Abstract
Purpose
This work investigated the levels, ecological and potential human health risk of heavy metals in soils and crop plants of an abandoned open municipal solid waste dumpsite being used for agricultural crop planting at Ugwuaji in Enugu, Nigeria.
Method
Samples of soils and plants were collected from the site and heavy metals (Pb, Cr, Cd, Zn, Al and Fe) contents and some soil physicochemical parameters were determined after wet digestion with Nitric acid/perchloric acid mixture. The digested samples and all the supernatants were analyzed for heavy metals using Atomic Absorption Spectrophotometer (Shimadzu AA7000 made in Japan) fitted with deuterium lamp for background correction.
Results
The heavy metal concentrations in the soils (mg/kg) ranged from 20.825 to 62.501; 7.25 to 11.806; 54.074 to 275.559; 10.861 to 40.139; 1835.751 to 2225.768; and 7166.724 to 11,601.940 for Pb, Cd, Cr, Fe, Al and Zn respectively. The Cr, Fe, Al, and Zn contents (mg/kg) in the plants samples were within the recommended limits but Cd levels were higher.
Conclusion
The ecological and health risk assessments revealed contamination and high human health risk associated to Cd. Apart from Cd, other heavy metals under investigation in the soil posed low ecological risk. The target hazard quotient (THQ) values for Cd in the plants samples were all above one except for for Ocimum gratissimum, indicating that people consuming the plants cultivated in the vicinity of the dumpsite may experience adverse effects due to Cd toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a growing awareness of the impacts of solid wastes of anthropogenic origin on the environment [1, 2]. Most often in Nigeria, dumpsites are agricultural lands left to lie fallow and these carelessly disposed wastes have devastating effect on all aspect of the environment- soil, water and air.
The physicochemical properties of soil usually affect the type of vegetation obtainable in such soil. For instance, soil structure and acidity affects the absorption and accumulation of mineral elements by plants [3]. Furthermore, the concentrations and types of heavy metals in soil and consequently in crops around dumpsites are influenced by the wastes types, run-off, topography, and level of scavenging [4].
Heavy metal contamination of agricultural soil may pose health risk to humans via the food chain and can also lead to reduction in food quality [5]. Crops and vegetables grown in soils contaminated with heavy metals have greater tendency to accumulate these metals. Consumption of vegetable has increased in recent years due to its health benefits [6] and uptake of metals by vegetables is a major pathway for soil-metals to enter the food chain and bio-accumulates leading to human health risk [7]. The health risks usually depend on the chemical composition of the waste material, its physical characteristics, vegetables cultivated and the consumption rate. Heavy metals from the contaminated soils enter the plants and finally to the human tissues via inhalation, diets and manual handling. The metals can bind to vital cellular components, such as structural proteins, enzymes, and nucleic acids, and interfere with their functioning. Long time exposure to heavy metals can have carcinogenic, central and peripheral nervous system, and circulatory effects. Hence there is the need to continuously monitor the level of heavy metal bioaccumulation in the edible crops to ensure that the accepted levels are not exceeded.
Waste management in Enugu city is becoming an increasing problem daily and a complex task. In Enugu, activities generate wastes in various forms; liquids, solids and gaseous and they increase with increasing population in the state. The more prevalent method of disposal of wastes have been to first collect them from their sources, and then deposit them in the waste bins or sites situated in various areas of the city by the government. Some throw them in the surrounding deep erosion gullies in their areas. The Enugu State Waste Management Agency (ESWAMA) collects all the deposited wastes from the various dumping site in the city and dump them in the major open municipal dumpsite (which is the study area), hence doing more harm than good to the overall sanitation status of the people living in the area. Wastes carry different metals which are then transferred to plants by different ways.
The aims of the study were to evaluate the soil level, bioaccumulation and health hazards of some heavy metals in agricultural plants grown in abandoned site of central open municipal solid waste dumpsites.
