VKAs) and oral anticoagulant therapy are under-utilised for stroke prevention in patients with non-valvular atrial fibrillation (AF), and patients who decline or cannot tolerate such treatment are often prescribed aspirin instead. Apixaban has been shown in the AVERROES trial to be superior to aspirin in preventing stroke and systemic embolism without significantly increasing the risk of major bleeding among patients with AF who are unsuitable for VKA therapy. This study estimates the economic implications and potential cost effectiveness of apixaban compared with aspirin in such individuals from the perspective of healthcare payers in Belgium.
Methods
A Markov model was developed to evaluate the clinical and economic impact of apixaban compared with aspirin in patients unsuitable for VKA therapy. The clinical events modelled include ischaemic and haemorrhagic stroke, systemic embolism, intracranial haemorrhage, other major bleeding, clinically relevant non-major bleeding, myocardial infarction, cardiovascular hospitalisation and treatment discontinuations obtained from AVERROES. Outcomes included life-years and quality-adjusted life-years (QALYs) gained, costs and incremental cost-effectiveness ratios (ICERs) over a lifetime.
Results
Apixaban was projected to increase life expectancy and QALYs compared with aspirin, with an associated increase in drug acquisition costs. The estimated ICER was €7,334 per QALY gained with apixaban compared with aspirin.
Conclusions
Apixaban is a cost-effective alternative to aspirin for patients with AF in Belgium who decline or cannot tolerate VKA treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Apixaban is the only new oral anticoagulant that has been investigated in a randomised controlled trial in patients for whom vitamin K antagonist therapy was unsuitable, and this cost-effectiveness study adds key evidence for assessing its value as a treatment option in this population. |
Our analysis shows higher incremental costs for patients treated with apixaban attributable to higher drug acquisition costs that are partly offset by a reduction in medical costs. |
Due to an increase in life expectancy and quality-adjusted life expectancy with apixaban, the drug is predicted to be a cost-effective alternative to aspirin over a range of scenarios. |
1 Introduction
An estimated one in every 45 people in Belgium has atrial fibrillation (AF) [1], and, consequently, a fivefold increased risk of experiencing stroke and other thromboembolic events [2]. Belgium’s standard of care for preventing these vascular outcomes is conventional oral anticoagulant (OAC) therapy involving vitamin K antagonists (VKAs) [3]. However, such use of VKA therapy is complicated by its various well-known limitations, including multiple drug–food and drug–drug interactions, the high risk of adverse events and the need for frequent monitoring [3]. The result is that many patients with AF decline or cannot tolerate VKA therapy and, if considered to be at low thromboembolic risk, might be prescribed aspirin instead [4–7]. It is not surprising, therefore, that evidence indicates underutilisation of OAC therapy for AF in Belgium [4–7]. For example, a prospective study involving 885 people with a CHADS2 [congestive heart failure, hypertension, age ≥75 years old, diabetes mellitus, prior stroke or transient ischaemic attack (TIA) or thromboembolism] score of 1 or more found that only 47 % overall were on OACs (ranging from 39 % of those with a score of 1 to 69 % with a score of 6) [7]. In addition, antiplatelet monotherapy was being used even in a significant proportion (35 %) of those at moderate to high thromboembolic risk [7].
Such treatment patterns are challenged by current guidelines from the European Society of Cardiology (ESC), which take into account the availability of the new generation of OACs (NOACs). Specifically, these guidelines recommend a shift in practice to treating low-risk patients with OACs, rather than antiplatelet therapy, on the grounds that aspirin is supported only by weak evidence of effective stroke prevention in AF [8] and has bleeding risks similar to those of the NOACs [9]. The guidelines also recommend antiplatelet therapy, such as acetylsalicylic acid (referred to as aspirin), only for those patients who refuse to take or cannot tolerate any form of OAC [9].
Key evidence underlying these recommendations includes a large multinational randomised study of the NOAC apixaban (an orally active inhibitor of coagulation factor Xa)—the AVERROES (Apixaban [5 mg twice daily] Versus Acetylsalicylic Acid [81–324 mg] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) trial [10]. This showed that, compared with aspirin, apixaban significantly reduced the risk of stroke or systemic embolism with no statistically significant difference in the incidence of major bleeding [10], making it an attractive alternative when VKA therapy is unsuitable for patients.
