Abstract
Background
Large randomized trials have shown conflicting evidence regarding the cardiovascular safety of dipeptidyl-peptidase 4 (DPP-4) inhibitors. Systematic reviews have been limited by incomplete data and inclusion of observational studies. This study aimed to systematically evaluate the cardiovascular safety of DPP-4 inhibitors in patients with type 2 diabetes.
Methods
Electronic databases were searched for randomized trials that compared DPP-4 inhibitors versus placebo and reported cardiovascular outcomes. The main outcome assessed in this analysis was heart failure. Other outcomes included all-cause mortality, cardiovascular mortality, myocardial infarction, and ischemic stroke. Summary odds ratios (ORs) were primarily constructed using Peto’s model.
Results
A total of 90 trials with 66,730 patients were included. Compared with placebo, DPP-4 inhibitors were associated with a non-significant increased risk of heart failure [OR 1.11, 95% confidence interval (CI) 0.99–1.25, P = 0.07] at a mean of 108 weeks. The risk of all-cause mortality (OR 1.03, 95% CI 0.94–1.12, P = 0.53), cardiovascular mortality (OR 1.02, 95% CI 0.92–1.14, P = 0.72), myocardial infarction (OR 0.98, 95% CI 0.88–1.09, P = 0.69), and ischemic stroke (OR 0.99, 95% CI 0.85–1.15, P = 0.92) was similar between both groups.
Conclusion
In patients with type 2 diabetes, the safety profile of DPP-4 inhibitors is similar to placebo. As a class, there is only weak evidence for an increased risk of heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Large randomized trials have shown conflicting evidence regarding the cardiovascular safety of dipeptidyl-peptidase 4 (DPP-4) inhibitors. |
This meta-analysis demonstrated that the safety profile of DPP-4 inhibitors is similar to placebo. |
Patients and providers can feel reassured that DPP-4 inhibitors are well tolerated and can represent a valuable component in the armamentarium of anti-glycemic therapy. |
1 Introduction
In the USA, diabetes mellitus affects approximately 21 million adults [1]. Dipeptidyl-peptidase 4 (DPP-4) inhibitors have emerged as a new class of incretin-based medications for the management of type 2 diabetes mellitus [2]. They improve glucose control without inducing hypoglycemia or weight gain [3]. Some authors had suggested that these medications might exert a cardiovascular protective effect [4]. The American Diabetes Association and the European Association for the Study of Diabetes recommend this class as a second-line agent in patients with type 2 diabetes [5]. In the largest randomized trial designed to explore the cardiovascular safety of this class of medications, there was a 27% relative increased risk for heart failure hospitalizations with saxagliptin compared with placebo [6]. In two other large randomized trials testing alogliptin and sitagliptin, the risk of heart failure was similar compared with placebo [7, 8]. Furthermore, data from real world registries have yielded inconsistent results regarding the risk of heart failure with this class of medications [9–13]. Previous meta-analyses were limited by incomplete evaluation of cardiovascular safety outcomes [14–16], non-comprehensive evaluation of data [17–21], or inclusion of data from observational studies [22]. Given the uncertainty about the cardiovascular safety of this class of medications, we aimed to conduct a comprehensive meta-analysis of placebo-controlled randomized trials to test the cardiovascular safety of this class of medications.
2 Methods
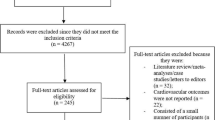
We searched the MEDLINE database without language restriction from inception until August 2015 using the keywords and Medical Subject Headings illustrated in Fig. 1. We also searched the Web of Science and the Cochrane Register of Controlled Trials databases using similar keywords. This meta-analysis was registered at the PROSPERO international prospective register of systematic reviews (CRD42015024674) [23].
We selected randomized controlled clinical trials that compared any of the DPP-4 inhibitors (i.e., anagliptin, alogliptin, dutogliptin, linagliptin, omarigliptin, sitagliptin, saxagliptin, teneligliptin, and vildagliptin) with placebo in patients with type 2 diabetes. We required that the published report for the study explicitly reported any cardiovascular outcome (namely, heart failure, all-cause mortality, cardiovascular mortality, myocardial infarction, or ischemic stroke). We excluded trials that compared DPP-4 inhibitors with any other comparator agent (e.g., metformin, sulfonylurea, or thiazolidinediones) in order to test the relative safety of DPP-4 inhibitors with placebo. We also excluded trials that had unequal distribution of a second oral hypoglycemic agent in either arm. For trials with multiple comparison arms, we combined the DPP-4 inhibitor arms irrespective of the doses. For trials that had a second phase with an active agent (i.e., another oral hypoglycemic agent), we reported outcomes only for the placebo-controlled phase. For these trials, we excluded the ones that reported cardiovascular outcomes only at the end of the second phase.