Materials and methods
Study area
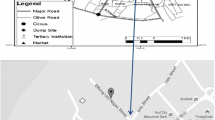
This study was carried out in the vicinity of an abandoned municipal solid waste dumpsite along Enugu/Port Harcourt road, Enugu state (Ugwuaji dumpsite). The city of Enugu is located between longitude 7o 6′ E and 7o 54′ E and latitudes 5o 56′ N and 6o 52′ N. It falls within the humid tropical rainforest belt of the southern Nigeria. The soil consists of hydro-orphic soil which is mineral rich soil and whose morphology is influenced by seasonal water logging caused by underlying impervious shale. The annual rainfall varies between 100 to 200 m with its peak occurring between mid-March and September. The rainfall average is 1412 mm per month, with the lowest rainfall in February. The temperature is generally high throughout the year with monthly maximum temperature ranging between 20.3 °C and 32.16 °C. A map showing the study area with the sampling locations is presented in Fig. 1.
Sampling
For the study, eight (8) sampling sites/stations created and labeled S1, S2, S3, S4, S5, S6, S7, and S8(control). These stations S1 to S7 were located at the farms and 500 m apart while S8 was on an isolated farm 2 km away from the main location. At each sampling station, 5 sampling points were created for soil collections. The soil collections points were created in such a manner as to be representatives of the stations (i.e., at the apices of the farmland and the center). Composites were made from these collections at each station. At each sampling point, a mini profile pit was dug to clearly reveal the soil profile with the aid of a measuring tape (after removing the overlying wastes) to mark the sampling depth which was 0 - 15 cm. A clean plastic shovel was used to collect the soil samples at the specified depth. A mass of 500 g of soil was collected from each site and put in a cleaned cellophane bag.
The edible part of Zea mays mature seeds, vernonia amygdaline, ocimumum gratissimum and Talinum triangulare were collected from the sampling stations. Three (3) samples of each plant were collected at random and a composite sample was made for each plant. The soil and plants were sampled bimonthly for a period of twelve (12) months to give a total of 48 soil and 24 plants samples.
Sample preparation
The soil samples were screened of debris and stones, air-dried by spreading on previously washed and dried polythene sheets. The dried soil samples were pulverized using mortar and pestle, sieved with 2 mm mesh sieve, mixed by coning and quartering and stored in previously washed, dried and labeled polythene containers. The soil samples were divided for use in total metal and physicochemical parameters determination.
Nitric-perchloric acids digestion {HNO3:HClO4}(4:1 ratio) was performed, following the procedure recommended by the AOAC [8]. Two gram (2 g) of each of the soil sample was placed in a 250 digestion tube and 20 ml of conc. HNO3 (67% BDH, UK) added. The mixture was boiled gently for 30–45 min to oxidize all the easily oxidizable matter. After cooling, 10 ml of 70% HClO4 acid (Merck, Germany) was added and the mixture boiled gently until dense white fumes appears. After cooling, 30 ml of deionized water was added and the mixture boiled further to release white fumes. The solution was cooled, and filtered through whatman No 42 filter paper and < 0.43 mm Millipore paper and transferred quantitatively to a 50 ml volumetric flask and was reached to mark with demonized water. A blank was similarly prepared. The extract solutions were immediately transferred to acid-pre-washed polythene bottles.
The plant samples were separated, thoroughly washed in the laboratory with tap water and rinsed with de-ionized water, drained and air- dried. They were dried at 65 °C, pulverized, sieved and stored in polyethylene bottles prior to digestion. Nitric-perchloric acids {HNO3:HClO4} digestion was carried out on plant samples as was the case of soil samples. The extract solutions were immediately transferred to acid-pre-washed polythene bottles. The digested samples and all the supernatants were analyzed for heavy metals (Pb, Cd, Cr, Fe, Zn, and Al,) using Atomic Absorption Spectrophotometer (Shimadzu AA7000 made in Japan) fitted with deuterium lamp for background correction.
Quality assurance
Glass wares were washed and rinsed properly with deionized water. Reagents of pure analytical grade were used. Deionized water was used throughout the study. Reagent blank determinations (deionized water and acids) were used to correct the instrument readings. The most sensitive wavelength for each element was selected for analysis, and calibration of AAS was done using multi-elemental solution prepared by serial dilution of 2, 4, 6, 8, and 10. Accuracy of the instrument was checked through the determination of LOD and LOQ using digested reagent blank.
