Abstract
Background
Earlier meta-analyses have demonstrated a significant reduction in major adverse cardiovascular events (MACE) with dipeptidyl peptidase 4-inhibitor (DPPI) use, as compared with placebo or alternative anti-diabetic therapies. However, the large phase III/IV trials, namely SAVOR-TIMI 53 and the EXAMINE trials, failed to demonstrate any significant differences in MACE between DPPI and placebo. We aimed to perform an updated meta-analysis of randomized controlled trials (RCTs) to investigate the differences in cardiovascular death, myocardial infarction (MI), and stroke between DPPI and placebo/alternative agents.
Methods
We searched the MEDLINE, EMBASE, and Cochrane databases for relevant phase III/IV RCTs. Unpublished trials with results available on national clinical trials registers were also included. RCTs with follow-up duration ≥24 weeks were included if they compared DPPI with placebo or an alternative anti-diabetic agent.
Results
A total of 82 RCTs including 73,678 patients were included. We did not observe any significant difference in the pooled odds of cardiovascular death, MI, or stroke in the composite DPPI arm as compared with the control arm. Similarly, the pooled odds of all-cause death and MACE were statistically similar between the two groups. None of the clinical outcomes studied demonstrated evidence of statistical heterogeneity or publication bias. Due to a larger sample size and a longer duration of follow-up, both SAVOR-TIMI 53 and EXAMINE trials had a considerably larger contribution to the pooled estimates in our meta-analysis, driving the updated pooled estimates towards null for all clinical outcomes assessed.
Conclusions
DPPI use was not associated with increased incidence of cardiovascular mortality, MI, stroke, or MACE compared with placebo or alternative anti-diabetic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diabetes is associated with significantly increased cardiovascular morbidity and mortality in patients with and without established heart disease [1, 2]. In the recent past, there has been concern regarding cardiovascular safety of several anti-diabetic drugs such as rosiglitazone, muraglitazar, and sulfonylureas [3–5]. In July 2008, the Endocrinologic and Metabolic Drugs Advisory Committee of the US FDA revised their approval criteria for all new anti-diabetic drugs [6]. This committee recommended that the sponsors would need to conclusively demonstrate that the new anti-diabetic therapy would not result in an unacceptably higher cardiovascular risk prior to approval. The last decade has witnessed the use of several new classes of anti-diabetic agents, including dipeptidyl peptidase 4 inhibitors (DPPI). Several phase II/III trials have demonstrated improved glycemic control with DPPI as compared with placebo [7]. A recent meta-analysis of 70 randomized controlled trials (RCTs) demonstrated a significant reduction in overall major adverse cardiovascular events (MACE) in patients randomized to DPPI as compared with placebo or an alternative anti-diabetic agent [8]. Notably, the authors reported a 40 % reduced odds of mortality and 36 % reduced odds of acute myocardial infarction (MI) in patients using DPPI as compared with the control group [8]. In response to the FDA guidance, two large, multicenter RCTs (SAVOR-TIMI 53 [Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus], EXAMINE [Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care]) were recently published assessing cardiovascular safety of saxagliptin and alogliptin, respectively, in comparison with placebo [9, 10]. Despite a significant reduction in glycated hemoglobin over the follow-up period, both these trials demonstrated that use of DPPI did not significantly decrease the incidence of MACE in comparison with the placebo arm. In this manuscript, we aimed to carry out an updated meta-analysis to present cumulative estimates on the cardiovascular safety of DPPI drugs.
2 Methods
2.1 Data Sources and Searches
A computerized literature search of the MEDLINE, EMBASE, and Cochrane databases was conducted using medical subject heading (MeSH) terms and keywords including dipeptidyl peptidase 4 inhibitors, DPPI, DPP4-I, alogliptin, linagliptin, saxagliptin, sitagliptin, and vildagliptin coupled with outcomes searched using the terms death, mortality, myocardial infarction, MI, stroke, cerebrovascular accident, major adverse cardiovascular event, or MACE. Results of unpublished trials, if available, were retrieved using the national clinical trials register (http://www.clinicaltrials.gov) [11]. The literature search was conducted through November 2013.
2.2 Study Selection
We evaluated all phase III and phase IV RCTs reporting the safety and efficacy of DPPI in patients with diabetes published in the English language. All trials performed in patients with type I or type II diabetes were considered. Trials were considered for inclusion if they compared DPPI with placebo or an alternative anti-diabetic agent. Only trials with a follow-up duration of ≥24 weeks were included in our study. Cardiovascular death, MI, and stroke were considered as co-primary outcomes. Unlike the prior meta-analysis, we did not include MACE as a primary outcome due to non-uniform reporting across the trials, along with significant differences in operational definitions of MACE [8]. Secondary safety endpoints included all-cause death and MACE. RCTs failing to report at least one of our study outcomes were excluded from our analysis.
2.3 Data Extraction
Full text articles were retrieved for all title–abstracts that met the inclusion criteria. Data extraction was subsequently performed independently by two authors (SA, AP). All discrepancies about study inclusion or outcomes were resolved by the senior author (VM). Only good-quality trials with a Jadad score ≥3 were included in our analysis [12]. In cases of multiple publications arising from a single trial, only the trial with the longest follow-up was included for the analysis.
2.4 Data Synthesis and Statistical Analysis
Statistical analysis was conducted using ‘metan’ function in Stata version 13.1 (Stata Corporation, College Station, TX, USA). The meta-analysis has been reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta Analyses (PRISMA) guidelines [13]. Due to a relatively small proportion of primary events in each study, we used the Peto odds ratio (OR) method for pooling effect estimates across studies per recommendations of the Cochrane Collaboration [14]. Even though correction for zero cell counts is not usually necessary while using this method, we verified the veracity of our results by calculating pooled risk difference estimates, which would conclusively account for all studies including those with zero cell counts. Risk differences are unaffected by zero cell counts and hence do not eliminate studies with zero cell counts from pooled analysis.