Teams of two paired reviewers extracted data on general study data, study design, sample size, patient characteristics, interventional strategies, and cardiovascular outcomes from the included studies. Two reviewers further reviewed the extracted data to ensure accuracy. Reviewers resolved any discrepancies by discussion. For all clinical outcomes, we tabulated the number of events that occurred in each arm. Since it is mandated that parties submit a summary of the results including serious adverse events to clinicaltrial.gov, we searched this registry for each of the included studies to ensure that we collected any possible cardiovascular outcome. We evaluated the quality of the included trials on the basis of adequate description of treatment allocation, blinded outcome assessment, and description of losses to follow-up [24].
The main outcome evaluated in this analysis was heart failure. We defined heart failure as any reported case of “heart failure,” “cardiac failure,” or “hospitalization for heart failure.” We also evaluated all-cause mortality, cardiovascular mortality, myocardial infarction, and ischemic stroke (defined as ischemic stroke or transient ischemic attack). If a study reported outcomes at different follow-up periods, we preferentially extracted data for the longest reported follow-up.
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to conduct a high-quality meta-analysis [25]. We analyzed the outcomes with an intention-to-treat analysis. Since we anticipated that the outcomes are rare [26], we constructed the summary estimate odds ratios (ORs) primarily with Peto’s model [27]. We performed a secondary analysis using random effects summary risk ratios (RRs) with a DerSimonian and Laird model [28]. We performed the overall analysis for each outcome using the data reported from the longest follow-up period. We examined the statistical heterogeneity using I 2 statistic [29]. We evaluated publication bias with Egger’s method [30]. All P values were two-tailed, with statistical significance set at 0.05, and confidence intervals (CIs) were calculated at the 95% level for the overall estimate effect. We conducted all analyses with STATA software version 14 (STATA Corporation; College Station, TX, USA).
For the outcome of heart failure, we further performed a pre-specified sensitivity analysis including only the high-quality trials, and another sensitivity analysis including only trials that enrolled >1000 subjects. We conducted subgroup analyses according to the follow-up time (i.e., ≤24 weeks, 24–52 weeks, >52–104 weeks, and >104 weeks). Random effects meta-regression analyses were pre-specified for the outcome of heart failure with the type of DPP-4 inhibitor, age, male gender, hemoglobin A1c (HbA1c) level, duration of diabetes, and body mass index (BMI).
3 Results
The electronic search yielded 1040 articles, which we screened by reviewing the title and/or abstract; 137 articles were deemed potentially eligible. Upon further review of the full article, 90 trials with 66,730 patients were included in the final analysis (Fig. 1) [6–8, 31–117]. Table 1 summarizes the baseline characteristics of the included trials. Sitagliptin was the most frequent medication tested (i.e., in 24 trials), while 15 trials evaluated saxagliptin. All of the included studies were multicenter and double blinded. The follow-up time ranged from 2 to 156 weeks. Supplemental Table 1 reports the quality of the included studies (see the electronic supplementary material, online resource 1). The primary outcome for most of the included studies was the change in the HbA1c level at the end of the follow-up period. Only three trials evaluated cardiovascular outcomes as the primary outcome [6, 8, 50].
Twenty-five trials evaluated the outcome of heart failure (nine of these studies had zero events in both arms). The mean follow-up was 108 ± 45 weeks (median follow-up time 109 weeks). We utilized data regarding heart failure events for the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial from the pre-specified analysis that evaluated heart failure [7]. Compared with placebo, DPP-4 inhibitors were associated with a non-significant increase in the risk of heart failure using both Peto’s method (OR 1.11, 95% CI 0.99–1.25, P = 0.07) (Fig. 2) and the DerSimonian and Laird model (RR 1.11, 95% CI 0.99–1.24, P = 0.09, I 2 = 0%). There was no evidence of publication bias with Egger’s test (P = 0.23). The pre-specified sensitivity analysis including only high-quality studies yielded similar results (OR 1.13, 95% CI 0.99–1.28, P = 0.07, I 2 = 27%), as well as the pre-specified analysis limited to trials that enrolled >1000 subjects (OR 1.12, 95% CI 1.00–1.26, P = 0.06, I 2 = 42%) (Supplemental Figure 1). Subgroup analyses according to the follow-up time showed that the risk of heart failure was comparable to placebo at ≤24 weeks (OR 0.48, 95% CI 0.13–1.73), at 24–52 weeks (OR 2.64, 95% CI 0.54–12.87), and at >52–104 weeks (OR 0.35, 95% CI 0.02–7.60), but a non-significant increase in heart failure at >104 weeks (OR 1.12, 95% CI 0.99–1.26) (P for interaction = 0.27). Meta-regression analysis did not identify a difference in treatment effect based on the type of DPP-4 inhibitors, age, male gender, HbA1c level, duration of diabetes, and BMI (P = 0.76, 0.34, 0.24, 0.23, 0.66, and 0.10, respectively).