Statistical analysis
Laboratory results were subjected to statistical analysis using package for social science (SPSS) version 20.0 for windows. Analysis of variance (ANOVA) and Correlation matrix studies were carried out.
Ecological risk assessment
Seven indices were used namely; contamination factor (Cf) and degree of contamination (Cd), modified degree of contamination, pollution load index, Geo-accumulation index, Potential ecological risk index (PERI),.Enrichment factor (EF), transfer factor. Human health risk assessment was also determined. The background level values used for all the elements (Pb, Cd, Cr, Fe, Zn and Al), was the guideline value developed by of CCME [9].
The contamination factor, Cfi, was calculated using Eq. 1 developed by Hakanson [10]:
where, Cfi is the contamination factor for the element i; Co is the average concentration of metal “i” in seven sampling stations and Cn is the concentration of the individual metal in the CCME guideline values. The sum of individual contamination factor of the pollutant gives the degree of contamination (Cd), computed from Eq. 2:
The degree of contamination estimates the extent of overall contamination in soil surface layers the sampling site. A modified model for estimating the overall degree of contamination was proposed by Abrahim and Parker [11] as in (3).
where n is the number of metals determined and mCd is the modified degree of contamination. The advantage of this modification is that it allows for as many elements as possible in contamination determination.
The pollution load index (PLI) allows comparison of pollution loads between locations at different periods [12]. The PLI was obtained from Eq. 4 using CCME background values.
Geo-accumulation index (Igeo) proposed by Mueller [13] have been widely used in assessment of metals pollution soils and sediments.
The model equation is:
where Cm is the mean concentration of metal m in soil and Bm is the geochemical background concentration (value) of metal m, either taken from the literature (average crustal abundance) or directly determined from a geologically similar material, while 1.5 is a factor for possible variation in the background concentration due to lithologic differences. Igeo is classified into seven descriptive classes as follows: < 0 = practically uncontaminated; 0 < Igeo ≤ 1 indicates uncontaminated to moderately contaminated state,1 < Igeo ≤ 2 indicates moderately contaminated state, 2 ≤ Igeo ≤ 3 indicates moderately to heavily contaminated state, 3 < Igeo ≤ 4 indicates heavily contaminated, 4 < Igeo ≤ 5 indicates heavily to extremely contaminated state. The latter is an open-end class that is indicative of all values greater than 5, and an Igeoof 6 is said to be indicative of 100-fold enrichment of a metal with respect to the baseline value [13].
The potential ecological risk index (ERI) method assesses the level of risk posed by the heavy metals using the heavy metal toxic factors [10]. The ERI is defined as:
where Ci is the average concentration of individual metal in the dumpsite, Ti is the heavy metal toxic response factor for a given metal, Bi is the guideline value for the metal and n is the number of metals. Toxic response factor used are: Cd(30), Zn(1), Cr(2), Pb(5) in mg/kg [14]. Heavy metals guideline values of the Canadian soil quality guideline [9] were used, and there are as follows: Zn(200), Pb(70), Cd(1.4),and Cr(64), in mg/kg. ERI were classified as follows: low contamination (ERI ≤ 50), moderate contamination (50 ≤ ERI ≤ 100), considerable contamination (100 ≤ ERI ≤ 200), and high contamination (ERI ≤ 200).
The transfer factor coefficient was calculated by dividing the concentration of heavy metals in vegetables by the total heavy metals concentration in the soil [15].
where, Cplantis the metal concentration in plant samples mg/kg fresh weight and Csoil is the metal concentration in soil samples in mg/kg.
If the ratios >1, the plants have accumulated elements, the ratios around 1 indicate that the plants are not influenced by the elements, and ratios <1 show that plants exclude the elements from the uptake. If the plants have higher TF values, they can be used for phytoremediation.
Enrichment Factor (EF), introduced by Alina Kabata-Pendias [16] was used to assess the degree of anthropogenic influence on element load in the soil, and also to differentiate between elements originating from the natural or anthropogenic activities. The enrichment factor (EF) or accumulation factor for an element was calculated using the formula.
where Xi, Fe are the concentrations of element ‘i’ and Fe respectively.