While the AVERROES study and ESC guidelines offer straightforward therapeutic messages regarding care for AF in Belgium, the health economic consequences of using apixaban in this setting are much less clear. Previous cost-effectiveness studies have demonstrated that the drug is a dominant treatment option or cost-effective treatment option to aspirin in the USA and UK, respectively [11, 12] but this evidence cannot be assumed to hold for other countries, as geographic variations in healthcare resource use and clinical practice suggest that findings may not be generalisable in this way [13]. Ultimately, assessing how closely health economic outcomes for different countries correlate with each other requires specific evaluations to be conducted for those territories.
Therefore, the primary objective of this study was to estimate the economic implications of using apixaban compared with aspirin for the prevention of stroke in the management of patients with non-valvular AF (NVAF) who decline or cannot tolerate VKA treatment in Belgium.
2 Methods
A previously developed economic model [12] was utilised for the purposes of this study, to estimate the long-term clinical outcomes for Belgian patients with AF receiving NOAC or antiplatelet therapy to prevent thromboembolic events over their lifetime. This evaluation was conducted from the perspective of the National Institute for Health and Disability Insurance (RIZIV/INAMI) in Belgium. The model was adapted by updating background mortality estimates, treatment patterns as well as resource use and cost items to reflect a Belgian setting.
2.1 Model Design
Like earlier cost-effectiveness studies in AF [14–16], this analysis used a Markov model approach. In such a model, patients are considered to be in distinct, mutually exclusive, states of health. They may remain in their current state or experience a specific event that puts them into a subsequent state, during a discrete period of time known as a model cycle. The likelihood that a patient will experience one of these outcomes during a cycle is referred to as the transition probability for that consequence. By applying the relevant transition probabilities to the cohort over a series of cycles, the model can predict how the patients would be distributed between the different health states at the end of each cycle, and the consequent costs, life-years and quality-adjusted life-years (QALYs) that would have accrued at that point.
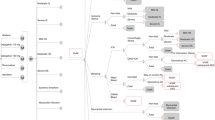
In the current analysis, the model (schematically depicted in Fig. 1) had a cycle length of 6 weeks to adequately capture the significant risk of AF-related events even within this short timeframe. Patients start in the NVAF state and can transition to the specific health states based on their risks of experiencing the following events: ischaemic or unspecified stroke (referred to as ‘ischaemic stroke’); myocardial infarction (MI); systemic embolism; intracranial haemorrhage (ICH) including haemorrhagic strokes; other major bleeds; clinically relevant non-major bleeds (CRNMBs); or death. The transition probability for haemorrhagic strokes was derived by assuming that cases of this condition accounted for a proportion of all cases of ICH, with the remaining cases being regarded as ‘other ICHs’. Major bleeds other than ICHs are referred to as ‘other major bleeds’.
Schematic representation of the Markov model. All patients remain in the ‘NVAF’ state until one of stroke, bleed, systemic embolism, MI, treatment discontinuation or death occurs. The transition probabilities of these events occurring depend on the treatment. For patients on second-line aspirin, ‘NVAF subsequent ASA’, the events are identical; however, patients cannot experience any further discontinuation. Triangles indicate which health state the patient enters after an event. Health states coloured in grey are absorbing health states. AC anticoagulant, ASA aspirin, CRNM clinically relevant non-major, HS haemorrhagic stroke, ICH intracranial haemorrhage, IS ischaemic stroke, M represents a Markov process with 11 health states that are identical for each of the two treatment options, NVAF non-valvular atrial fibrillation, Tmt treatment
Patients experiencing non-fatal ischaemic or non-fatal haemorrhagic stroke are classified into one of three categories of severity based on the score on the modified Rankin Scale (mRS)—mild (mRS: 0–2), moderate (mRS: 3–4) and severe (mRS: 5)—and are subjected to the risk of one recurrent stroke event in subsequent model cycles. Those experiencing a recurrent stroke are transitioned to the most severe health state between primary and recurrent strokes and remain there until death, i.e. if a patient in the mild stroke health state experiences a severe recurrent stroke the patient would transition to the severe stroke health state after the recurrent event. Only one recurrent event is modelled. Patients experiencing MI or systemic embolism are not subjected to the risk of any further events and remain in that health state until they die. As stroke, MI or systemic embolism are modelled as semi-absorbing health states, patients experiencing these events are no longer of a risk of experiencing another other event, i.e. a patient who experiences a stroke cannot experience an MI.