Compared with placebo, DPP-4 inhibitors were associated with a similar risk of all-cause mortality (OR 1.03, 95% CI 0.94–1.12, P = 0.53, I 2 = 0%) (Fig. 3), cardiovascular mortality (OR 1.02, 95% CI 0.92–1.14, P = 0.72, I 2 = 0%) (Supplemental Figure 2), myocardial infarction (OR 0.98, 95% CI 0.88–1.09, P = 0.69, I 2 = 10%) (Supplemental Figure 3), and ischemic stroke (OR 0.99, 95% CI 0.85–1.15, P = 0.92, I 2 = 20%) (Supplemental Figure 4). There was no evidence of publication bias for the secondary outcomes. In Table 2, we summarize the summary estimates for the outcomes assessed in this meta-analysis.
4 Discussion
In this comprehensive meta-analysis of 90 double-blind, multicenter, placebo-controlled randomized clinical trials with 66,730 patients; we demonstrated that DPP-4 inhibitors were associated with a non-significant increase in the risk of heart failure at a mean of 108 weeks. We performed various sensitivity and meta-regression analyses to further explore any potential explanation for this observed finding. Our results suggested that any potential increase in the risk of heart failure was driven by one large trial [6]. Reassuringly, we also demonstrated that DPP-4 inhibitors were associated with a similar risk of all-cause mortality, cardiovascular mortality, myocardial infarction, and ischemic stroke compared with placebo.
In the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI) 53 trial, saxagliptin neither increased nor decreased the composite of cardiovascular death, myocardial infarction, or ischemic stroke compared with placebo [6]. There was an unexpected 27% increased relative risk of hospitalization for heart failure in the saxagliptin arm. A post hoc analysis of this trial revealed that the risk of hospitalization for heart failure was increased among patients with elevated levels of natriuretic peptides at baseline, previous heart failure, or chronic kidney disease [118]. It remains unclear how saxagliptin might predispose to heart failure; a pooled analysis of 20 trials suggested that there was no evidence of fluid retention or weight gain with saxagliptin [21]. One plausible explanation for the increased heart failure risk in the SAVOR-TIMI 53 trial was the relatively large number of subjects with a prior history of heart failure at baseline (~13%). Most of the large randomized trials evaluating oral hypoglycemic agents in general had a lower number of subjects with previous heart failure history [119]. In the two other large randomized trials evaluating the cardiovascular outcomes with DPP-4 inhibitors [i.e., Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) and EXAMINE], the risk of hospitalization for heart failure was not increased with either sitagliptin or alogliptin, respectively [7, 8, 50]. We performed a sensitivity analysis for these three large trials and found that there was a non-significant increase in the risk of heart failure, again driven by the results of the SAVOR-TIMI 53 trial. In a large multicentre cohort of 1,499,650 diabetic patients, incretin-based drugs [i.e., DPP-4 inhibitors and glucagon-like peptide-1 (GLP-1) analogs] were not associated with an increased risk of hospitalization for heart failure, as compared with oral antidiabetic drugs [120]. Our analysis of placebo-controlled randomized trials further supports that DPP-4 inhibitors as a class have only weak evidence for an increased risk of heart failure.
A recent systematic review evaluated the risk of heart failure with DPP-4 inhibitors and concluded that the risk of heart failure is uncertain with DPP-4 inhibitors [22]. However, that analysis was limited by the inclusion of observational studies that can be prone to bias. In addition, the authors used placebo and active agents in the comparator arm, which could have affected their results. In the present analysis, we included only placebo-controlled randomized trials in order to conduct a robust analysis. Furthermore, we assessed a wide spectrum of cardiovascular outcomes besides heart failure (i.e., all-cause mortality, cardiovascular mortality, myocardial infarction, and ischemic stroke), in order to provide a comprehensive analysis on the cardiovascular safety of DPP-4 inhibitors.
