Abstract
Background
Apabetalone is a selective bromodomain and extra-terminal (BET) inhibitor which modulates lipid and inflammatory pathways implicated in atherosclerosis. The impact of apabetalone on attenuated coronary atherosclerotic plaque (AP), a measure of vulnerability, is unknown.
Methods
The ApoA-1 Synthesis Stimulation and intravascular Ultrasound for coronary atheroma Regression Evaluation (ASSURE; NCT01067820) study employed serial intravascular ultrasound (IVUS) measures of coronary atheroma in 281 patients treated with apabetalone or placebo for 26 weeks. AP was measured at baseline and follow-up. Factors associated with changes in AP were investigated.
Results
AP was observed in 31 patients (11%) [27 (13.0%) in the apabetalone group and four (5.5%) in the placebo group]. The apabetalone group demonstrated reductions in AP length by − 1 mm [interquartile range (IQR) − 4, 1] (p = 0.03), AP arc by − 37.0° (IQR − 59.2, 8.2) (p = 0.003) and the AP index by − 34.6 mm° (IQR − 52.6, 10.1) (p = 0.003) from baseline. The change in AP index correlated with on-treatment concentration of high-density lipoprotein (HDL) particles (r = − 0.52, p = 0.006), but not HDL cholesterol (r = − 0.11, p = 0.60) or apolipoprotein A-1 (r = − 0.16, p = 0.43). Multivariable analysis revealed that on-treatment concentrations of HDL particles (p = 0.03) and very low-density lipoprotein particles (p = 0.01) were independently associated with changes in AP index.
Conclusions
Apabetalone favorably modulated ultrasonic measures of plaque vulnerability in the population studied, which may relate to an increase in HDL particle concentrations. The clinical implications are currently being investigated in the phase 3 major adverse cardiac event outcomes trial BETonMACE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our findings indicate that apabetalone has a potential effect on vulnerable atherosclerotic plaque in the coronary artery. |
Reduced high-density lipoprotein (HDL) particles can be superior to HDL cholesterol and apolipoprotein A-1 as a marker of progression of high-risk plaque. |
In addition, triglyceride-rich lipoprotein may be another important factor to predict the development of attenuated plaque. |
1 Introduction
There remains a considerable residual risk of cardiovascular events despite widespread use of established medical therapies. Raising high-density lipoprotein (HDL) cholesterol levels or activity have become potential therapeutic targets. Induction of endogenous synthesis of apolipoprotein A-1 (apoA-1), the major protein carried on HDL particles, represents an attractive approach to lipid modification. A bromodomain and extra-terminal (BET) inhibitor, apabetalone, by transcriptional regulation increases endogenous synthesis of apoA-1, with early studies demonstrating favorable effects on HDL quantitative measures (HDL cholesterol, apoA-1, total and large HDL particles) [1], systemic cholesterol efflux capacity [2] and inflammatory factors implicated in atherosclerosis and plaque stability [3].
Attenuated coronary atherosclerotic plaque (AP) acquired from gray-scale intravascular ultrasound (IVUS) has been proposed to identify vulnerable plaque, containing necrotic and lipid components that underscore destabilization of lesions and adverse cardiovascular events [4]. In a previous observational IVUS study examining 120 patients with coronary artery disease, lower HDL cholesterol levels were associated with a higher incidence of AP, which raises the concern that HDL-cholesterol–raising therapy may have favorable effects on atherosclerotic plaque instability in the coronary artery. Therefore, we hypothesized that apabetalone may modulate atherosclerotic plaque composition, especially IVUS-derived vulnerable plaque characteristics of AP. The ApoA-1 Synthesis Stimulation and Intravascular Ultrasound for Coronary Atheroma Regression Evaluation (ASSURE; NCT01067820) study evaluated the effect of apabetalone on atherosclerotic plaque burden in patients with coronary artery disease and demonstrated a trend to reduce atherosclerotic coronary plaque, which paralleled observations in placebo-treated patients. In this analysis, we investigated the prevalence of AP in the ASSURE study cohort and the potential impact of apabetalone and its biochemical effects on AP with serial imaging.
2 Methods
2.1 Study Design
The ASSURE study design has been previously described [5]. Briefly, ASSURE was a prospective, randomized, multicenter, double-blind clinical trial. Patients with angiographic coronary artery disease and low HDL cholesterol level (< 45 mg/dl in females and < 40 mg/dl in males) were randomized to treatment for 26 weeks with apabetalone 100 mg or placebo administered twice daily. Subjects received treatment with either atorvastatin 10–40 mg daily or rosuvastatin 5–20 mg daily during the study.