Patients are also subjected to the risk of discontinuing their first-line treatment, and if this happens, they transition to the ‘NVAF with subsequent aspirin treatment’ state, in which their risks of events in the following cycle are updated to those of their second-line therapy. Patients can also transition to this state upon treatment discontinuation resulting from other ICH or other major bleeds. That is, after other ICH, patients can either stay on current treatment or discontinue, and after other major bleeds patients can have a 6-week treatment interruption or discontinue to second-line treatment.
In addition to the above-mentioned events, cardiovascular hospitalizations are modelled in the background, i.e. patients in the NVAF health states are exposed to the risk of cardiovascular hospitalization and on occurrence are applied a cost and utility decrement. Patients experiencing a cardiovascular hospitalization do not transition to any health state but remain in the NVAF health state.
The model iterates through multiple cycles until all patients in the cohort end up in the death state.
2.2 Population
The model considers patients with AF who are known or expected to be unsuitable for VKA, and are treated with aspirin. Patient characteristics were matched to those of participants in the AVERROES trial (Table 1) [10]. Of note, the proportion of patients in this trial with a high baseline risk for stroke (CHADS2 ≥2) was similar to that in a volunteer screening study in Belgium (64 vs. 58 %) [1].
2.3 Risk of Clinical Events
Key inputs for the model included the risks of clinical events associated with apixaban and aspirin for patients for whom VKA therapy is unsuitable. These values were obtained by secondary analysis of the AVERROES trial [10], as detailed in the earlier publication of this model [12]. Table 1 presents the absolute risks, severity and case-fatality rates of events by population and treatment. The increase of the risk of stroke and bleeding over time was presented by increasing the rates of these events progressively from cycle to cycle per decade of life, an approach similar to those used in other models [16, 17]. In the base case, stroke risks were increased by a factor of 1.46 and bleeding risks by 1.97 per decade [18, 19]. The risk of recurrent stroke, which was not dependent on treatment, was based on a 10-year cumulative recurrence risk, as presented in a 2009 population-based study, and is reported in the Electronic Supplementary Material, Appendix A [20].
2.4 Second-Line Treatment
Upon the occurrence of stroke or systemic embolism, patients treated with apixaban were assumed to continue on their initial treatment whilst patients treated with aspirin or second-line aspirin were assumed to switch to VKA treatment. This assumption is based on discussions with two national experts, advising that despite unsuitability, patients experiencing stroke whilst on aspirin treatment would require anticoagulation with higher stroke prevention power. As this would only impact costs and would have no impact on subsequent transitions, VKA treatment was considered a conservative assumption due to the lower associated costs. Patients in whom first-line treatment with apixaban was discontinued owing to bleeding events (e.g. other ICH, other major bleeds) or reasons unrelated to the events modelled were assumed to be subsequently treated with aspirin. Patients in whom first-line treatment with aspirin was discontinued were assumed to be restarted on aspirin (second-line), to ensure a fair comparison between apixaban and aspirin: the alternative of starting such patients on second-line treatments other than aspirin would obscure the outcomes of first-line treatments. The estimated proportions of patients who switch treatment after temporary bleeding events came from six clinical experts surveyed in an advisory board (2012) in Belgium. Based on this consultation, it was assumed that, after the occurrence of other ICHs, 20 % of patients would have a temporary interruption of treatment for a period of a cycle (i.e. 6 weeks), while the remaining 80 % would be switched to aspirin therapy. It was also assumed that among people on first-line apixaban who experienced other major bleeds, 50 % would then be switched to treatment with aspirin, while the rest would continue on apixaban. Clinical event rates for second-line aspirin (Table 1) were based on a subgroup of patients in the AVERROES trial who were previously prescribed but failed to continue on VKA and, hence, were treated with aspirin.