The present analysis has some limitations. First, the follow-up duration was variable among the included studies; thus we performed several subgroup analyses according to the follow-up time and demonstrated that the results were almost similar among these subgroups. Second, we performed our primary analysis with a fixed effects model (i.e., Peto’s). We determined that Peto’s model would be a good model for this particular analysis, given that the outcomes that we assessed were rare [26]. Furthermore, a secondary analysis with a DerSimonian and Laird model showed that the results were fairly robust irrespective of the methodology used. Third, the definition of heart failure was variable among the included studies; however, we observed no heterogeneity with statistical testing. Fourth, most of the included studies were small and not designed to address cardiovascular outcomes; however, all the included studies were designed to test the safety of the medication. In addition, we performed a sensitivity analysis limited to the three trials that tested cardiovascular outcomes as the primary outcome, which yielded similar results. Fifth, a considerable number of the studies had a significant drop-out rate at the end of the follow-up period; therefore we performed a sensitivity analysis excluding these low-quality studies. Finally, a lack of access to patient level data precluded a full evaluation to identify patient characteristics (e.g., renal disease, prior history of heart failure) associated with the potential risk for heart failure; however, we performed multiple meta-regression analyses using the available study-level data and found that none of the tested demographics were significant.
5 Conclusion
In patients with type 2 diabetes, DPP-4 inhibitors are relatively well tolerated compared with placebo. As a class, there is only weak evidence for an increased risk of heart failure.
References
Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517–25.
Scheen AJ. Cardiovascular effects of gliptins. Nat Rev Cardiol. 2013;10:73–84.
Kawalec P, Mikrut A, Łopuch S. The safety of dipeptidyl peptidase-4 (DPP-4) inhibitors or sodium-glucose cotransporter 2 (SGLT-2) inhibitors added to metformin background therapy in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2014;30:269–83.
Murohara T. Dipeptidyl peptidase-4 inhibitor: another player for cardiovascular protection. J Am Coll Cardiol. 2012;59:277–9.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–76.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42.
Fadini GP, Avogaro A, Degli Esposti L, Russo P, Saragoni S, Buda S, Rosano G, Pecorelli S, Pani L. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP-4 inhibitors or other oral glucose-lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health-DB Database. Eur Heart J. 2015;36:2454–62.
Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH, Chen SJ, Yeh CM, Chen TJ, Lin SJ, Chiang CE. Sitagliptin and the risk of hospitalization for heart failure: a population-based study. Int J Cardiol. 2014;177:86–90.
Weir DL, McAlister FA, van Senthilsel A, Minhas-Sandhu JK, Eurich DT. Sitagliptin use in patients with diabetes and heart failure: a population-based retrospective cohort study. JACC Heart Fail. 2014;2:573–82.
Ou SM, Shih CJ, Chao PW, Chu H, Kuo SC, Lee YJ, Wang SJ, Yang CY, Lin CC, Chen TJ, Tarng DC, Li SY, Chen YT. Effects on clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015;163:663–72.
Fu AZ, Johnston SS, Ghannam A, Tsai K, Cappell K, Fowler R, Riehle E, Cole AL, Kalsekar I, Sheehan J. Association between hospitalization for heart failure and dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes: an observational study. Diabetes Care. 2016;39:726–34.
Monami M, Iacomelli I, Marchionni N, Mannucci E. Dipeptydil peptidase-4 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2010;20:224–35.
Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008:CD006739.
Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4:753–68.
Savarese G, Perrone-Filardi P, D’Amore C, Vitale C, Trimarco B, Pani L, Rosano GM. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors in diabetic patients: a meta-analysis. Int J Cardiol. 2015;181:239–44.
Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2014;24:689–97.
Schweizer A, Dejager S, Foley JE, Couturier A, Ligueros-Saylan M, Kothny W. Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large phase III type 2 diabetes population. Diabetes Obes Metab 2010;12:485–94.
Williams-Herman D, Engel SS, Round E, Johnson J, Golm GT, Guo H, Musser BJ, Davies MJ, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord. 2010;10:7.
Iqbal N, Parker A, Frederich R, Donovan M, Hirshberg B. Assessment of the cardiovascular safety of saxagliptin in patients with type 2 diabetes mellitus: pooled analysis of 20 clinical trials. Cardiovasc Diabetol. 2014;13:33.
