Abstract
Background
Inhibition of bromodomain and extra-terminal (BET) proteins can modulate lipoprotein and inflammatory factors that mediate atherosclerosis. The impact of the BET inhibitor, apabetalone, on cardiovascular events is unknown.
Objective
Our objective was to investigate the impact of apabetalone on cardiovascular event rates in a pooled analysis of clinical studies in patients with established coronary artery disease.
Methods
We conducted a pooled analysis of patients (n = 798) with coronary artery disease who participated in clinical trials (ASSERT, ASSURE, SUSTAIN) that evaluated the impact of 3–6 months of treatment with apabetalone on lipid parameters and coronary atherosclerosis. The incidence of major adverse cardiovascular events (death, myocardial infarction, coronary revascularization, hospitalization for cardiovascular causes) in the treatment groups was evaluated.
Results
At baseline, patients treated with apabetalone were more likely to be Caucasian, have a history of dyslipidemia, and be undertreated with ß-blocker and anti-platelet agents. Treatment with apabetalone produced the following dose-dependent changes compared with placebo: increases in apolipoprotein A-I (apoA-I) of up to 6.7% (P < 0.001), increases in high-density lipoprotein cholesterol (HDL-C) of up to 6.5% (P < 0.001), increases in large HDL particles of up to 23.3% (P < 0.001), and decreases in high-sensitivity C-reactive protein (hsCRP) of − 21.1% (P = 0.04). Apabetalone treatment did not affect atherogenic lipoproteins compared with placebo. Patients treated with apabetalone experienced fewer major adverse cardiovascular events than those treated with placebo (5.9 vs. 10.4%; P = 0.02), a finding that was more prominent in patients with diabetes (5.4 vs. 12.7%; P = 0.02), with baseline HDL-C < 39 mg/dl (5.5 vs. 12.8%; P = 0.01), or with elevated hsCRP levels (5.4 vs. 14.2%; P = 0.02).
Conclusion
Pooled analysis of short-term studies demonstrated fewer cardiovascular events among patients treated with the BET protein inhibitor, apabetalone, than among those treated with placebo. BET protein inhibition warrants further investigation as a novel approach to cardiovascular risk reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Apabetalone targets lipid and inflammation pathways implicated in cardiovascular disease. |
Apabetalone was associated with fewer major cardiovascular events than placebo in available trials. |
This benefit was more prominent in patients with diabetes and elevated inflammatory markers. |
1 Introduction
Despite evidence-based approaches for secondary prevention of ischemic cardiovascular events, including statins, aspirin, ß-blockers, and inhibitors of the renin-angiotensin system, residual risk remains high [1]. This highlights the need for new therapeutic strategies to achieve further cardiovascular risk reduction.
Vascular inflammation is implicated in all stages of atherosclerosis [2], and elevated inflammatory biomarkers are associated with residual cardiovascular risk in statin-treated patients [3]. This suggests that additional anti-inflammatory approaches may be cardioprotective. A pro-thrombotic state also influences incident and recurrent ischemic cardiovascular events [4]. Bromodomains (BRDs) are a family of evolutionary conserved amino acid sequences in proteins that recognize acetylated lysine residues on chromatin-associated molecules and thereby modulate chromatin organization and gene transcription [5]. Bromodomain and extra-terminal (BET) proteins have been implicated in the regulation of inflammatory and thrombotic pathways involved in the pathologensis of ischemic cardiovascular events [5].
Apabetalone is a selective BET inhibitor that preferentially targets BET protein 4 (BRD4) [6,7,8]. This inhibitory activity is more prominent in perturbed states, where BET proteins are more abundant, permitting apabetalone to modulate gene transcription towards a baseline state [6,7,8]. Apabetalone was originally developed as a lipid-modifying agent, based on preclinical observations that it induced hepatic synthesis of apolipoprotein A-I (apoA-I) and enhanced the cholesterol efflux capacity of high-density lipoprotein (HDL) [9]. However, early studies evaluating the administration of apabetalone for 3–6 months in statin-treated patients with coronary artery disease revealed modest elevation of apoA-I and HDL cholesterol (HDL-C) [10, 11] that did not translate to regression of coronary atherosclerosis volume with short-term treatment [12]. More recent data suggested that apabetalone may have actions beyond those on lipoproteins. It has been shown in vitro to suppress the expression of multiple genes potentially involved in the pathogenesis of atherothrombotic events, including interleukin-6, monocyte chemoattractant protein-1, complement component 9, and thrombin [6,7,8, 13].