2.5 Mortality
Mortality was modelled as being either a direct consequence of events related to AF or its treatment, or due to other causes. Patients in the NVAF health state were initially exposed to a background risk of death based on the pattern of all-cause mortality observed in the AVERROES trial having excluded deaths attributable to stroke, bleeding, MI and systemic embolism within a period equivalent to the duration of the trial (i.e. 1.12 years). Beyond this initial trial period, background mortality was estimated using a Gompertz function that had been fitted to general mortality rates from the Belgian life tables [21] and a hazard ratio (HR) of death that reflected the increased mortality in patients with AF, attributable to causes other than those modelled, compared with the general population [22].
Case-fatality rates and the long-term HR of mortality versus the general population were applied to patients experiencing ischaemic stroke, haemorrhagic stroke, other ICHs, other major bleeds, systemic embolism and MI as detailed in the earlier publication.
2.6 Utilities
Quality-adjusted life expectancy was estimated as the product of the time spent in each health state and the corresponding utility for that state (where a utility of 1 denotes full health and 0 denotes death). Utilities were obtained from a UK-based utility catalogue [23], as detailed in Table 2.
2.7 Costs
Direct healthcare costs, at 2012 prices, were applied in the model. A daily price of €2.53 (5 mg twice daily) was considered for apixaban and €0.09 for aspirin [24]. The average daily dose of aspirin in the AVERROES trial was close to 150 mg; thus, the costs of two tablets of Asaflow® 80 mg were considered. Also applied in the model were routine care costs for disease monitoring [excluding international normalized ratio (INR) monitoring], and these were estimated to be €91 annually,Footnote 1 by using the RIZIV/INAMI tariffs [24] corresponding to four annual general practitioner (GP) visits—a consultation rate indicated by seven Belgian experts during an advisory board conducted in 2012.
The costs of care related to acute events were based on the average amount reimbursed by the public payer to the Belgian hospitals by All Patients Refined Diagnosis Related Group (APR-DRG) [25], and are detailed in Table 3. Costs of fatal strokes were calculated by assuming that the relative ratio of the costs of fatal strokes to the mean costs of stroke was equal to the relative ratio of costs of fatal MI to the mean costs of MI [16, 26]. Long-term maintenance costs of stroke and MI were obtained from published estimates [27].
For patients switching to VKA treatment due to a stroke event, a daily treatment price of €0.28 was applied and costs of routine care and INR monitoring were estimated to be €609 annually [3]. The daily treatment price reflected an average of the costs for various VKA treatments weighted according to their market share (IMS data, MAT 1Q2013). Monitoring costs with VKA treatment were calculated using unit costs per monitoring visit obtained from RIZIV/INAMI tariffs [24] and resource use from a recent health technology assessment [3], corresponding to an average of 17.6 annual GP visits. The face validity, technical validity and outcomes validity of the model was examined prior to adaptation [12].
2.8 Analyses
The analyses compared apixaban with aspirin among patients with NVAF who declined or were unable to tolerate VKA treatment. Event counts were predicted for a cohort of 1,000 such patients over their lifetime. The model also calculated life-years, QALYs, costs and incremental cost-effectiveness ratios (ICERs), in terms of cost per QALY gained, per average patient. Health outcomes and costs were discounted at 1.5 and 3.0 % per annum, respectively [28].
The primary analysis, known as the base case, involved using the model inputs and assumptions described above. Additionally, a one-way sensitivity analysis was performed in order to capture the effect of varying several model inputs of interest, such as the risk of ischaemic and unspecified stroke for aspirin. Furthermore, scenario analyses were also conducted to test the robustness of the base-case results. This included exploring the effect of changes in various model parameters, including the following: (1) initial CHADS2 scores of patients entering the model (i.e. for different subgroups classified by this score, and using estimated CHADS2 scores specific for the Belgian population); (2) discount rates (i.e. 0 and 5 % for both health and cost outcomes, as recommended in Belgian guidelines for pharmacoeconomic analysis) [28]; (3) assumptions around treatment discontinuation; (4) second-line treatment set to no treatment (i.e. instead of aspirin); and (5) assuming that apixaban provides no mortality benefit beyond that attributable to the events modelled, i.e. use of the same background mortality rates for patients treated with apixaban and aspirin.