Li L, Li S, Deng K, Liu J, Vandvik PO, Zhao P, Zhang L, Shen J, Bala MM, Sohani ZN, Wong E, Busse JW, Ebrahim S, Malaga G, Rios LP, Wang Y, Chen Q, Guyatt GH, Sun X. Dipeptidyl peptidase 4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. BMJ 2016;352:i610.
Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, Stewart L. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2.
Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42–6.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
O’Connor D, Green S, Higgins JP. 9.4.4.2. Peto odds ratio method. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Available: http://handbook.cochrane.org/chapter_9/9_4_4_2_peto_odds_ratio_method.htm. Accessed on 25 Feb 2016.
Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–71.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in metaanalyses. BMJ. 2003;327:557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Matthaei S, Catrinoiu D, Celiński A, Ekholm E, Cook W, Hirshberg B, Chen H, Iqbal N, Hansen L. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015;38:2018–24.
Mathieu C, Shankar RR, Lorber D, Umpierrez G, Wu F, Xu L, Golm GT, Latham M, Kaufman KD, Engel SS. A randomized clinical trial to evaluate the efficacy and safety of co-administration of sitagliptin with intensively titrated insulin glargine. Diabetes Ther. 2015;6:127–42.
Yang HK, Min KW, Park SW, Chung CH, Park KS, Choi SH, Song KH, Kim DM, Lee MK, Sung YA, Baik SH, Kim IJ, Cha BS, Park JH, Ahn YB, Lee IK, Yoo SJ, Kim J, Park IeB, Park TS, Yoon KH. A randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of anagliptin in drug-naïve patients with type 2 diabetes. Endocr J. 2015;62:449–62.
Wang W, Yang J, Yang G, Gong Y, Patel S, Zhang C, Izumoto T, Ning G. Efficacy and safety of linagliptin in Asian patients with type 2 diabetes mellitus inadequately controlled by metformin: a multinational 24-week, randomized clinical trial. J Diabetes. 2016;8:229–37.
Yang W, Xing X, Lv X, Li Y, Ma J, Yuan G, Sun F, Wang W, Woloschak M, Lukashevich V, Kozlovski P, Kothny W. Vildagliptin added to sulfonylurea improves glycemic control without hypoglycemia and weight gain in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7:174–81.
Sheu WH, Gantz I, Chen M, Suryawanshi S, Mirza A, Goldstein BJ, Kaufman KD, Engel SS. Safety and efficacy of omarigliptin (MK-3102), a novel once-weekly DPP-4 inhibitor for the treatment of patients with type 2 diabetes. Diabetes Care. 2015;38:2106–14.
Ning G, Wang W, Li L, Ma J, Lv X, Yang M, Wang W, Woloschak M, Lukashevich V, Kothny W. Vildagliptin as add-on therapy to insulin improves glycemic control without increasing risk of hypoglycemia in Asian, predominantly Chinese, patients with type 2 diabetes mellitus. J Diabetes. 2016;8:345–53.
Bajaj M, Gilman R, Patel S, Kempthorne-Rawson J, Lewis-D’Agostino D, Woerle HJ. Linagliptin improved glycaemic control without weight gain or hypoglycaemia in patients with type 2 diabetes inadequately controlled by a combination of metformin and pioglitazone: a 24-week randomized, double-blind study. Diabet Med. 2014;31:1505–14.
Thrasher J, Daniels K, Patel S, Whetteckey J, Woerle HJ. Efficacy and safety of linagliptin in black/African American patients with type 2 diabetes: a 6-month, randomized, double-blind, placebo-controlled study. Endocr Pract. 2014;20:412–20.
Pratley RE, Fleck P, Wilson C. Efficacy and safety of initial combination therapy with alogliptin plus metformin versus either as monotherapy in drug-naïve patients with type 2 diabetes: a randomized, double-blind, 6-month study. Diabetes Obes Metab. 2014;16:613–21.
White JL, Buchanan P, Li J, Frederich R. A randomized controlled trial of the efficacy and safety of twice-daily saxagliptin plus metformin combination therapy in patients with type 2 diabetes and inadequate glycemic control on metformin monotherapy. BMC Endocr Disord. 2014;14:17.
Odawara M, Hamada I, Suzuki M. Efficacy and safety of vildagliptin as add-on to metformin in Japanese patients with type 2 diabetes mellitus. Diabetes Ther. 2014;5:169–81.
Ahrén B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, Feinglos MN. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–88.
Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diabetes Obes Metab. 2014;16:418–25.
Moses RG, Kalra S, Brook D, Sockler J, Monyak J, Visvanathan J, Montanaro M, Fisher SA. A randomized controlled trial of the efficacy and safety of saxagliptin as add-on therapy in patients with type 2 diabetes and inadequate glycaemic control on metformin plus a sulphonylurea. Diabetes Obes Metab. 2014;16:443–50.
Lukashevich V, Del Prato S, Araga M, Kothny W. Efficacy and safety of vildagliptin in patients with type 2 diabetes mellitus inadequately controlled with dual combination of metformin and sulphonylurea. Diabetes Obes Metab. 2014;16:403–9.
Hage C, Brismar K, Efendic S, Lundman P, Rydén L, Mellbin L. Sitagliptin improves beta-cell function in patients with acute coronary syndromes and newly diagnosed glucose abnormalities–the BEGAMI study. J Intern Med. 2013;273:410–21.
Barnett AH, Charbonnel B, Li J, Donovan M, Fleming D, Iqbal N. Saxagliptin add-on therapy to insulin with or without metformin for type 2 diabetes mellitus: 52-week safety and efficacy. Clin Drug Investig. 2013;33:707–17.
Barnett AH, Huisman H, Jones R, von Eynatten M, Patel S, Woerle HJ. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1413–23.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35.
Strain WD, Lukashevich V, Kothny W, Hoellinger MJ, Paldánius PM. Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet. 2013;382:409–16.
Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:252–7.
McGill JB, Sloan L, Newman J, Patel S, Sauce C, von Eynatten M, Woerle HJ. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36:237–44.
Yki-Järvinen H, Rosenstock J, Durán-Garcia S, Pinnetti S, Bhattacharya S, Thiemann S, Patel S, Woerle HJ. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52-week randomized, double-blind study. Diabetes Care. 2013;36:3875–81.
Dobs AS, Goldstein BJ, Aschner P, Horton ES, Umpierrez GE, Duran L, Hill JS, Chen Y, Golm GT, Langdon RB, Williams-Herman DE, Kaufman KD, Amatruda JM, Ferreira JC. Efficacy and safety of sitagliptin added to ongoing metformin and rosiglitazone combination therapy in a randomized placebo-controlled 54-week trial in patients with type 2 diabetes. J Diabetes. 2013;5:68–79.
Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4:576–84.
Rosenstock J, Gross JL, Aguilar-Salinas C, Hissa M, Berglind N, Ravichandran S, Fleming D. Long-term 4-year safety of saxagliptin in drug-naive and metformin-treated patients with type 2 diabetes. Diabet Med. 2013;30:1472–6.
Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–92.
Alba M, Ahrén B, Inzucchi SE, Guan Y, Mallick M, Xu L, O’Neill EA, Williams-Herman DE, Kaufman KD, Goldstein BJ. Sitagliptin and pioglitazone provide complementary effects on postprandial glucose and pancreatic islet cell function. Diabetes Obes Metab. 2013;15:1101–10.
Kadowaki T, Kondo K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:810–8.
Kadowaki T, Tajima N, Odawara M, Nishii M, Taniguchi T, Ferreira JC. Addition of sitagliptin to ongoing metformin monotherapy improves glycemic control in Japanese patients with type 2 diabetes over 52 weeks. J Diabetes Investig. 2013;4:174–81.
Seino Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to sulfonylurea in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label, long-term extension study. J Diabetes Investig. 2012;3:517–25.
Ross SA, Rafeiro E, Meinicke T, Toorawa R, Weber-Born S, Woerle HJ. Efficacy and safety of linagliptin 2.5 mg twice daily versus 5 mg once daily in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, placebo-controlled trial. Curr Med Res Opin 2012;28:1465–74.
Lewin AJ, Arvay L, Liu D, Patel S, von Eynatten M, Woerle HJ. Efficacy and tolerability of linagliptin added to a sulfonylurea regimen in patients with inadequately controlled type 2 diabetes mellitus: an 18-week, multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2012;34:1909–19.
Kothny W, Shao Q, Groop PH, Lukashevich V. One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment. Diabetes Obes Metab. 2012;14:1032–9.
Barnett AH, Patel S, Harper R, Toorawa R, Thiemann S, von Eynatten M, Woerle HJ. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: an 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diabetes Obes Metab. 2012;14:1145–54.