Potential human health risk assessment
Risk assessment is defined as the processes of estimating the probability of occurrence of any given probable magnitude of adverse health effects over a specified time period and is a function of the hazard and exposure. The risk to human beings resulting from the consumption of plants grown from the study area was calculated by employing the estimated dietary intake (EDI, in mg/kg/person /day), target hazard quotient (THQ) and the total chronic hazard index (THI) as described by USEPA [16]. The average daily vegetable intake rate was estimated by carrying out a survey in which 200 people having average weight of 60 kg were asked for their daily intake of a particular vegetable from the experimental site [17]. This gives the total dose entering the human body through oral ingestion of vegetable as shown in eq. 9.
where C = heavy metal concentration in plants (mg/kg). D = daily intake of vegetable (kg/person/day/dry weight) and BW = body weight.
Target hazard quotient (THQ) was determined by the ratio of the estimate daily intake (EDI) to oral reference dose (RfD) for each metal [17] given in Eq. 10
where RfD = oral reference dose for each metal (mg per kg of body weight per day). It is an estimate of an oral exposure per day of the human population which does not cause deleterious effects during a life time in non-cancer health assessments [17]. The values of RfD for Cd is 0.001(mg kg−1 day−1) and for Zn is 0.3 (mg kg−1 day−1) [18].
In order to assess the overall potential health risks of non-carcinogenic effects arising from the consumption of more than one heavy metal in the vegetable, the total chronic hazard index (THI) was developed by [16] and is defined as the summation of all the individual target hazard quotients of all the metals examined as shown in Eq. 11:
If either value of target hazard quotients (THQ) or total hazard index (THI) is less than 1, then the consumers of the vegetables are considered to be safe, but if either the hazard quotients (THQ) or the hazard index is equal to 1 or above 1, the plants are considered unsafe for human consumers and can lead to potential human health risk [17]. The probability of experiencing long-term health hazard effects increases with the increasing THI value.
Results and discussion
Table 1 presents the results of the physicochemical properties of soils samples investigated. The concentrations (mean concentration ± standard deviation) of the heavy metals in the studied soil and plants are presented in Tables 2 and 3.
The soil pH falls within the range recommended for agricultural soil (i.e., 6.5–6.8) by WHO [19] and is similar to pH range of a similar study by Getachew et al. [20]. The pH is also lower than the pH of the previous study on the site by Obasi et al. [21] who reported a pH range of 7.1 to 8.6. This implies that the environment is becoming more acidic and the mobility of elements in the study area tends to increase with the decrease in pH. Consequently, the solubility of the metallic elements in the soil increases and they become more readily available to plants, leading to an increased root uptake of metals by plants Ovasogie and Ndiokwere. [22]. It has been found that soil pH is correlated with the availability of nutrients to plants [23]. Low pH would favour availability, mobility and redistribution of the metals in the soil.
The electrical conductivity of the soil in the present study ranges from 54.95 ± 0.64 μs/cmto 410.5 ± 0.7 μs/cm with the average of 160.4 ± 125.99 μs/cm. while the control (S8) have the lowest conductivity of 31.45 ± 0.03 μs/cm as shown in Table 1,indicating low to moderate salinity. This implies that soil from the vicinity of the dumpsite contains more dissolved solids than the control. Similar results were reported for some dumpsites at Zaira [24]. The result is higher than the previous findings on the area that reported 0.52 to 3.12 mScm−1 range of electrical conductivity (E.C) [21]. The significance is that considerable amount of dissolved inorganic materials are present in the dumpsite soil. Such materials can provide adsorptive sites for certain chemicals and biological agents which may eventually foster pollution of surrounding soils, vegetation and underground water within the area of the dumpsite. The high conductivity value of the waste soil may be attributed to the presence of metal scraps which is one of the constituents of the refuse dumpsite.
TOC represents the amount of carbon bound in an organic compound and is independent of the oxidation state of the organic matter. The presence of organic carbon increases the cation exchange capacity of the soil which retains nutrients assimilated by plants. The result is higher than the previous findings in the area that reported 1.02 to 4.25% range of electrical conductivity (E.C) with 2.64% the average [21].