In addition to these analyses using predetermined input values (so-called deterministic analyses), probabilistic sensitivity analysis was conducted to assess how the uncertainty around the input values affects the model’s prediction of the cost effectiveness of apixaban compared with aspirin. This involved running 2,000 iterations of the model while simultaneously varying key inputs for each iteration, by randomly selecting values from probability distributions of these inputs. Details on distributions used for each input in these sensitivity analyses are given in the Electronic Supplementary Material, Appendix A.
The results of the probabilistic sensitivity analysis were used to produce a scatter plot of the additional gain in QALYs versus the additional cost for apixaban compared with aspirin (i.e. the ICER for each simulation)—a graphical presentation known as the cost-effectiveness plane. The results were also used to generate a cost-effectiveness acceptability curve (CEAC), in which the x-axis represented threshold values of the ICER (i.e. the amount a decision maker would be willing to pay for an additional QALY) and the y-axis represented the proportion of simulations for which the ICER was below a given threshold. The CEAC therefore indicated the probability that apixaban would be considered cost effective at different levels of willingness to pay for the treatment advantage over aspirin.
3 Results
3.1 Deterministic Analyses
The model predicted that 1,000 patients treated with apixaban rather than aspirin would collectively experience fewer strokes (281 vs. 339 first and recurrent ischaemic strokes and 21 vs. 19 first and recurrent haemorrhagic episodes) and fewer systemic embolisms (24 vs. 35) over a lifetime horizon. However, they would have additional other ICHs (16 vs. 15), major bleeds (121 vs. 87), CRNMBs (324 vs. 258), MIs (94 vs. 92), treatment discontinuations (686 vs. 664) and cardiovascular hospitalisations (1,163 vs. 1,126).
The predicted increase in bleeding events was offset by a reduction in stroke and systemic embolism events that translated into gains of 0.42 life-years and 0.32 QALYs, and a reduction of €889 in all event-related costs, as detailed in Table 4. Similarly, monitoring and routine care-related costs were reduced by €245 because there were fewer strokes and, therefore, fewer patients switching to VKA treatment. The higher drug acquisition costs for apixaban resulted in an incremental total cost of €2,311 per patient treated with the drug. The ICER of apixaban relative to aspirin was estimated to be €7,334 per QALY gained, thus demonstrating apixaban to be a cost-effective alternative to aspirin when considering a threshold of €30,000 per QALY gained.
3.1.1 Sensitivity Analysis
Figure 2 depicts the deterministic sensitivity analysis results and, more specifically, the top ten parameters that had the most impact on the ICERs. The ICERs from all scenarios varied from €3,760 to €14,082 per QALY.
One-way sensitivity analysis of apixaban versus aspirin. The vertical line appearing in the middle of the graphs represents the base-case ICER for apixaban versus aspirin and the horizontal bars represent the ICER ranges for each scenario that is varied. ICER incremental cost-effectiveness ratio, MI myocardial infarction, QALY quality-adjusted life-year, PY person-year
3.1.2 Scenario Analyses
The results from the scenario analyses are detailed in Table 4. The ICERs for comparisons of apixaban with aspirin from all scenarios varied between €3,625 and €16,829, with the most influential parameters being the distribution of patients amongst different levels of stroke risk as determined by CHADS2 scores.
3.2 Probabilistic Analysis
Results from the probabilistic sensitivity analysis are detailed in Fig. 3. The cost-effectiveness plane for apixaban versus aspirin shows a cluster around the north-east quadrant, suggesting that apixaban was both more effective and more costly than aspirin. Using a threshold of €30,000 per QALY gained, apixaban was a cost-effective alternative to aspirin in 97 % of the iterations. Results from the CEAC suggest that apixaban offers a greater net benefit, at a willingness-to-pay threshold above €7,500 per QALY, in patients for whom VKA therapy is unsuitable.