Fixed effects modeling was primarily used to conduct outcomes meta-analysis from included studies. A fixed effect model of meta-analysis is based on a mathematical assumption that every study is evaluating a common treatment effect. This means that the effect of the treatment, allowing for the play of chance, was the same in all included studies. Sometimes this underlying assumption of a fixed effect meta-analysis (i.e. that diverse studies can be estimating a single effect) is too simplistic. In order to circumvent the issues that arise due to fixed effects modeling, analysis might need to be performed using random effects meta-analysis. In addition to the fixed effects modeling, we performed a sensitivity analysis using random effects modeling. We assessed for heterogeneity using the I 2 test (I 2 > 50 % with p < 0.05 implies significant heterogeneity). However, statistical tests for heterogeneity are scarcely reliable when the large majority of trials have very few events. Therefore, corroboration of results obtained using fixed effects modeling with those obtained using random effects model was deemed important for this analysis. The DerSimonian and Laird method of estimation of variance was utilized for the random effects modeling. This method is a variation of the inverse-variance method and incorporates an assumption that the different studies are estimating different, yet related, intervention effects. To undertake a random-effects meta-analysis, the standard errors of the study-specific estimates were adjusted to incorporate a measure of the extent of variation at the population level. This variation is often referred to as tau-squared (τ2, or Tau2). The amount of this population-level variation, and hence the adjustment, could be estimated from the intervention effects and standard errors of the studies included in the meta-analysis.
Although not the primary focus of our manuscript, we performed several sensitivity analyses with various cut-offs in the baseline characteristics to understand the impact of different characteristics across studies on pooled outcomes. In order to understand the collective impact of differing baseline characteristics across the included studies upon the pooled effect estimates, we performed a meta-regression analysis with primary outcome (cardiovascular death, MI, stroke) as the dependent variable of interest. The covariates incorporated into the model were mean age, proportion of males, mean diabetes duration, mean glycated hemoglobin, mean body mass index (BMI), and the follow-up duration of each study. Other variables, including traditional cardiovascular risk factors, could not be included into the regression model as these were not uniformly available in the majority of the included studies. Publication bias was assessed using the funnel plot method as well as Egger correlation testing [15]. We also performed cumulative meta-analysis in order to determine the differential impact of the SAVOR-TIMI 53 and EXAMINE trials on the effect estimates provided by the prior published meta-analysis [8–10]. Although the main intent of this meta-analysis was focused on studying the cardiovascular safety of the entire DPPI class, we have reported the pooled effect estimates for individual DPPI agents, without intra-class comparisons. All p-values were two-tailed with statistical significance specified at 0.05 and confidence intervals (CIs) computed at the 95 % level.
3 Results
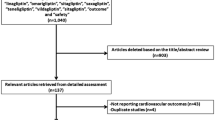
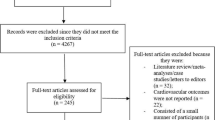
A total of 82 RCTs including 73,678 patients were included in our study. The recent RCTs (SAVOR-TIMI 53 and EXAMINE) contributed a total of 22,322 patients to our analysis [9, 10]. The characteristics of the included trials are shown in Table 1. The flow diagram for study selection is depicted in Fig. 1. Of all 82 included studies, 65 studies had been published in peer-reviewed journals. The remaining 17 studies were unpublished, with data extracted from the national clinical trials register [11]. The follow-up duration ranged between 24 and 104 weeks across the included trials. All included clinical trials were industry sponsored. The characteristics of included patients in each trial are shown in Table 2. We observed significant heterogeneity in the baseline characteristics of included subjects across the various trials. Mean glycated hemoglobin ranged from 6.5 % (50 mmol/mol) to 9.9 % (85 mmol/mol). Mean disease duration before randomization ranged between 1.2 and 16.3 years.
Table 3 demonstrates the pooled ORs for all the primary and secondary outcomes, stratified by the type of DPPI. We did not observe any significant difference in the pooled odds of cardiovascular death (OR 0.95 [95 % CI 0.82–1.09]), MI (OR 0.98 [95 % CI 0.86–1.10]), or stroke (OR 0.92 [95 % CI 0.77–1.11]) in the DPPI arm as compared with the control arm. Similarly, the pooled odds of all-cause death (OR 1.00 [95 % CI 0.90–1.13]) and MACE (OR 0.95 [95 % CI 0.86–1.04]) were statistically similar between the two groups. The stratified forest plots of all outcomes are shown in the supplementary material (supplementary figures 1–5). None of the clinical outcomes studied demonstrated evidence of statistical heterogeneity or publication bias (supplementary figure 6). Results obtained using random effects modeling were similar to those obtained using fixed effects modeling (Table 3). Figure 2 demonstrates the pooled risk differences between the DPPI arm and the control arm for all primary and secondary outcomes. Similar to the pooled analysis using the ORs, we did not observe any significant differences in the pooled risk differences between the two study groups. Although we did not aim to focus on the safety profile of individual DPPI agents, we did observe a significant improvement in stroke (OR 0.45 [95 % CI 0.23–0.89]) and MACE (OR 0.47 [95 % CI 0.25–0.87]) with linagliptin and a significant improvement in stroke (OR 0.23 [95 % CI 0.07–0.71]) with vildagliptin (Table 1). However, these comparisons are limited by the small number of patients.