2.2 Acquisition and Analysis of Intravascular Coronary Imaging
The presence of at least one epicardial coronary artery stenosis > 20% was required for study entry. Baseline IVUS was performed in a single, native coronary artery with no lumen stenosis > 50% and that had not undergone revascularization. Imaging was performed using either the s5™ (Volcano, Sacramento, CA, USA) or iLab™ (Boston Scientific, Boston, MA, USA) systems and screened by the core laboratory at C5Research. Patients meeting prespecified requirements for image quality were eligible for randomization. Following 26 weeks of treatment, patients underwent a second IVUS within the same artery. Anatomically matched arterial segments were selected for analysis on the basis of proximal and distal fiduciary points. The measurements of the lumen and external elastic membrane (EEM) in images within a matched artery segment were performed. Plaque was determined as the area between leading edges. At a vessel level, plaque burden was calculated as percent atheroma volume (PAV) and total atheroma volume (TAV), as previously described [5]. Changes in PAV, TAV, lumen and EEM volumes were calculated from measurements at 26 weeks minus the corresponding volume at baseline. Plaque regression was defined as any decrease in plaque burden from baseline.
Plaque attenuation was defined as a hypoechoic area with deep ultrasonic attenuation despite the absence of bright calcium. Cross-sectional images having plaque attenuation were identified within the entire imaged segment (Fig. 1), and then the arc of plaque attenuation was measured in degrees with a protractor centered on the lumen at every 0.1-mm interval. The length of plaque attenuation was measured on a longitudinal view. Attenuated plaque index (API) was defined as the mean arc of plaque attenuation multiplied by its length and subsequently normalized to account for difference in imaged segment length between subjects as follows:
Baseline and follow-up attenuation index were evaluated, and serial changes in these IVUS measures were compared to baseline values between treatment groups.
2.3 Biochemical Measures
All biochemical determinations were performed at a central laboratory (ACM, Rochester, NY, USA, and York, UK). Lipid profiles were determined by enzymatic assay. Levels of apoA-1 were determined by turbidimetric immunoassay (Boston Heart Diagnostics, Framingham, MA, USA). Measurements of lipoprotein particle number and size were performed by nuclear magnetic resonance (LipoScience, Raleigh, NC, USA) as previously described [6, 7]. Particle concentrations of lipoprotein subclasses of different sizes were calculated from the measured amplitudes of their spectroscopically distinct lipid methyl signals. Lipoprotein levels and safety laboratory measurements were obtained and any adverse reactions recorded at each study visit.
2.4 Statistical Analysis
Data analyses were performed using Stata 14.2 (Stata Corp, USA). Categorical data were expressed as frequency and percentages. Continuous data were presented as median and interquartile ranges (IQRs). Baseline patient characteristics and lipid and IVUS parameters were compared between AP-positive and AP-negative groups using Mann–Whitney test; changes in lipid parameters, C-reactive protein (CRP), and IVUS and AP characteristics were examined using Wilcoxon’s matched-pairs signed rank test. The correlations between lipid parameters and the inflammatory biomarker (CRP) and PAV and API were examined using the Spearman’s correlation test. The change in API was modeled using the multiple linear regression model, adjusting for the baseline API as a priori. Baseline, follow-up and change in lipid parameters [total cholesterol; HDL cholesterol; low-density lipoprotein (LDL) cholesterol; triglycerides; apoA-1; apoB; total HDL particles; large, medium and small HDL particles; HDL size; total LDL particles; large and small LDL particles; LDL particle size; intermediate density lipoprotein (IDL) particles; non-HDL cholesterol; very low-density lipoprotein (VLDL) and chylomicron triglyceride; VLDL and chylomicron particles; large VLDL and chylomicron particles; medium and small VLDL particles; and VLDL particle size], biochemistry parameters [alkaline phosphatase (ALP), creatine kinase, creatinine, CRP], baseline and follow-up IVUS parameters (PAV, normalized TAV), sex, age, weight, medical condition (hypertension and diabetes) and medication [angiotensin converting enzyme (ACE) inhibitor and beta-blocker] at the baseline were examined for inclusion in the final model. A predictor was included in the final model if it had significant effect on the change in API at a 5% level. If the follow-up measurement of a variable had significant effect, we also included the baseline measurement of the variable in the final model. A linear regression model was used for all subgroup analysis, where we divided data by median age, sex, median body mass index (BMI), medical condition (hypertension or diabetes), estimated glomerular filtration rate (eGFR) ≥ 60 ml/min/1.73 m2, statin type (atorvastatin vs. rosuvastatin), ALP using clinical cut-off (100 U/l), and median ALP. All tests were two-tailed and assessed at the 5% alpha level.