The high level of the total organic carbon status of the study area may be as a result of the degraded and composted wastes from the dumpsite. Soil organic matter enhances the usefulness of soil for agricultural purpose. It supplies essential nutrients and has unlimited capacity to hold water, absorbs cations and functions as a source of food for soil microbes (reference). The availability of organic matter in the study area followed the trend, S1 > S3 > S2 > S4 > S5 > S6 > S8 > S7. The higher amount of organic matter in the vicinity of dumpsite may be responsible for the increased soil pH in the dumpsite. The soil organic matter level in this study is higher than 4.24% reported by Obasi et al., [21].
The effective cation exchange capacity (CEC) of the study area ranges from 19.95 ± 0.050 meq/100 gto 19.95 ± 0.050 meq/100 g with an average of 26.93 ± 3.81 meq/100 g, while the CEC of the control soil was 17.58 ± 0.54 meq/100 g. Higher values of CEC as compared to that of the control site indicate average metal retention capability in the dumpsite vicinity. The result could be attributed to the higher clay and organic matter content in the dumpsite soil. Similar results were reported for some dumpsites at Bayelsa, Nigeria by Amos- Tautua et al. [25]. Cation exchange capacity helps to replenish the nutrients removed from the soil water by plant-uptake by providing a reservoir of nutrients, hence very important.
The results of the metals analysis show that the metal loads of the soil samples at the abandoned sites were higher than the control site samples. This could be attributed to the serious anthropogenic influence resulting from assorted wastes ranging from domestic, commercial and industrial wastes earlier dumped at the sites [26]. The result is similar to work by Al-Turki and Helai [27] who reported that lead and cadmium are normally abundant in upper layer soils as a result of anthropogenic activities. The study by Aja [14] indicated that the concentration of heavy metals in the dumpsite soil were higher in dry season than in rainy season due to more heavy metal loss as a result of run-off and infiltration in rainy season. The order of abundance of the six heavy metal monitored in the sites as compared with the control was Pb > Zn > Cr > Fe > Cd > Al.
The concentration of Pb in the various soil samples were lower than the maximum tolerable levels proposed for agricultural soil (90–400 mg/kg) set by WHO [28]. This is in agreement with the results obtained from similar study by Umoh and Etim [29] for soil from dumpsite within Ikot- Ekpene in Akwa-Ibom State Nigeria. Lead bearing wastes emanate from battery production and recycling industries, paints and electronic materials. Moreover Pb is a highly immobile element which exists in association with clay minerals, Mn-oxides, Al and Fe hydroxides, and organic material [30]. Long term exposure to Pb is risky, since bioaccumulation and bio-magnification can take place.
Cadmium level in the soil samples were higher than the natural limits of 0.01–3.0 mg/kg in agricultural soil, as recommended by EU [31] but lower than the upper limits (85 mg/kg) as recommended by WHO [28].This result is similar to findings by Njoku and Ayoka [32] and Amos-Tautua [25]. The presence of Cd in the environment is of worry because it is nearly the most eco-toxic metal. Cd has adverse effects on animal and plant metabolism. Environmental input of Cd come from smelting industries, fertilizers production wastes and paint, plastics, ceramics production processes.
The level of zinc was within the permissible limit of 300–400 as recommended by WHO in agricultural soil. The major anthropogenic sources of Zn include wastes from non-ferric metal industry, construction industries and agro practice [33]. Zinc is a highly mobile element whose toxicity results in carcinogenic, neurologic and hematological complications. Other health effects include hypertension, organs function disorders in animals [33].
The WHO/FAO [34] and Canadian soil quality guidelines [9] recommended value of Chromium for agricultural plants and soil are 0.85 mg/kg and 64 mg/kg respectively. In samples detected, the Cr levels were below the recommended limits. Chromium was not detected in S1, S3, and S5 which could be as a result of low mobility of Cr due to the topography of the stations. Chromium is a low mobility metal whose mobility also depends on pH [35]. Stations 1, 3 and 5 are less acidic and in elevated area.