The impact of apabetalone on cardiovascular events remains unknown. The objective of the current report was to investigate the impact of apabetalone on cardiovascular event rates in a pooled analysis of clinical studies completed to date in patients with established coronary artery disease.
2 Methods
2.1 Study Inclusion
The current analysis encompasses data from three trials. The ASSERT (ApoA-I Synthesis Stimulation Evaluation in Patients Requiring Treatment for Coronary Artery Disease) study evaluated the impact of apabetalone 50–150 mg twice daily compared with placebo on lipid and lipoprotein parameters in 299 statin-treated patients with coronary artery disease [11]. The SUSTAIN (Study of Quantitative Serial Trends in Lipids with Apolipoprotein A-I Stimulation) trial evaluated the impact of treatment with apabetalone 100 mg twice daily compared with placebo for 24 weeks on lipid and lipoprotein parameters in 172 statin-treated patients [10]. The ASSURE (ApoA-I Synthesis Stimulation and Intravascular Ultrasound for Coronary Atheroma Regression Evaluation) study compared the impact of treatment with apabetalone 100 mg twice daily or placebo for 26 weeks on progression of coronary atherosclerosis using serial intravascular ultrasound in 323 patients with angiographic coronary artery disease and low HDL-C levels [12]. Each study involved the evaluation of patients for safety parameters and cardiovascular events 30 days following the treatment phase.
2.2 Statistical Analysis
Continuous parameters are expressed as mean ± standard deviation, or median (interquartile range) when not normally distributed. Categorical parameters are expressed as percentages. All patients treated with apabetalone were compared with those receiving placebo in terms of clinical characteristics, biochemical parameters, and investigator-reported major adverse cardiovascular events (MACE: death, myocardial infarction, coronary revascularization, and hospitalization for cardiovascular causes). Cumulative event rates (as percentages) are presented for the first MACE and for the first event of each type of MACE. For statistical analysis, we used the t test for independent samples to compare normally distributed continuous variables between two treatment groups. The Chi squared test or Fisher’s exact test was used to examine the difference in categorical variables, and the Wilcoxon matched-pairs signed-ranks test was used to examine the change in lipid parameters between baseline and follow-up. Analysis of covariance on the ranks of the percent changes in lipid parameters with the ranks of the baseline values as covariates on rank transformed percentage change in lipid parameters was used to compare the percentage change in lipid parameters between two groups, and log rank test was used to compare the MACE between two groups. All statistical analyses were performed using Stata version 14.2 (Stata Corp LP, TX, USA). A P value < 0.05 was considered significant.
3 Results
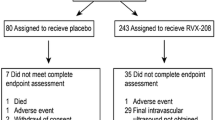
No patients were lost to follow-up, so we could determine cardiovascular event rates for all 798 patients (Fig. 1). Baseline clinical characteristics and concomitant medication use are summarized in Table 1. Given the relatively small sample size of each contributory trial, there were some differences in baseline characteristics between apabetalone and placebo groups. Patients treated with apabetalone were older (61.4 ± 9.9 vs. 59.7 ± 11.3 years; P = 0.03) and were more likely to be male (75.5 vs. 69.0%; P = 0.05) and Caucasian (87.9 vs. 79.8%; P = 0.003) and have a history of dyslipidemia (64.4 vs. 46.7%; P < 0.001). Apabetalone-treated patients were more likely to be treated with a ß-blocker (70.1 vs. 59.5%; P < 0.001) and an antiplatelet agent (90.6 vs. 82.6%; P < 0.001).
The effects of treatment with apabetalone on lipids and lipoproteins are summarized in Table 2. Apabetalone produced dose-dependent increases in apoA-I of up to 6.7% (P < 0.001) and HDL-C of up to 6.5% (P < 0.001), which were associated with increases in the concentration of large HDL particles of up to 23.3% (P < 0.001) and HDL particle size of 1.1% (P < 0.001). Apabetalone produced a dose-dependent decrease in high-sensitivity C-reactive protein (hsCRP) of − 21.1% (P = 0.04). Low-density lipoprotein (LDL) parameters and triglycerides did not differ according to treatment with apabetalone or placebo.