3 Results
3.1 Baseline Characteristics According to AP
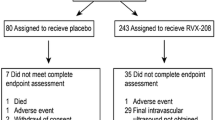
The disposition of patients in the study is summarized in Fig. 2. Six hundred seventy-six patients were screened, with 243 patients randomized to the apabetalone group and 80 to the placebo group. After 26 weeks of treatment, 281 patients (87.0%) remained in the study and underwent gray-scale IVUS imaging at baseline and follow-up. At baseline, 27 patients (11.1%) had evidence of attenuated plaque in the apabetalone group at baseline, whereas four patients (5.0%) had it in the placebo group at baseline. Table 1 summarizes patient characteristics, stratified according to the presence of attenuated plaque in the apabetalone group, with no statistically significant differences observed. Table 2 summarizes baseline lipid parameters, CRP, and IVUS parameters according to the presence of attenuated plaque in the apabetalone group, with no statistically significant differences observed. The attenuated plaque group had a greater PAV [43.8% (IQR 38.2, 49.2) vs. 37.5% (IQR 32.6, 43.7), p = 0.007] and TAV [245.0 mm3 (IQR 215.4, 273.7) vs. 193.6 mm3 (IQR 143.0, 257.2), p = 0.005].
3.2 Biochemical and IVUS Measurements in Patients with Attenuated Plaque in the Apabetalone Group
Table 3 presents baseline, follow-up levels and changes in lipid parameters and CRP in the apabetalone groups (n = 27). Apabetalone treatment was associated with a decrease in LDL cholesterol [− 11 mg/dl (IQR − 27, 4), p = 0.02] and CRP [− 1.8 mg/l (IQR − 4.1, 0), p < 0.001] and an increase in HDL cholesterol [8 mg/dl (IQR 4, 11), p < 0.001], apoA-1 [16.2 mg/dl (IQR 10.5, 27), p < 0.001], total HDL particles [4.7 mol/l (IQR 1.2, 6.7), p < 0.001], small HDL particles [2.6 mol/l (IQR − 9, 5.5), p = 0.01] and large HDL particles [1.5 mol/l (IQR 0, 2.3), p < 0.001]. Table 4 presents IVUS measurements of plaque burden and attenuated plaque characteristics in the apabetalone group. PAV was increased significantly compared to baseline [1.1% (IQR − 0.3, 1.9), p = 0.03]; however, atheroma volume throughout the whole analyzed segment [2.4 mm3 (IQR − 11.0, 17.4), p = 0.26] and in the most diseased 10-mm segment [0.1 mm3 (IQR − 4.7, 4.6), p = 0.89] did not significantly change during the course of the study. A decrease in attenuated plaque length [− 1 mm (IQR − 4, 1), p = 0.03], arc [− 37° (IQR − 59.2, 8.2), p = 0.003] and index [− 34.6 mm° (IQR − 52.6, 10.1), p = 0.003] was observed.
3.3 Relationship Between Follow-Up Lipoprotein and Inflammatory Biomarker Levels and Changes in PAV and Characteristics of AP
Table 5 describes the association between follow-up lipoprotein and inflammatory biomarker levels and changes in the PAV and API in the apabetalone group. Changes in API correlated inversely with follow-up HDL particle concentration (r = − 0.52, p = 0.006) (Fig. 3), but not HDL cholesterol (r = − 0.11, p = 0.60) nor apoA-1 levels (r = − 0.16, p = 0.43). Follow-up LDL cholesterol levels correlated significantly with changes in PAV (r = 0.44, p = 0.02), but not changes in API (r = − 0.11, p = 0.60). Multivariable analysis revealed that follow-up HDL particles (p = 0.028) and small VLDL particles (p = 0.011) were independently associated with changes in API (Table 6).