Figure 3 demonstrates the results of the cumulative meta-analysis that was performed to assess the differential impact of the latest trials [9, 10] on the pooled estimates provided by the prior published meta-analysis [8]. As seen in Fig. 3, the prior published meta-analysis demonstrated a significant benefit of DPPI in terms of reduced all-cause mortality, MI, and MACE [8]. Both the recent trials demonstrated a lack of difference in clinical outcomes, included in our analysis, between the DPPI and the placebo arms. The total ‘weight’ of all trials included in the prior published meta-analysis amounted to 10.5, 15.2, and 6.8 % for these outcomes, respectively [8]. Similarly, the total ‘weight’ from the prior published meta-analysis for cardiovascular death and stroke was 6.5 and 23.0 %, respectively. In our analysis, we observed that a large majority of the total ‘weight’ arose from the latest trials, which resulted in driving the updated pooled estimates towards null for all clinical outcomes. Supplementary table 1 demonstrates the differences in pooled baseline characteristics of the trials included in the prior published meta-analysis [8] and the pivotal SAVOR-TIMI 53 and EXAMINE trials [9, 10]. Both SAVOR-TIMI 53 and EXAMINE trials included patients that were older compared with the earlier trials [9, 10]. In addition, there was a higher proportion of males in these two trials as compared with the earlier published trials [9, 10]. Furthermore, the duration of diabetes preceding randomization as well as the follow-up duration in the SAVOR-TIMI 53 and the EXAMINE trials were considerably higher than in the earlier published trials [9, 10]. It was not possible to study the differences in the distribution of other traditional cardiovascular risk factors between the trials included in the prior published meta-analysis [8] and the current meta-analysis as these were not uniformly available in the majority of included trials.
Cumulative meta-analysis to assess the differential impact of the latest phase IV trials [9, 10] on the pooled odds ratios provided by the prior published meta-analysis [8] for all primary and secondary outcomes. In this figure, ‘earlier pooled’ refers to the results obtained from the prior published meta-analysis [8], which included 80 trials and 51,356 patients. ‘NEJM-White et al.’ refers to the results obtained from the EXAMINE trial [10], which included 5,380 patients. ‘NEJM-Scirica et al.’ refers to the results obtained from the SAVOR-TIMI 53 trial, which included 16,492 patients [9]. CI confidence interval, NEJM New England Journal of Medicine, MACE major adverse cardiovascular event, OR odds ratio
3.1 Sensitivity Analysis
Supplementary table 2 demonstrates the results of the sensitivity analysis based on various cut-offs in the baseline characteristics to understand the impact of different characteristics across studies on pooled outcomes. We observed a significant reduction in cardiovascular death in the DPPI cohort, among all trials with mean BMI <31 kg/m2 (OR 0.76 [95 % CI 0.58–0.99]). Although this cohort included the EXAMINE trial, it did not include the SAVOR-TIMI 53 trial [9, 10]. In addition, there was a significant reduction in stroke (OR 0.52 [95 % CI 0.34–0.80]) and MACE (OR 0.52 [95 % CI 0.36–0.75]) in the DPPI cohort, among all trials with mean age <60 years. Similarly, there was a significant reduction in stroke (OR 0.33 [95 % CI 0.17–0.64]) and MACE (OR 0.59 [95 % CI 0.38–0.90]) in the DPPI cohort, among all trials with mean glycated hemoglobin <8.0 %. Besides this, we observed a significant reduction in stroke (OR 0.59 [95 % CI 0.38–0.92]) in the DPPI cohort, among all trials with percentage of males <60 %. Furthermore, the follow-up duration in each trial had a major impact on the pooled effect estimates. We observed a significant reduction in MACE (OR 0.63 [95 % CI 0.40–0.90]) in the DPPI cohort, among all trials with follow-up duration of ≤52 weeks.
3.2 Meta-Regression Analysis
Figure 4 demonstrates the results of the meta-regression analysis using cardiovascular death as the primary outcome of interest. Using meta-regression, we found that there was a statistically significant direct influence of BMI on the pooled estimate of cardiovascular mortality (meta-regression coefficient 1.57 [95 % CI 1.07–2.31], p = 0.02). This implies that the OR for cardiovascular mortality between the DPPI and the control groups increased with corresponding increase in mean BMI across the included studies. Although not statistically significant, there was a trend towards a direct influence of age on the pooled estimate of cardiovascular mortality (meta-regression coefficient 1.16 [95 % CI 0.99–1.37], p = 0.06). Besides this, we demonstrated that there was a statistically significant inverse influence of follow-up duration on the pooled estimate of cardiovascular mortality (meta-regression coefficient 0.29 [95 % CI 0.10–0.89], p = 0.03). This implies that the OR for cardiovascular mortality between the DPPI and the control groups decreased with an increase in the follow-up duration across the included studies. There was no influence of gender (meta-regression coefficient 1.06 [95 % CI 0.98–1.15], p = 0.2), diabetes duration (meta-regression coefficient 0.82 [95 % CI 0.64–1.05], p = 0.1), and glycated hemoglobin level (meta-regression coefficient 1.17 [95 % CI 0.38–3.71], p = 0.8), upon the pooled OR for cardiovascular mortality. Furthermore, we did not find any statistically significant impact of any of the above-mentioned variables on pooled estimates of stroke, MI, all-cause death, or MACE.