Cardiovascular event rates are summarized in Table 3 and Fig. 2. Patients treated with apabetalone were less likely to experience MACE (5.9 vs. 10.4%; P = 0.02). Although not statistically significant, patients treated with apabetalone experienced fewer coronary revascularization procedures (3.6 vs. 5.3%; P = 0.11) and hospitalizations for cardiovascular cause (1.1 vs. 3.8%; P = 0.06) than did those receiving placebo. In exploratory subgroups, MACE occurred less frequently in association with apabetalone than with placebo among patients with diabetes (5.4 vs. 12.7%; P = 0.02) but not among those without diabetes (6.2 vs. 9.0%; P = 0.30). Similarly, MACE occurred less frequently in association with apabetalone than with placebo in patients with baseline HDL-C < 39 mg/dl (5.5 vs. 12.8%; P = 0.01) or with baseline hsCRP levels > 2 mg/l (5.4 vs. 14.2%; P = 0.02), but MACE did differ according to treatment assignment in patients with HDL-C ≥39 mg/dl (6.1 vs. 9.0%; P = 0.44) or with normal hsCRP levels (6.6 vs. 4.5%; P = 0.79). Upon adjustment for differences in baseline risk factors and study duration, apabetalone treatment continued to be associated with fewer cardiovascular events in the pooled cohort [hazard ratio (HR) 0.51; 95% confidence interval (CI) 0.27–0.93; P = 0.03], in patients with diabetes (HR 0.38; 95% CI 0.15–0.99; P = 0.04), and in patients with elevated baseline hsCRP levels (HR 0.39; 95% CI 0.19–0.83; P = 0.01).
Time to first cardiovascular events in patients treated with placebo and apabetalone in the whole cohort (top panel) and stratified according to the presence of diabetes (middle panel) and baseline high-sensitivity C-reactive protein level (bottom panel). CRP C-reactive protein, RRR relative risk reduction
In general, apabetalone was well tolerated. Apabetalone treatment was associated with an increased incidence of liver transaminase elevation, without concomitant elevation in total bilirubin or cases of Hy’s law. Transaminase elevation more than three times the upper limit of normal was observed in approximately 8% of patients receiving apabetalone, compared with no patients receiving placebo. Transaminase elevation typically occurred after 4–12 weeks of exposure to apabetalone and rapidly returned to baseline levels either with continued treatment [when maximum alanine transaminase (ALT) level was less than five times the upper limit of normal] or upon cessation of treatment (when maximum ALT level was more than eight times the upper limit of normal), as specified in the study protocols.
4 Discussion
By virtue of its selective BET protein inhibition, apabetalone modulates lipid, inflammatory, and thrombotic pathways implicated in atherosclerotic cardiovascular disease [6,7,8, 13]. While we have previously reported that apabetalone did not promote coronary atheroma regression compared with placebo [12], we herein present hypothesis-generating data for a reduction in cardiovascular events, based upon outcomes observed in three studies ranging in duration from 12 to 26 weeks. The present findings suggest that apabetalone may produce cardiovascular benefit in high-risk patients and provide the rationale for ongoing evaluation of this agent in larger outcomes trials.
While initially developed as an inducer of endogenous apoA-I synthesis, further in vitro investigation revealed that apabetalone favorably modulated inflammatory and thrombogenic pathways that may influence the risk of atherothrombotic events [6,7,8, 13]. The current clinical finding that apabetalone administration is associated with fewer cardiovascular events, despite prior findings of no discernible effect on coronary plaque volume, suggests a potential influence on factors affecting atherosclerotic plaque stability.
A benefit of apabetalone appeared to be more pronounced in patients with low HDL-C, diabetes, and elevated hsCRP levels. This suggests that high-risk patients, who are more likely to harbor factors that impact progression of atherosclerosis and a greater propensity to undergo plaque rupture, might be more likely to benefit from treatment with apabetalone. Although apabetalone increased levels of HDL-C, it did not influence blood glucose and had a marginal benefit on hsCRP levels. Therefore, diabetes and hsCRP may mark an inflammatory state that is favorably modified by apabetalone, even if glucose regulation and CRP expression are not directly affected by the drug.