Results of the probabilistic sensitivity analysis for apixaban versus aspirin. a Scatter plot representing incremental costs and QALYs for apixaban versus aspirin. The line represents a cost-effectiveness threshold representing the maximum amount society is willing to pay for a QALY gain (i.e. €30,000). Apixaban is a cost-effective alternative in cases that fall to the right of this line; apixaban is not a cost-effective alternative in cases that fall to left of this line. b Cost-effectiveness acceptability curve for apixaban and aspirin. QALY quality-adjusted life-year
4 Discussion
This study translated the health benefits observed in the AVERROES trial into predicted long-term health and economic outcomes among patients with AF for whom VKA therapy is unsuitable, from the perspective of healthcare payers in Belgium. Specifically, the analysis showed that the reduction in clinical events with the use of apixaban compared with aspirin led to an increase in life-years and QALYs. Although the use of apixaban over a lifetime increased drug acquisition costs due to a longer expected life span and lower treatment discontinuation rates, most of this additional expenditure was offset by a reduction in the avoided event-related costs. The resulting ICER was €7,334 per QALY, indicating that the added benefits from treatment with apixaban can be achieved at reasonable additional cost, and is cost effective in comparison with aspirin for stroke prevention assuming healthcare decision makers have a willingness-to-pay threshold of €30,000 per QALY gained.
In comparison with aspirin, the lifetime model simulates that patients treated with apixaban experienced somewhat more bleeds, MIs, cardiovascular hospitalisations and treatment discontinuations. However, compared with aspirin, apixaban carries a slightly lower risk of ICH (0.344 vs. 0.348 per 100 patient-years) and patients treated with apixaban are projected to live longer; thus, the number of predicted ICHs was slightly higher (16 vs. 15 in a cohort of 1,000 patients) due to longer exposure to risk. Subsequently, those taking apixaban are also more likely to experience bleeding that necessitates switching to second-line aspirin: this treatment change exposes them to a higher risk of MI overall, even though apixaban carries a lower risk of MI. The higher number of cardiovascular hospitalisation events with apixaban can be attributed partly to the longer life expectancy associated with use of the drug (and, therefore, the longer exposure to risk). It may also reflect that, owing to treatment discontinuations, more patients in the apixaban group are potentially exposed to second-line aspirin, which is associated with a higher rate of cardiovascular hospitalisations than first-line treatment with either apixaban or aspirin, as detailed in Table 1.
In sensitivity testing, the predicted ICER was lower than for the base case in a scenario that incorporated CHADS2 scores derived from a subgroup of patients in Belgium with AF and hypertension (i.e. around 50 % of the total AF population [1]). Therefore, the base-case results can be regarded as conservative. Scenario analysis also demonstrated that even in patients with a very low risk of stroke, apixaban was still a cost-effective alternative to aspirin, with an ICER of €16,829.
Overall, our model was comparable to earlier versions that have evaluated the cost effectiveness of treatment options for prevention of thromboembolic events in patients with AF, by taking account of the risks of stroke and bleeding events as well as the influence of these outcomes on treatment discontinuation [11, 14–16, 29]. However, distinguishing strengths of our model include its detailed design for considering the severity of strokes and bleeding events and mimicking the use of anticoagulants under real-world conditions. In addition, its projections with regards to the burden caused by thromboembolic events over a lifetime are more advanced than in earlier models adapted to the Belgian setting [30], as a result of the inclusion of long-term maintenance costs for patients with stroke, systemic embolism and MI. Our model did not include TIAs as these were not reported as outcomes in the AVERROES trial. However, a costing component in relation to TIAs was captured through inclusion of cardiovascular hospitalisations. Earlier anticoagulation models used background mortality life tables to estimate survival of patients with AF [14–16, 29]. By contrast, our study included an HR of 1.34 to adjust for mortality in the NVAF health state, to take into account the increased mortality in patients with AF beyond that related to the modelled events [22, 31]. If, instead, we had used general mortality for patients in the NVAF health state, like the earlier models, scenario analysis suggested that the generated results would be expected to favour apixaban because of this drug’s higher incremental gains in QALYs compared with aspirin. Similarly, the estimates in our evaluation represent improvements over previous studies because of the inclusion of treatment-specific other-cause mortality rates during the initial 1.1 years of time elapsed in the model to reflect the mortality benefit that occurred over the same duration in the AVERROES trial, beyond what was attributable to the reduction in the modelled clinical events. Finally, the analysis used conservative approaches in modelling the effects of apixaban, by assuming that continuing the use of OACs after a first stroke offered no protection against recurrence of such an event.