4 Discussion
The current study evaluated the impact of a BET protein inhibitor, apabetalone, which is reported to favorably modulate lipid and inflammatory mediators of plaque vulnerability, on coronary atherosclerosis. We showed that apabetalone had a potential effect to reduce features of high-risk coronary atherosclerotic plaque, as evidenced by a reduction in attenuated plaque indices. These changes in plaque composition were observed in the absence of any demonstrable reduction in plaque burden within the short treatment period of 26 weeks. Although the studied vessels showed no overall change in plaque burden, change in PAV was associated with follow-up LDL cholesterol and change in attenuated plaque was related to a larger proportion of HDL particles at follow-up, as opposed to measures of HDL cholesterol and apoA-1. The observation is consistent with preliminary studies demonstrating that reduced HDL particle number is associated with cardiovascular risk [6, 8]. Small VLDL particles also were associated with the development of attenuated plaque, suggesting that triglyceride-rich lipoproteins may play an important role in the progression of high-risk atherosclerotic plaque.
The feature of ultrasonic attenuation acquired from grey-scale IVUS has been frequently observed at culprit lesions of patients with acute coronary syndrome (ACS) and has been demonstrated to be associated with greater transient deterioration in coronary flow, larger infarct size, and higher incidence of fatal arrhythmia following percutaneous coronary intervention (PCI) in patients with ACS [9,10,11]. These findings were supported by several histological studies implicating that plaque debris retrieved from the distal portions of infarcted related arteries using distal protection device [12] and from the lesions with plaque attenuation by the directional coronary atherectomy during PCI [13] in patients with ACS consisted of necrotic core, inflammatory cell and old and fresh thrombi. Additionally, according to a recent histological study examining a large series of human coronary specimens with both cardiovascular and non-cardiovascular deaths, IVUS attenuation, especially superficial echo attenuation, was likely associated with an advanced fibroatheroma containing a large necrotic core [4]. Therefore, the IVUS feature of ultrasonic attenuation with the absence of calcification in the atherosclerotic plaque has been thought to be associated with a high risk for plaque rupture. The prevalence of plaque attenuation in the present study (9.6%) was consistent with a previous report that demonstrated the presence of plaque attenuation in non-culprit segments in patients with stable settings [14]. The overall study is modest in size; as a result, the small prevalence of plaque attenuation in the placebo group is likely to be a play of chance. Given that ultrasonic attenuation within atherosclerotic coronary plaque is more frequently observed at the lesions containing greater atheroma burden [13], baseline IVUS data in the main analysis of the ASSURE study demonstrating a lower amount of plaque volume in the placebo group compared with the treatment group (TAV 154.8 vs. 199.9 mm3, p < 0.001) [5] is thought to be partly responsible for the difference in prevalence of attenuation plaque between placebo and treatment groups in the current study.
Apabetalone acts through an epigenetic mechanism, increasing apoA-1 synthesis and increasing HDL particle concentration. The ASSURE study demonstrated a trend of plaque regression with apabetalone, which paralleled observations in placebo-treated patients [5]. A pooled analysis of phase 2 studies of apabetalone in cardiovascular disease patients demonstrated a reduction in major cardiovascular events compared with placebo-treated patients [15]. The discrepancy between apabetalone’s moderate effect on plaque volume and reduction in cardiovascular disease events suggests that apabetalone may have additional properties, including modulation of inflammatory, immunoregulatory and coagulative factors implicated in atherosclerosis [3, 17, 18]. In the current analysis, while the imaged vessels showed no change in plaque burden, all attenuated plaque features decreased in size, suggesting that apabetalone may have the potential to stabilize high-risk atherosclerotic plaque. This finding is consistent with a previous animal study that demonstrated that interventions targeting HDL had a potential effect on the histological composition of atherosclerotic plaques [19].
HDL particles have received considerable interest, as they play a central role in the promotion of reverse cholesterol transport in addition to other biological activities indicating anti-inflammatory, anti-oxidant, anti-thrombotic and anti-apoptotic activities [20, 21]. Population studies have demonstrated that HDL cholesterol levels were inversely correlated with cardiovascular risk [22] and low HDL cholesterol levels were associated with progression and vulnerability of atherosclerotic plaques [23, 24]. However, recent evidences have raised doubts with regard to the hypotheses that elevating HDL cholesterol levels is necessarily therapeutic [25,26,27]. Given that HDL has also been recognized to circulate as a heterogeneous population of particles differing in size, shape and composition, this has led to the proposal that other HDL-associated metrics might provide better assessments of the relationship between HDL metabolism and cardiovascular disease. Indeed, some evidences have demonstrated that HDL particle concentration was superior to HDL cholesterol levels as a predictor of cardiovascular disease [6, 8]. Similarly, cholesterol efflux capacity, a functional, non-quantitative measure of HDL, has emerged as a better risk marker of cardiovascular disease than HDL cholesterol levels [28]. However, whether each HDL subclass would be the source of overall HDL cardioprotection has not been fully understood. In the current study, although high total HDL particle concentration was associated with the reduction of plaque attenuation, there were no significant correlations between each HDL subclass and change in plaque attenuation. Further investigations are required to understand the relationship between HDL and atherosclerotic coronary plaque instability.