A number of caveats and limitations should be noted with regard to this report. The current analysis represents a pooling of data from three modestly sized clinical trials that evaluated the impact of apabetalone on lipid parameters and coronary atherosclerosis. None of these studies was sufficiently powered to formally evaluate the impact of apabetalone on cardiovascular events. Moreover, the relatively small sample size in each trial predisposed to several observed imbalances in baseline characteristics; we cannot exclude the possibility that these imbalances influenced the rates of MACE in the apabetalone and placebo groups. Furthermore, patients enrolled in phase II studies evaluating lipid and imaging outcomes are often those at lower risk than those enrolled in large outcomes trials.
Notwithstanding these limitations, the current findings, in conjunction with prior preclinical data, provide a strong rationale for the ongoing BET-on-MACE trial (http://www.clinicaltrials.gov NCT02586155). This phase III trial will compare the effects of apabetalone with placebo on cardiovascular death, myocardial infarction, or stroke in patients with type 2 diabetes mellitus and recent acute coronary syndrome.
References
Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225–8.
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26.
Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8.
Catena C, Novello M, Lapenna R, et al. New risk factors for atherosclerosis in hypertension: focus on the prothrombotic state and lipoprotein(a). J Hypertens. 2005;23:1617–31.
Meslamani J, Smith SG, Sanchez R, Zhou MM. Structural features and inhibitors of bromodomains. Drug Discov Today Technol. 2016;19:3–15.
McLure KG, Gesner EM, Tsujikawa L, et al. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS One. 2013;8:e83190.
Wasiak S, Gilham D, Tsujikawa LM et al. Downregulation of the complement cascade in vitro, in mice and in patients with cardiovascular disease by the BET protein inhibitor apabetalone (RVX-208). J Cardiovasc Transl Res. 2017.
Wasiak S, Gilham D, Tsujikawa LM, et al. Data on gene and protein expression changes induced by apabetalone (RVX-208) in ex vivo treated human whole blood and primary hepatocytes. Data Brief. 2016;8:1280–8.
Bailey D, Jahagirdar R, Gordon A, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55:2580–9.
Di Bartolo BA, Scherer DJ, Nicholls SJ. Inducing apolipoprotein A-I synthesis to reduce cardiovascular risk: from ASSERT to SUSTAIN and beyond. Arch Med Sci: AMS. 2016;12:1302–7.
Nicholls SJ, Gordon A, Johansson J, et al. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol. 2011;57:1111–9.
Nicholls SJ, Puri R, Wolski K, et al. Effect of the BET protein inhibitor, RVX-208, on progression of coronary atherosclerosis: results of the phase 2b, randomized, double-blind, multicenter, ASSURE Trial. Am J Cardiovasc Drug. 2016;16:55–65.
Gilham D, Wasiak S, Tsujikawa LM, et al. RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis. 2016;247:48–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The studies were funded by Resverlogix.
Conflicts of interest
SJN has received research support from AstraZeneca, Amgen, Anthera, Eli Lilly, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron, and LipoScience and is a consultant for AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. KKR reports grants and/or personal fees from Pfizer, MSD, Astra Zeneca, Sanofi, Aegerion, Regeneron, Abbvie, Kowa, Cerenis, Medicines Company, Lilly, Esperion, Amgen, Cipla, Algorithm, Takeda, Boehringer Ingelheim, and Novo Nordisk within the last 12 months outside of the submitted work. GGS, through his institution, has received research support from Cerenis, The Medicines Company, Resverlogix, Roche, and Sanofi. JOJ, AG, MS, CH, EK, and NW are employees of Resverlogix. SWK has no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nicholls, S.J., Ray, K.K., Johansson, J.O. et al. Selective BET Protein Inhibition with Apabetalone and Cardiovascular Events: A Pooled Analysis of Trials in Patients with Coronary Artery Disease. Am J Cardiovasc Drugs 18, 109–115 (2018). https://doi.org/10.1007/s40256-017-0250-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-017-0250-3