Only limited trial data were available from trials on the efficacy of the modelled treatments in preventing recurrent strokes. Consequently, model inputs for rates of stroke recurrence were derived from published UK population estimates [20]. These rates were in the lower range of such values compared with those used in other studies [32], and so resulted in conservative estimation of the number of recurrent strokes, which was likely to favour aspirin with poorer efficacy in preventing these outcomes. Of note, a recent study on patients with prior TIA or stroke enrolled in the AVERROES trial found that the benefits of apixaban in reducing stroke and systemic embolism events in such individuals were consistent with, and possibly greater than, those seen in patients without a prior stroke or TIA [33], indicating that use of treatment-specific recurrence rates in the model would benefit apixaban.
Our analysis had several limitations. Firstly, clinical event rates were derived from the AVERROES trial, and so might not reflect efficacy of apixaban under real-world conditions. Secondly, no Belgian-specific utilities were identified. Instead, in keeping with Belgian guidelines encouraging consistency between the methodology used to derive utilities for all health states, particularly the EQ-5D, utilities for our analysis were based on a UK EQ-5D catalogue [23], on the assumption that they would be similar to those specifically for a Belgian population. Thirdly, CHADS2 risk scores in the model were not updated over time. An alternative way of reflecting the increase in the risks of stroke and bleeding over time was adopted, as mentioned in the Methods section. Fourthly, in line with the AVERROES trial, an average daily dose of aspirin 160 mg was considered, which might be higher than the daily dose in current Belgian practice. The impact on the acquisition cost of aspirin was, however, minimal. Finally, the number of treatment lines allowed in the model was limited to two to avoid overcomplicating the model and also due to the unavailability of data on the efficacy of these treatments when being used as the third or subsequent treatment.
5 Conclusion
Overall, our model indicates that apixaban has demonstrated an advantage over aspirin with regards to the prevention of stroke events and gain in QALYs among patients in Belgium with AF who decline or cannot tolerate VKA treatment. Also, these added benefits appear to be achieved at a reasonable additional cost. We therefore conclude that apixaban is a cost-effective alternative to aspirin in this setting.
Notes
This cost was set to 0 for patients switching to VKA treatment to avoid double counting with INR monitoring costs.
References
Claes N, Van Laethem C, Goethals M, Goethals P, Mairesse G, Schwagten B, et al. Prevalence of atrial fibrillation in adults participating in a large-scale voluntary screening programme in Belgium. Acta Cardiol. 2012;67(3):273–8.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147(9):1561–4.
KCE. Utilisation des coagulomètres portables chez les patients sous anticoagulants oraux: Health Technology Assessment [database on the Internet]. 2009. https://kce.fgov.be/sites/default/files/page_documents/d20091027348.pdf. Accessed 11 Aug 2012.
Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638–45, e4.
De Breucker S, Herzog G, Pepersack T. Could geriatric characteristics explain the under-prescription of anticoagulation therapy for older patients admitted with atrial fibrillation? A retrospective observational study. Drugs Aging. 2010;27(10):807–13.
Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26(22):2422–34.
Thijs V, Van der Niepen P, Van de Borne P, et al. Oral anticoagulant use, atrial fibrillation and CHADS2 score in primary care patients with hypertension: the Belgica-Stroke Study [abstract and poster no. 967]. XIX European Stroke Conference; 25–28 May 2010; Barcelona.
Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–67.
Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719–47.
Connolly S, Eikelboom J, Joyner C, Diener H, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17.
Lee S, Anglade MW, Meng J, Hagstrom K, Kluger J, Coleman CI. Cost-effectiveness of apixaban compared with aspirin for stroke prevention in atrial fibrillation among patients unsuitable for warfarin. Circ Cardiovasc Qual Outcomes. 2012;5(4):472–9.
Dorian P, Kongnakorn T, Phatak H, Rublee DA, Kuznik A, Lanitis T, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J. 2014;35(28):1897–906.
Drummond M, Barbieri M, Cook J, Glick HA, Lis J, Malik F, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12(4):409–18.
Sorensen SV, Dewilde S, Singer DE, Goldhaber SZ, Monz BU, Plumb JM. Cost-effectiveness of warfarin: trial versus “real-world” stroke prevention in atrial fibrillation. Am Heart J. 2009;157(6):1064–73.
Lee S, Anglade MW, Pham D, Pisacane R, Kluger J, Coleman CI. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110(6):845–51.
Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation. 2011;123(22):2562–70.
Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1–11.
Ariesen M, Claus S, Rinkel G, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–5.
Pisters R, Lane DA, Marin F, Camm AJ, Lip GY. Stroke and thromboembolism in atrial fibrillation. Circ J. 2012;76(10):2289–304.
Mohan KM, Crichton SL, Grieve AP, Rudd AG, Wolfe CD, Heuschmann PU. Frequency and predictors for the risk of stroke recurrence up to 10 years after stroke: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2009;80(9):1012–8.
Human Mortality Database, 2009 Belgian life tables. http://www.mortality.org/. Accessed 12 May 2012.
Friberg L, Hammar N, Pettersson H, Rosenqvist M. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort-Study of Atrial Fibrillation (SCAF). Eur Heart J. 2007;28(19):2346–53.
Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011;31(6):800–4.
National Institute for Health and Disability Insurance (RIZIV/INAMI) [database on the Internet]. http://www.inami.fgov.be. Accessed 23 Aug 2012.
National Databank Medische Diagnose 2009 [database on the Internet]. https://tct.fgov.be/webetct/etct-web/. Accessed 11 Aug 2012.
Lamotte M, Annemans L, Kawalec P, Zoellner Y. A multi-country health economic evaluation of highly concentrated N-3 polyunsaturated fatty acids in secondary prevention after myocardial infarction. Pharmacoeconomics. 2006;24(8):783–95.
Annemans LLM, Levy E, et al. Cost effectiveness analysis of clopidogrel versus aspirin in patients with atherothrombosis based on the CAPRIE trial. J Med Econ. 2003;6:55–68.
Centre fédéral d’expertise des soins de santé (2008). Recommandations pour les évaluations pharmacoéconomiques en Belgique. KCE reports 78B. http://kce.fgov.be/sites/default/files/page_documents/d20081027324.pdf. Accessed 11 Aug 2012.
Lafata JE, Martin SA, Kaatz S, Ward RE. The cost-effectiveness of different management strategies for patients on chronic warfarin therapy. J Gen Intern Med. 2000;15(1):31–7.
Wouters H, Thijs V, Annemans L. Cost-effectiveness of dabigatran etexilate in the prevention of stroke and systemic embolism in patients with atrial fibrillation in Belgium. J Med Econ. 2013;16(3):407–14.
Boggon R, Lip GY, Gallagher AM, van Staa TP. Resource utilization and outcomes in patients with atrial fibrillation: a case control study. Appl Health Econ Health Policy. 2012;10(4):249–59.
Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42(5):1489–94.
Diener HC, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GY, et al. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol. 2012;11(3):225–31.
Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156(16):1829–36.
Acknowledgments
This study was funded by Pfizer and Bristol Myers Squibb.
We acknowledge the following physicians for their inputs on treatment pathways in Belgian settings (Advisory Board on 19 June 2012): Profs. H. Heidbuchel and V. Thijs (UZ Gasthuisberg, Leuven), Dr. G. Mairesse (Clinique Sud Luxembourg, Arlon), Dr. M. Goethals (HH Roeselare, Roeselare), Dr. A. Peeters (UCL—St-Luc, Brussels), and Dr. L. Pierard (ULg—Sart Tilman, Liège).
The authors would like to thank Koo Wilson from Pfizer for her contribution in model development and preparation of the manuscript as well as David Jakouloff, ex-employee from Bristol Myers Squibb, for his contribution in model development, and Ike Iheanacho from Evidera for editorial assistance which was funded by Pfizer and Bristol Myers Squibb.
Disclosures
Tereza Lanitis and Thitima Kongnakorn are employees of Evidera and were paid consultants to Pfizer in connection with the development of this manuscript and of the model.
Profs. Lieven Annemans and Vincent Thijs received an honorarium from Pfizer for advice in connection with the inputs of the health economic model.
Sophie Marbaix is an employee of Pfizer.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kongnakorn, T., Lanitis, T., Annemans, L. et al. Cost Effectiveness of Apixaban Versus Aspirin for Stroke Prevention in Patients with Non-Valvular Atrial Fibrillation in Belgium. Clin Drug Investig 34, 709–721 (2014). https://doi.org/10.1007/s40261-014-0224-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0224-z