HDL particles account for a much larger fraction of the variation in cholesterol efflux capacity than HDL cholesterol levels [29], suggesting that an increase in total HDL particle number and improved cholesterol efflux could be partly responsible for the reduction in AP. In addition, the apabetalone effect on reducing BET driven transcription, resulting in a reduction in mediators of vascular inflammation and calcification, may contribute to our findings [17]. In a previous animal experimental study, apabetalone inhibited progression of atherosclerosis via a combination of lipid change including raising HDL cholesterol, decreasing LDL cholesterol and a reduction of proinflammatory cytokines and adhesion molecules, suggesting that anti-inflammatory activities may also contribute to plaque stabilization [30].
Accumulating evidence suggests that triglyceride-rich lipoproteins play a role in coronary artery disease [30, 31]. Our finding that VLDL concentration associates with AP development is intriguing and requires further investigation. The degree to which this contributes to the current findings of changes in measures of plaque vulnerability and that triglyceride lowering therapies may favorably lower cardiovascular disease risk remains to be determined in clinical trials.
A number of caveats should be noted. The current analysis was small, with very few patients in the placebo group having AP at baseline. Accordingly, a between group comparison with apabetalone was not possible. Patients treated with the highest doses of atorvastatin and rosuvastatin were excluded; thus the impact of apabetalone combination with potent statin therapy remains unknown. Acoustic shadowing artifacts with IVUS imaging can limit the ability to detect and quantify AP burden. The low prevalence of AP at baseline also suggests a limited therapeutic effect in the broader ASSURE study population, but does suggest potential benefits on higher risk patients. Further investigation with a larger number of subjects with ACS may provide great understanding of the impact of apabetalone on high-risk atherosclerotic plaque.
In summary, apabetalone has a potential effect on vulnerable atherosclerotic plaque in the coronary artery. Our observations raise the possibility that reduced HDL particles contribute to the development of attenuated plaque, and that HDL particles can be superior to HDL cholesterol and apoA-1 as a marker of progression of high-risk plaque. In addition, triglyceride-rich lipoprotein may be another important factor to predict the development of attenuated plaque. How these findings translate to cardiovascular events requires further investigation in larger studies.
References
Nicholls SJ, Gordon A, Johansson J, et al. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol. 2011;57:1111–9.
Bailey D, Jahagirdar R, Gordon A, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55:2580–9.
Gilham D, Wasiak S, Tsujikawa LM, et al. RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis. 2016;247:48–57.
Pu J, Mintz GS, Biro S, et al. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2294 human coronary artery segments. J Am Coll Cardiol. 2014;63:2220–33.
Nicholls SJ, Puri R, Wolski K, et al. Effect of the BET protein inhibitor, RVX-208, on progression of coronary atherosclerosis: results of the phase 2b, randomized, double-blind, multicenter, ASSURE trial. Am J Cardiovasc Drugs. 2016;16:55–65.
Mackey RH, Greenland P, Goff DC Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2012;60:508–16.
Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–9.
Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–63.
Tanaka A, Kawarabayashi T, Nishibori Y, et al. No-reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation. 2002;105:2148–52.
Wu X, Mintz GS, Xu K, et al. The relationship between attenuated plaque identified by intravascular ultrasound and no-reflow after stenting in acute myocardial infarction: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011;4:495–502.
Shiono Y, Kubo T, Tanaka A, et al. Impact of attenuated plaque as detected by intravascular ultrasound on the occurrence of microvascular obstruction after percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2013;6:847–53.
Sutsch G, Kiowski W, Bossard A, et al. Use of an emboli containment and retrieval system during percutaneous coronary angioplasty in native coronary arteries. Schweiz Med Wochenschr. 2000;130:1135–45.
Kimura S, Kakuta T, Yonetsu T, et al. Clinical significance of echo signal attenuation on intravascular ultrasound in patients with coronary artery disease. Circ Cardiovasc Interv. 2009;2:444–54.