Pan CY, Yang W, Tou C, Gause-Nilsson I, Zhao J. Efficacy and safety of saxagliptin in drug-naïve Asian patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab Res Rev. 2012;28:268–75.
Frederich R, McNeill R, Berglind N, Fleming D, Chen R. The efficacy and safety of the dipeptidyl peptidase-4 inhibitor saxagliptin in treatment-naïve patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetol Metab Syndr. 2012;4:36.
Fonseca V, Staels B, Morgan JD 2nd, Shentu Y, Golm GT, Johnson-Levonas AO, Kaufman KD, Goldstein BJ, Steinberg H. Efficacy and safety of sitagliptin added to ongoing metformin and pioglitazone combination therapy in a randomized, placebo-controlled, 26-week trial in patients with type 2 diabetes. J Diabetes Complications. 2013;27:177–83.
Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin. 2012;28:513–23.
Kawamori R, Inagaki N, Araki E, Watada H, Hayashi N, Horie Y, Sarashina A, Gong Y, von Eynatten M, Woerle HJ, Dugi KA. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012;14:348–57.
Bergenstal RM, Forti A, Chiasson JL, Woloschak M, Boldrin M, Balena R. Efficacy and safety of taspoglutide versus sitagliptin for type 2 diabetes mellitus (T-emerge 4 trial). Diabetes Ther. 2012;3:13.
Eto T, Inoue S, Kadowaki T. Effects of once-daily teneligliptin on 24-h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2012;14:1040–6.
Haak T, Meinicke T, Jones R, Weber S, von Eynatten M, Woerle HJ. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2012;14:565–74.
Yang W, Guan Y, Shentu Y, Li Z, Johnson-Levonas AO, Engel SS, Kaufman KD, Goldstein BJ, Alba M. The addition of sitagliptin to ongoing metformin therapy significantly improves glycemic control in Chinese patients with type 2 diabetes. J Diabetes. 2012;4:227–37.
Yang W, Pan CY, Tou C, Zhao J, Gause-Nilsson I. Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Res Clin Pract. 2011;94:217–24.
Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study. Curr Med Res Opin. 2011;27:1781–92.
Kashiwagi A, Kadowaki T, Tajima N, Nonaka K, Taniguchi T, Nishii M, Ferreira JC, Amatruda JM. Sitagliptin added to treatment with ongoing pioglitazone for up to 52 weeks improves glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig. 2011;2:381–90.
Hollander PL, Li J, Frederich R, Allen E, Chen R. Safety and efficacy of saxagliptin added to thiazolidinedione over 76 weeks in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2011;8:125–35.
Forst T, Uhlig-Laske B, Ring A, Ritzhaupt A, Graefe-Mody U, Dugi KA. The oral DPP-4 inhibitor linagliptin significantly lowers HbA1c after 4 weeks of treatment in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2011;13:542–50.
Nowicki M, Rychlik I, Haller H, Warren M, Suchower L, Gause-Nilsson I, Schützer KM. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J ClinPract. 2011;65:1230–9.
Lukashevich V, Schweizer A, Shao Q, Groop PH, Kothny W. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obes Metab. 2011;13:947–54.
Kaku K, Itayasu T, Hiroi S, Hirayama M, Seino Y. Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label long-term extension study. Diabetes Obes Metab. 2011;13:1028–35.
Henry RR, Smith SR, Schwartz SL, Mudaliar SR, Deacon CF, Holst JJ, Duan RY, Chen RS, List JF. Effects of saxagliptin on β-cell stimulation and insulin secretion in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:850–8.
Owens DR, Swallow R, Dugi KA, Woerle HJ. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011;28:1352–61.
Taskinen MR, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, Woerle HJ. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13:65–74.
Vilsbøll T, Rosenstock J, Yki-Järvinen H, Cefalu WT, Chen Y, Luo E, Musser B, Andryuk PJ, Ling Y, Kaufman KD, Amatruda JM, Engel SS, Katz L. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167–77.
Pattzi HM, Pitale S, Alpizar M, Bennett C, O’Farrell AM, Li J, Cherrington JM, Guler HP. Dutogliptin, a selective DPP4 inhibitor, improves glycaemic control in patients with type 2 diabetes: a 12-week, double-blind, randomized, placebo-controlled, multicentre trial. Diabetes Obes Metab. 2010;12:348–55.
Iwamoto Y, Taniguchi T, Nonaka K, Okamoto T, Okuyama K, Arjona Ferreira JC, Amatruda J. Dose-ranging efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Endocr J. 2010;57:383–94.