Multivariable meta-regression analysis demonstrating the impact of baseline characteristics of patients on the pooled odds ratio of cardiovascular mortality across the included randomized controlled trials. The multivariable meta-regression model consisted of six variables, including mean age, proportion of males, mean body mass index, mean glycated hemoglobin, mean diabetes duration, and mean follow-up duration in each study. We have graphically demonstrated the impact of most important variables that were found to be significant or that demonstrated a trend towards statistical significance. Panel a demonstrates the impact of mean age in each trial on the pooled odds ratio of cardiovascular mortality. Panel b demonstrates the impact of mean body mass index in each trial on the pooled odds ratio of cardiovascular mortality. Panel c demonstrates the impact of mean follow-up duration in each trial on the pooled odds ratio of cardiovascular mortality. The size of each circle represents the weight of each study included in the estimation of pooled odds ratio. The largest circles represent the SAVOR-TIMI 53 and the EXAMINE trials. The line in each panel represents the regression line obtained after the multivariable meta-regression analysis. The slope of the line and the p-value for the coefficient are shown in the top right-hand corner of each panel
4 Discussion
In this comprehensive meta-analysis, we have collated the currently available cardiovascular safety data following the use of DPPI agents in patients with diabetes. The present meta-analysis contradicts the trends that have been seen in prior meta-analyses on this topic [8, 79–81]. We observed that there was no significant difference in pooled cardiovascular mortality, MI, and MACE between patients randomized to DPPI as compared with those randomized to placebo or alternate anti-diabetic therapy. In addition, we failed to observe any significant difference in the incidence of stroke or all-cause mortality between patients treated with DPPI and the control group. The pivotal randomized trials comparing alogliptin with placebo (EXAMINE) and saxagliptin with placebo (SAVOR-TIMI 53) demonstrated similar cardiovascular risk in the two study groups on follow-up [9, 10]. The EXAMINE trial randomized 5,380 patients and followed them for a median period of 1.5 years [10]. The SAVOR-TIMI 53 trial randomized 16,492 patients, with a median follow-up period of 2.1 years [9]. Due to a larger sample size and a longer duration of follow-up, these trials had a considerably larger contribution to the pooled estimates in our meta-analysis, significantly altering the results compared with prior meta-analyses [3, 81].
The current meta-analysis comprehensively studied the impact of differing baseline characteristics across the included trials in influencing the pooled effect estimates. We have also attempted to explain the rationale behind the observed differences between the current meta-analysis and the prior published meta-analysis [8]. Our meta-analysis demonstrated significant differences in mean age, proportion of males, mean diabetes duration, and follow-up duration between the prior published meta-analysis and the current meta-analysis [8]. All of these differences in the baseline characteristics were introduced after the inclusion of the SAVOR-TIMI 53 and the EXAMINE trials, which included markedly different populations than the prior trials largely aimed at evaluating the efficacy of DPPI agents [9, 10]. This disparity in clinical characteristics arises from the primary intent to evaluate cardiovascular safety in EXAMINE and SAVOR-TIMI 53 as opposed to the desire to evaluate glycemic efficacy in the smaller phase II and phase III trials [9, 10]. Sensitivity analysis demonstrated a significant benefit of DPPI agents over alternative anti-diabetic therapy among those with a favorable risk factor profile for cardiovascular disease. Specifically, we observed significantly reduced risk of cardiovascular death among those trials with mean BMI <31 kg/m2. Similarly, there was a reduced risk of stroke and MACE among trials with mean glycated hemoglobin <8 % and trials with mean age <60 years. Interestingly, follow-up duration of included trials was a major factor influencing pooled results on both the sensitivity analysis and the meta-regression analysis. Although there was a benefit observed among trials with a follow-up duration of ≤52 weeks, this difference was not evident when the analysis was performed using trials with longer a duration of follow-up. This underscores the importance of including trials with sufficiently long follow-up while performing meta-analyses of cardiovascular outcomes with anti-diabetic agents.
Over the last decade, we have seen major turmoil in the field of cardiovascular safety of anti-diabetic agents. Diabetes has been associated with increased cardiovascular morbidity and mortality. The occurrence of increased cardiovascular-related adverse events with several anti-diabetic agents has led to several policy alterations leading to the approval of anti-diabetic drugs [3, 5, 82]. In 2008, the FDA issued specific mandates for the industry, requiring that pre-approval and post-approval studies for all new anti-diabetic agents rule out an excessive cardiovascular-related risk [6]. Irrespective of the signals towards cardiovascular risk in phase I or phase II studies, the mandate has been applied to all classes of anti-diabetic agents.
Prior to the publication of the pivotal randomized trials (SAVOR TIMI-53, EXAMINE), meta-analyses of the previous phase II/III trials have demonstrated discordant findings with the incidence of cardiovascular morbidity either reduced [8, 79, 80] or unaltered [83, 84]. In fact, the most recent meta-analysis demonstrated a significant reduction in all-cause mortality, MI, and MACE in patients randomized to DPPI as compared with those receiving placebo or alternative anti-diabetic therapies [8]. The results of this particular analysis were deemed plausible due to several mechanisms besides improved glycemic control. These include enhanced endothelial function, enhanced endothelial progenitor cell availability, and glucagon-like peptide (GLP-1)-mediated myocardial protection [85–87]. The current meta-analysis demonstrates the impact of several baseline variables including BMI, age, and follow-up duration on the differences in cardiovascular mortality between the two study groups. We have demonstrated that there were significant differences in several baseline characteristics like age, proportion of males, mean diabetes duration, and follow-up duration between the prior published meta-analysis [8] and the current meta-analysis. Although the traditional cardiovascular risk factors and comorbidities were not uniformly available across all included RCTs, it is possible to speculate that there were differences in these baseline characteristics that were partially responsible for the differences in outcomes between various trials. The patient populations included in the SAVOR-TIMI 53 and EXAMINE trials comprised older patients with a longer duration of diabetes and a much higher prevalence of previous cardiovascular events, renal failure, comorbidities, and current insulin treatment than most of the other trials that were intended to study the efficacy of DPPI agents rather than cardiovascular safety of these agents. Besides this, the incidence of hypoglycemic events was noted to be significantly higher in the SAVOR-TIMI 53 trial [9]. Considering that hypoglycemia has been associated with poorer cardiovascular outcomes, it is imperative that the incidence of hypoglycemia be considered in evaluation of cardiovascular safety of these agents. However, despite the evidence of increased hypoglycemia, the cardiovascular events were the same in both treatment groups.