Bayturan O, Tuzcu EM, Nicholls SJ, et al. Attenuated plaque at nonculprit lesions in patients enrolled in intravascular ultrasound atherosclerosis progression trials. JACC Cardiovasc Interv. 2009;2:672–8.
Nicholls SJ, Ray KK, Johansson JO, et al. Selective BET protein inhibition with apabetalone and cardiovascular events: a pooled analysis of trials in patients with coronary artery disease. Am J Cardiovasc Drugs. 2018;18:109–15.
Wasiak S, Gilham D, Tsujikawa LM, et al. Downregulation of the complement cascade in vitro, in mice and in patients with cardiovascular disease by the BET protein inhibitor apabetalone (RVX-208). J Cardiovasc Transl Res. 2017;10:337–47.
Wasiak S, Gilham D, Tsujikawa LM, et al. Data on gene and protein expression changes induced by apabetalone (RVX-208) in ex vivo treated human whole blood and primary hepatocytes. Data Brief. 2016;8:1280–8.
Nicholls SJ, Cutri B, Worthley SG, et al. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 2005;25:2416–21.
Brewer HB Jr. HDL metabolism and the role of HDL in the treatment of high-risk patients with cardiovascular disease. Curr Cardiol Rep. 2007;9:486–92.
Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72.
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–14.
von Birgelen C, Hartmann M, Mintz GS, Baumgart D, Schmermund A, Erbel R. Relation between progression and regression of atherosclerotic left main coronary artery disease and serum cholesterol levels as assessed with serial long-term (> or = 12 months) follow-up intravascular ultrasound. Circulation. 2003;108:2757–62.
Johnsen SH, Mathiesen EB, Fosse E, et al. Elevated high-density lipoprotein cholesterol levels are protective against plaque progression: a follow-up study of 1952 persons with carotid atherosclerosis the Tromso study. Circulation. 2005;112:498–504.
Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99.
Investigators A-H, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67.
Landray MJ, Haynes R, Group HTC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12.
Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35.
Monette JS, Hutchins PM, Ronsein GE, et al. Patients with coronary endothelial dysfunction have impaired cholesterol efflux capacity and reduced HDL particle concentration. Circ Res. 2016;119:83–90.
Jahagirdar R, Zhang H, Azhar S, et al. A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis. 2014;236:91–100.
Hodis HN, Mack WJ. Triglyceride-rich lipoproteins and the progression of coronary artery disease. Curr Opin Lipidol. 1995;6:209–14.
Mack WJ, Krauss RM, Hodis HN. Lipoprotein subclasses in the Monitored Atherosclerosis Regression Study (MARS) Treatment effects and relation to coronary angiographic progression. Arterioscler Thromb Vasc Biol. 1996;16:697–704.
Acknowledgements
Peter J. Psaltis is supported by a Future Leader Fellowship from the National Heart Foundation of Australia and project Grant funding from the National Health and Medical Research Council and National Heart Foundation of Australia. Stephen J. Nicholls is supported by a Principal Research Fellowship from the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Peter J. Psaltis has received research support from Abbott Vascular and speaker honoraria from AstraZeneca, Merck and Bayer. Stephen J. Nicholls has received speaking honoraria from AstraZeneca, Pfizer, Merck Schering-Plough and Takeda, consulting fees from AstraZeneca, Abbott, Atheronova, Esperion, Amgen, Novartis, Omthera, CSL Behring, Boehringer Ingelheim, Pfizer, Merck Schering-Plough, Takeda, Roche, NovoNordisk, LipoScience and Anthera and research support from Anthera, AstraZeneca, Cerenis, EliLilly, InfraReDx, Roche, Resverlogix, Novartis, Amgen, and LipoScience. Jan Johansson, Ewelina Kulikowski, Norman Wong and Michael Sweeney are employees of Resverlogix Corp. Other authors (Daisuke Shishikura, Yu Kataoka, Satoshi Honda, Kohei Takata, Susan W. Kim, and Jordan Andrews) have nothing to disclose.
Rights and permissions
About this article
Cite this article
Shishikura, D., Kataoka, Y., Honda, S. et al. The Effect of Bromodomain and Extra-Terminal Inhibitor Apabetalone on Attenuated Coronary Atherosclerotic Plaque: Insights from the ASSURE Trial. Am J Cardiovasc Drugs 19, 49–57 (2019). https://doi.org/10.1007/s40256-018-0298-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-018-0298-8