Stenlöf K, Raz I, Neutel J, Ravichandran S, Berglind N, Chen R. Saxagliptin and metformin XR combination therapy provides glycemic control over 24 hours in patients with T2DM inadequately controlled with metformin. Curr Med Res Opin. 2010;26:2355–63.
Kikuchi M, Haneda M, Koya D, Tobe K, Onishi Y, Couturier A, Mimori N, Inaba Y, Goodman M. Efficacy and tolerability of vildagliptin as an add-on to glimepiride in Japanese patients with Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2010;89:216–23.
Aaboe K, Knop FK, Vilsbøll T, Deacon CF, Holst JJ, Madsbad S, Krarup T. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12:323–33.
Forst T, Uhlig-Laske B, Ring A, Graefe-Mody U, Friedrich C, Herbach K, Woerle HJ, Dugi KA. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes. Diabet Med. 2010;27:1409–19.
Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63:46–55.
Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11:167–76.
Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11:1145–52.
Heise T, Graefe-Mody EU, Hüttner S, Ring A, Trommeshauser D, Dugi KA. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab. 2009;11:786–94.
Nonaka K, Tsubouchi H, Okuyama K, Fukao Y, Johnson-Levonas AO, Amatruda JM. Effects of once-daily sitagliptin on 24-h glucose control following 4 weeks of treatment in Japanese patients with type 2 diabetes mellitus. Horm Metab Res. 2009;41:232–7.
Goodman M, Thurston H, Penman J. Efficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Horm Metab Res. 2009;41:368–73.
Mohan V, Yang W, Son HY, Xu L, Noble L, Langdon RB, Amatruda JM, Stein PP, Kaufman KD. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract. 2009;83:106–16.
Raz I, Chen Y, Wu M, Hussain S, Kaufman KD, Amatruda JM, Langdon RB, Stein PP, Alba M. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin. 2008;24:537–50.
Kikuchi M, Abe N, Kato M, Terao S, Mimori N, Tachibana H. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83:233–40.
Garcia-Soria G, Gonzalez-Galvez G, Argoud GM, Gerstman M, Littlejohn TW 3rd, Schwartz SL, O’Farrell AM, Li X, Cherrington JM, Bennett C, Guler HP. The dipeptidyl peptidase-4 inhibitor PHX1149 improves blood glucose control in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:293–300.
DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315–7.
Covington P, Christopher R, Davenport M, Fleck P, Mekki QA, Wann ER, Karim A. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther. 2008;30:499–512.
Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376–86.
Garber AJ, Foley JE, Banerji MA, Ebeling P, Gudbjörnsdottir S, Camisasca RP, Couturier A, Baron MA. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047–56.
Scherbaum WA, Schweizer A, Mari A. Efficacy and tolerability of vildagliptin in drug-naïve patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes Metab. 2008;10:675–82.
Rosenstock J, Foley JE, Rendell M, Landin-Olsson M, Holst JJ, Deacon CF, Rochotte E, Baron MA. Effects of the dipeptidyl peptidase-IV inhibitor vildagliptin on incretin hormones, islet function, and postprandial glycemia in subjects with impaired glucose tolerance. Diabetes Care. 2008;31:30–5.
Nonaka K, Kakikawa T, Sato A, Okuyama K, Fujimoto G, Kato N, Suzuki H, Hirayama Y, Ahmed T, Davies MJ, Stein PP. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008;79:291–8.
Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–5.
Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166–74.
Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–87.
Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–45.
Hanefeld M, Herman GA, Wu M, Mickel C, Sanchez M, Stein PP. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin 2007;23:1329–39.
Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61:171–80.
Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–8.
Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–88.
McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–51.
Filion KB, Azoulay L, Platt RW, Dahl M, Dormuth CR, Clemens KK, Hu N, Paterson JM, Targownik L, Turin TC, Udell JA, Ernst P. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. 2016;374:1145–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Dr. Anthony A. Bavry discloses the following relationship: honorarium from American College of Cardiology. The other authors have no conflicts of interest to declare.
Additional information
I. Y. Elgendy and A. N. Mahmoud equally contributed to the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elgendy, I.Y., Mahmoud, A.N., Barakat, A.F. et al. Cardiovascular Safety of Dipeptidyl-Peptidase IV Inhibitors: A Meta-Analysis of Placebo-Controlled Randomized Trials. Am J Cardiovasc Drugs 17, 143–155 (2017). https://doi.org/10.1007/s40256-016-0208-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-016-0208-x