Besides establishing cardiovascular safety, both SAVOR-TIMI 53 and the EXAMINE trials underscored the importance of large, phase III/IV cardiovascular outcome trials in assessing cardiovascular safety. Despite a robust methodology and inclusion of good-quality RCTs, the prior published meta-analysis failed to demonstrate equivalence of cardiovascular endpoints between the two study groups. The trials included were designed to study the glycemic improvement, were shorter in duration and enrolled patients with lower cardiovascular risk. Besides this, most of the phase II/III trials did not prospectively adjudicate adverse cardiovascular events. Furthermore, comparison of cardiovascular risk of the new therapy with an active anti-diabetic agent is often challenged by questionable cardiovascular safety profiles of the comparator. Therefore, performance of a properly powered RCT with appropriate follow-up and a formalized adjudication process is of paramount importance in the determination of the safety profile of new drugs. Even in our meta-analysis, we see discordant findings for drugs that have small RCTs available for inclusion in our study. For example, we observed a significant benefit of linagliptin and vildagliptin use in reduction of stroke. However, these comparisons included only 6,039 randomized patients for the linagliptin stratum and 4,347 patients for the vildagliptin stratum, which constituted 10.7 and 7.7 % of the total randomized patients for the stroke endpoint, respectively. Similarly, we observed a significant benefit of linagliptin in reduction of MACE as compared with placebo/alternative anti-diabetic therapy. However, the linagliptin stratum only contained 1,778 patients, which constituted 5.9 % of the total randomized patients for the MACE endpoint. Two large, ongoing, phase III, multicenter RCTs aim to compare adverse cardiovascular outcomes between DPPI agents and alternative anti-diabetic therapy or placebo. The CAROLINA (NCT01243424; Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Patients with Type 2 Diabetes) trial plans to recruit 6,000 patients with pre-existing cardiovascular disease or at high risk for incident cardiovascular disease, with glycated hemoglobin between 6.5 and 8.5 %, and randomize them to linagliptin or glimepiride. The results of this trial are expected by September 2018 and would likely clarify the discrepancies in linagliptin-related outcomes that we have observed in our meta-analysis. Besides this, the TECOS trial (NCT00790205; Trial to Evaluate Cardiovascular Outcomes after Treatment with Sitagliptin) plans to recruit 14,000 patients with pre-existing cardiovascular disease or at high risk for incident cardiovascular disease, with glycated hemoglobin between 6.5 and 8.0 %, and randomize them to sitagliptin or placebo. The results of this trial are expected by December 2014.
Whether the results of SAVOR TIMI-53 and EXAMINE are able to be extrapolated to other DPPI agents is a matter of speculation at this time [9, 10]. However, most clinicians would expect a ‘class effect’ of DPPI agents with respect to cardiovascular safety. In fact, the pharmacodynamic profile of all DPPI agents is very similar across the whole class, with only minor pharmacokinetic differences between individual agents [8]. Therefore, we suspect that the beneficial effects of linagliptin and vildagliptin with respect to stroke and MACE are likely secondary to a lack of large phase IV post-marketing trials specifically looking at cardiovascular endpoints; further underscoring the need for these post-marketing trials in the drug development/approval process.
Another important observation that resulted from the SAVOR-TIMI 53 and EXAMINE trials was the lack of cardiovascular benefit despite a modest improvement in glycated hemoglobin over follow-up [9, 10]. The mean glycated hemoglobin (HbA1c) in the treatment and placebo arms of EXAMINE and SAVOR-TIMI 53 was 8.0 % in both trials [9, 10]. These results were consistent with those observed in other major RCTs such as ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Controlled Evaluation), VADT (Veterans Affairs Diabetes Trial), and ACCORD (Action to Control Cardiovascular Risk in Diabetes) [88–90]. These trials suggest that the benefit of glucose control within the 6.0–8.0 % range has a minimal effect on attenuating cardiovascular risk. However, a small benefit for glycemic control in this subset of diabetic subjects may still be noted, and a meta-analysis of these RCTs demonstrated a small but statistically significant benefit in reduction of adverse cardiovascular events [91]. In addition, the DCCT/EDIC (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications) study demonstrated long-term beneficial effects on the risk of cardiovascular disease in type I diabetes, raising the possibility of a legacy effect [92]. These findings have several major implications. First, HbA1c possibly does not serve as a valid surrogate for assessment of cardiovascular risk in patients with diabetes who have mild-moderate elevations of HbA1c. Second, the optimal approach towards attenuation of cardiovascular risk amongst patients with diabetes should include an aggressive modification of traditional cardiovascular risk factors rather than an overwhelming impetus on intensive glycemic control [82].
4.1 Strengths and Limitations
We have attempted to summarize a large body of contemporary evidence, derived from phase III and phase IV RCT data. The large number of patients included in our study serves to increase the strength, validity, and generalizability of our results. By comparing our results with those of earlier meta-analyses, we have demonstrated a need for properly powered randomized studies with appropriate follow-up and a formalized adjudication process in determination of cardiovascular safety of new anti-diabetic agents.
This study was a ‘trial-level’ meta-analysis and not a ‘patient-level’ analysis, implying that time-to-event analysis was not possible. In addition, not all trials were geared towards assessment of cardiovascular events or possessed formalized adjudication for cardiovascular and cerebrovascular events. Most of the ‘weight’ in the pooled estimates is derived from the recent pivotal trials, thereby assuring us of the accuracy of our findings. Since it was possible to have underestimated the differences in cardiovascular safety between the DPPI agents and the alternative anti-diabetic therapy due to the heterogeneity introduced by a large contribution of patients and outcomes from the recent RCTs, we performed sensitivity analyses using random-effects modeling to verify the results obtained using the fixed-effects modeling strategy.
5 Conclusions
Our meta-analysis has demonstrated that there was no significant difference in pooled cardiovascular mortality, MI, and MACE between patients randomized to DPPI and those randomized to placebo or alternate anti-diabetic therapy. In addition, we did not observe any excess risk of stroke or all-cause mortality with the use of DPPI over the comparison group. Due to a larger sample size and a longer duration of follow-up, both SAVOR-TIMI 53 and EXAMINE trials had a considerably larger contribution to the pooled estimates in our meta-analysis, significantly altering the results compared with prior meta-analyses.
References
Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB Sr, Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119(13):1728–35.
Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010; 375(9733):2215–22.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71.
Feinglos MN, Bethel MA. Therapy of type 2 diabetes, cardiovascular death, and the UGDP. Am Heart J. 1999;138(5 Pt 1):S346–52.
Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581–6.
Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf.
Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother. 2012;46(11):1453–69.
Monami M, Ahrén B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15(2):112–20.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mozenson O, McGuire DK, Ray KK, Leiter LA, Raz I; the SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med. 2013;369:1317–26.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F; the EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35.
http://www.clinicaltrials.gov. Accessed 3 Sept 2013.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Higgins JPT, Green S, editors. Handbook of systematic reviews of interventions 4.2.6 (updated September 2006). In: The Cochrane Library. Issue 4, 2006. Chichester UK: Wiley.
Egger M, Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–34.
DeFronzo RA, Fleck PR, Wilson CA, Mekki Q, Alogliptin Study 010 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31(12):2315–7.
Pratley RE, Reusch JE, Fleck PR, Wilson CA, Mekki Q, Alogliptin Study 009 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2009;25(10):2361–71.
Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q, Alogliptin Study 007 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11(2):167–76.
Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q, Alogliptin Study 008 Group. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63(1):46–55.
Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11(12):1145–52.
DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Efficacy and tolerability of the DPP-4 inhibitor alogliptin combined with pioglitazone, in metformin-treated patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(5):1615–22.
Bosi E, Ellis GC, Wilson CA, Fleck PR. Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study. Diabetes Obes Metab. 2011;13(12):1088–96.
Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2012;14(6):565–74.
Taskinen MR, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, Woerle HJ. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13(1):65–74.
Owens DR, Swallow R, Dugi KA, Woerle HJ. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28(11):1352–61.
Barnett AH, Huisman H, Jones R, von Eynatten M, Patel S, Woerle HJ. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet. 2013 (Epub Ahead of Print).
Thrasher J, Daniels K, Patel S, Whetteckey J. Black/African American patients with type 2 diabetes mellitus: study design and baseline patient characteristics from a randomized clinical trial of linagliptin. Expert Opin Pharmacother. 2012;13(17):2443–52.
Barnett AH, Patel S, Harper R, Toorawa R, Thiemann S, von Eynatten M, Woerle HJ. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: an 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diabetes Obes Metab. 2012 (Epub Ahead of Print).
Kawamori R, Inagaki N, Araki E, Watada H, Hayashi N, Horie Y, Sarashina A, Gong Y, von Eynatten M, Woerle HJ, Dugi KA. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012;14(4):348–57.
Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, von Eynatten M, Dugi KA, Woerle HJ. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380(9840):475–83.
Pfützner A, Paz-Pacheco E, Allen E, Frederich R, Chen R, CV181039 Investigators. Initial combination therapy with saxagliptin and metformin provides sustained glycaemic control and is well tolerated for up to 76 weeks. Diabetes Obes Metab. 2011;13(6):567–76.
Rosenstock J, Aguilar-Salinas C, Klein E, Nepal S, List J, Chen R, CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin. 2009;25(10):2401–11.
Hollander PL, Li J, Frederich R, Allen E, Chen R, CV181013 Investigators. Safety and efficacy of saxagliptin added to thiazolidinedione over 76 weeks in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2011;8(2):125–35.
Frederich R, McNeill R, Berglind N, Fleming D, Chen R. The efficacy and safety of the dipeptidyl peptidase-4 inhibitor saxagliptin in treatment-naïve patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetol Metab Syndr. 2012;4(1):36.
DeFronzo RA, Hissa MN, Garber AJ, Luiz Gross J, Yuyan Duan R, Ravichandran S, Chen RS, Saxagliptin 014 Study Group. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32(9):1649–55.
Chacra AR, Tan GH, Ravichandran S, List J, Chen R, CV181040 Investigators. Safety and efficacy of saxagliptin in combination with submaximal sulphonylurea versus up-titrated sulphonylurea over 76 weeks. Diab Vasc Dis Res. 2011;8(2):150–9.
Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin. 2012;28(4):513–23.
Göke B, Gallwitz B, Eriksson JG, Hellqvist Å, Gause-Nilsson I. Saxagliptin vs. glipizide as add-on therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: long-term (52-week) extension of a 52-week randomised controlled trial. Int J Clin Pract. 2013;67(4):307–16.
Hermans MP, Delibasi T, Farmer I, Lohm L, Maheux P, Piatti P, Malvolti E, Jörgens S, Charbonnel B. Effects of saxagliptin added to sub-maximal doses of metformin compared with uptitration of metformin in type 2 diabetes: the PROMPT study. Curr Med Res Opin. 2012;28(10):1635–45.
Pan CY, Yang W, Tou C, Gause-Nilsson I, Zhao J. Efficacy and safety of saxagliptin in drug-naïve Asian patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab Res Rev. 2012;28(3):268–75.
Yang W, Pan CY, Tou C, Zhao J, Gause-Nilsson I. Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Res Clin Pract. 2011;94(2):217–24.
Fonseca V, Staels B, Morgan JD 2nd, Shentu Y, Golm GT, Johnson-Levonas AO, Kaufman KD, Goldstein BJ, Steinberg H. Efficacy and safety of sitagliptin added to ongoing metformin and pioglitazone combination therapy in a randomized, placebo-controlled, 26-week trial in patients with type 2 diabetes. J Diabetes Complications. 2013;27(2):177–83.
Charbonnel B, Steinberg H, Eymard E, Xu L, Thakkar P, Prabhu V, Davies MJ, Engel SS. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia. 2013;56(7):1503–11.
Vilsbøll T, Rosenstock J, Yki-Järvinen H, Cefalu WT, Chen Y, Luo E, Musser B, Andryuk PJ, Ling Y, Kaufman KD, Amatruda JM, Engel SS, Katz L. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(2):167–77.
Chan JC, Scott R, Arjona Ferreira JC, Sheng D, Gonzalez E, Davies MJ, Stein PP, Kaufman KD, Amatruda JM, Williams-Herman D. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab. 2008;10(7):545–55.
Raz I, Chen Y, Wu M, Hussain S, Kaufman KD, Amatruda JM, Langdon RB, Stein PP, Alba M. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin. 2008;24(2):537–50.
Dobs AS, Goldstein BJ, Aschner P, Horton ES, Umpierrez GE, Duran L, Hill JS, Chen Y, Golm GT, Langdon RB, Williams-Herman DE, Kaufman KD, Amatruda JM, Ferreira JC. Efficacy and safety of sitagliptin added to ongoing metformin and rosiglitazone combination therapy in a randomized placebo-controlled 54-week trial in patients with type 2 diabetes. J Diabetes. 2013;5(1):68–79.
Williams-Herman D, Johnson J, Teng R, Golm G, Kaufman KD, Goldstein BJ, Amatruda JM. Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(5):442–51.
Yoon KH, Steinberg H, Teng R, Golm GT, Lee M, O’Neill EA, Kaufman KD, Goldstein BJ. Efficacy and safety of initial combination therapy with sitagliptin and pioglitazone in patients with type 2 diabetes: a 54-week study. Diabetes Obes Metab. 2012;14(8):745–52.
Yang W, Guan Y, Shentu Y, Li Z, Johnson-Levonas AO, Engel SS, Kaufman KD, Goldstein BJ, Alba M. The addition of sitagliptin to ongoing metformin therapy significantly improves glycemic control in Chinese patients with type 2 diabetes. J Diabetes. 2012;4(3):227–37.
Arechavaleta R, Seck T, Chen Y, Krobot KJ, O’Neill EA, Duran L, Kaufman KD, Williams-Herman D, Goldstein BJ. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2011;13(2):160–8.
Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE, DURATION-2 Study Group. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomized trial. Lancet. 2010;376(9739):431–9.
Aschner P, Chan J, Owens DR, Picard S, Wang E, Dain MP, Pilorget V, Echtay A, Fonseca V, EASIE investigators. Insulin glargine versus sitagliptin in insulin-naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open-label trial. Lancet. 2012;379(9833):2262–9.
Aschner P, Katzeff HL, Guo H, Sunga S, Williams-Herman D, Kaufman KD, Goldstein BJ, Sitagliptin Study 049 Group. Efficacy and safety of monotherapy of sitagliptin compared with metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(3):252–61.
Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H, Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49(11):2564–71.
Pratley R, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Garber A, Thomsen AB, Hartvig H, Davies M, 1860 LIRA-DPP-4 Study Group. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65(4):397–407.
Seck TL, Engel SS, Williams-Herman DE, Sisk CM, Golm GT, Wang H, Kaufman KD, Goldstein BJ. Sitagliptin more effectively achieves a composite endpoint for A1C reduction, lack of hypoglycemia and no body weight gain compared with glipizide. Diabetes Res Clin Pract. 2011;93(1):e15–7.
Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013 (Epub ahead of print).
Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P, Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28(10):1556–68.
Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9(5):733–45.
Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, González JG, Chan M, Wolka AM, Boardman MK, DURATION-4 Study Group. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35(2):252–8.
Strain WD, Lukashevich V, Kothny W, Hoellinger MJ, Paldánius PM. Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet. 2013;382(9890):409–16.
Scherbaum WA, Schweizer A, Mari A, Nilsson PM, Lalanne G, Jauffret S, Foley JE. Efficacy and tolerability of vildagliptin in drug-naïve patients with type 2 diabetes and mild hyperglycaemia*. Diabetes Obes Metab. 2008;10(8):675–82.
Garber AJ, Foley JE, Banerji MA, Ebeling P, Gudbjörnsdottir S, Camisasca RP, Couturier A, Baron MA. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10(11):1047–56.
Rosenstock J, Niggli M, Maldonado-Lutomirsky M. Long-term 2-year safety and efficacy of vildagliptin compared with rosiglitazone in drug-naïve patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11(6):571–8.
Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Care. 2007;30(2):217–23.
Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11(5):506–15.
Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50(6):1148–55.
Pan C, Yang W, Barona JP, Wang Y, Niggli M, Mohideen P, Wang Y, Foley JE. Comparison of vildagliptin and acarbose monotherapy in patients with Type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med. 2008;25(4):435–41.
Pan C, Xing X, Han P, Zheng S, Ma J, Liu J, Lv X, Lu J, Bader G, Institution Investigators. Efficacy and tolerability of vildagliptin as add-on therapy to metformin in Chinese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(8):737–44.
Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30(4):890–5.
Bolli G, Dotta F, Colin L, Minic B, Goodman M. Comparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Obes Metab. 2009;11(6):589–95.
Ferrannini E, Fonseca V, Zinman B, Matthews D, Ahrén B, Byiers S, Shao Q, Dejager S. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11(2):157–66.
Goodman M, Thurston H, Penman J. Efficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Horm Metab Res. 2009;41(5):368–73.
Foley JE, Sreenan S. Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naïve patients with type 2 diabetes. Horm Metab Res. 2009;41(12):905–9.
Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naïve patients with Type 2 diabetes. Diabet Med. 2007;24(9):955–61.
Schweizer A, Dejager S, Bosi E. Comparison of vildagliptin and metformin monotherapy in elderly patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Obes Metab. 2009;11(8):804–12.
Lukashevich V, Schweizer A, Shao Q, Groop PH, Kothny W. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obes Metab. 2011;13(10):947–54.
Schernthaner G, Barnett AH, Emser A, Patel S, Troost J, Woerle HJ, von Eynatten M. Safety and tolerability of linagliptin: a pooled analysis of data from randomized controlled trials in 3572 patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(5):470–8.
Doucet J, Chacra A, Maheux P, Lu J, Harris S, Rosenstock J. Efficacy and safety of saxagliptin in older patients with type 2 diabetes mellitus. Curr Med Res Opin. 2011;27(4):863–9.
Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27(Suppl 3):57–64.
Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs-insights from the rosiglitazone experience. N Engl J Med. 2013 (Epub ahead of print).
Williams-Herman D, Engel SS, Round E, Johnson J, Golm GT, Guo H, Musser BJ, Davies MJ, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord. 2010;10:7.
Schweizer A, Dejager S, Foley JE, Couturier A, Ligueros-Saylan M, Kothny W. Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large Phase III type 2 diabetes population. Diabetes Obes Metab. 2010;12(6):485–94.
Takasawa W, Ohnuma K, Hatano R, Endo Y, Dang NH, Morimoto C. Inhibition of dipeptidyl peptidase 4 regulates microvascular endothelial growth induced by inflammatory cytokines. Biochem Biophys Res Commun. 2010;401(1):7–12.
Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3(2):195–201.
Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, Agostini C, Tiengo A, Avogaro A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010;33(7):1607–9.
ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
ACCORD Study Group, Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH Jr, Byington RP, Rosenberg YD, Friedewald WT. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–28.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.
Tkác I. Effect of intensive glycemic control on cardiovascular outcomes and all-cause mortality in type 2 diabetes: Overview and metaanalysis of five trials. Diabetes Res Clin Pract. 2009;86(Suppl 1):S57–62.
Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53.
Acknowledgments
Guarantor: Shikhar Agarwal MD MPH FACP.
Conflicts of interest
None.
Author contributions
SA: Data collection, extraction, and analysis; manuscript writing.
AP: Data collection, extraction, and analysis; manuscript writing.
VM: Senior author, conception of idea, manuscript writing, critical appraisal, and proof reading.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
40256_2014_70_MOESM1_ESM.pdf
Supplementary figure 1: Forest plot comparing the odds of cardiovascular death in patients randomized to dipeptidyl peptidase 4-inhibitors compared with those randomized to placebo or alternative anti-diabetic therapy
40256_2014_70_MOESM2_ESM.pdf
Supplementary figure 2: Forest plot comparing the odds of myocardial infarction in patients randomized to dipeptidyl peptidase 4-inhibitors compared with those randomized to placebo or alternative anti-diabetic therapy
40256_2014_70_MOESM3_ESM.pdf
Supplementary figure 3: Forest plot comparing the odds of stroke in patients randomized to dipeptidyl peptidase 4-inhibitors compared with those randomized to placebo or alternative anti-diabetic therapy
40256_2014_70_MOESM4_ESM.pdf
Supplementary figure 4: Forest plot comparing the odds of all-cause mortality in patients randomized to dipeptidyl peptidase 4-inhibitors compared with those randomized to placebo or alternative anti-diabetic therapy
40256_2014_70_MOESM5_ESM.pdf
Supplementary figure 5: Forest plot comparing the odds of major adverse cardiovascular events in patients randomized to dipeptidyl peptidase 4-inhibitors compared with those randomized to placebo or alternative anti-diabetic therapy
40256_2014_70_MOESM6_ESM.pdf
Supplementary figure 6: Funnel plot for comparison of cardiovascular death between dipeptidyl peptidase 4-inhibitors and placebo/alternative anti-diabetic therapy. The dotted line represents the pseudo 95 % confidence intervals for the effect estimate (vertical line in the center)
Rights and permissions
About this article
Cite this article
Agarwal, S., Parashar, A. & Menon, V. Meta-Analysis of the Cardiovascular Outcomes with Dipeptidyl Peptidase 4 Inhibitors: Validation of the Current FDA Mandate. Am J Cardiovasc Drugs 14, 191–207 (2014). https://doi.org/10.1007/s40256-014-0070-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-014-0070-7