The concentrations of Fe in all the soil samples were several times higher than the maximum permissible limit of 400 mg/kg set by WHO [28], and its concentration was several times higher than the previous work on the area reported by Ogbonna et al. [4]. The concentration of Al in the collected soil samples exceeded the established guidelines of 4.1–5.5 mg/kg [36] as reported by Anyeni et al., [37]. Excess amount of Al can be toxic for plant and animals. They may cause poor root growth and proliferation in the soil and hinder other forms of life. The presence of Al in the site might come from a wide range of sources of household items, electronics and even from plant tissues ashes.
The results in crops showed that bitter leaf (venonia amigdalina), scent leaf (Ocimum gratissimum), water leaf (talinum triangulae), and maize seed (Zea mays) have Cd concentration of 9.611 ± 1.722 mg/kg, 8.695 ± 0.079 mg/kg, 2.000 ± 0.098 mg/kg and 6.722 ± 1.70 mg/kg respectively and are all above the acceptable limits of 0.10 mg/kg as recommended by FAO/WHO [34]. Cd is a non-essential element and actually displaces Zn, leading to Zn deficiency. It accumulates principally in the kidney and liver [38]. Cd and several Cd compounds are known as carcinogens. Zn concentration in the plant samples ranges from 10.042 ± 0.728 mg/kg in bitter leaves to 18.472 ± 0.83 mg/kg in water leaves. Zn concentrations in the plant samples were in the order of; water-leaves > Zea mays> scent leaves > bitter-leaves. These high Zn concentrations found in the plants samples are similar to the observed results by Odukoya and co-workers [39] which can be attributed to domestic refuse, wastes from construction materials, motor emissions and motor vehicle wear. Cr concentrations in the plants samples were in the order of; scent leaves > water-leaves > bitter-leaves. Except for maize seed (Zea mays), the chromium concentration for all the plant samples were above the permissible limit of 0.08 mg/kg and 0.85 mg/kg as recommended by FAO/WHO [34]. Cr (III) is an essential element required for normal sugar and fat metabolism. It is effective to the management of diabetes and it is a cofactor with insulin.
The concentrations of iron in water leaves, bitter leaves scent leaves and Zea mays follow the trend Zea mays > scent leaves > bitter leaves > water leaves. The values recorded for Fe in all the plant samples were below the maximum permissible limit of 48 mg/kg as recommended by FAO/WHO [34], except for Zea mays. Fe serves vital functions, but as we age, excess store of the metal may build up and become toxic. Initially, the symptoms are not worrisome, but as the Fe level keeps on increasing, symptoms like constipation, poor sex drive, fatigue, headache, dizziness, and abnormal cramps become more serious. The concentration of Al was not detected in bitter-leaves, but in scent leaves, Zea mays, and water leaves, Al was all above the maximum permissible limit set by FAO/WHO [34].
Statistical analysis
The Correlation study for the studied metals in the soil and plants indicated positive correlation between the soil parameters and the heavy metals. Positive correlation exists between T.O.C (%) and C.E.C (meg/100 g), SOM (%) and C.E.C (meg/100 g) concentration in the soil samples with a correlation coefficient value of 0.930 and 0.930 respectively. A significant negative correlation was also observed between E.C (μs/cm)and T.O.C(%),E.C (μs/cm)and SOM(%),E.C (μs/cm)and C.E.C(meg/100 g) with correlation coefficient value of −0.513, −0513 and 0.633. Positive correlation exists between Pb (Lead) and Cd (Cadmium), Pb (Lead) and Cr (Chromium), Pb (Lead) and Zn (Zinc), Cd (Cadmium) and Al (Aluminium), Cd (Cadmium) and Zn (Zinc) concentration in the soil samples with a correlation coefficient value of 0.925, 0.723, 0.781, 0.507 and 0.759 respectively. A significant negative correlation was also observed between Cr (Chromium) and Fe (Iron) with correlation coefficient value of −0.516. Furthermore, positive correlation between Cr (Chromium) and Fe (Iron), Al (Aluminium) and Zn (Zinc) concentration in the plant samples with a correlation coefficient value of 0.987 and 0.759 respectively while significant negative correlation was also observed between Cd (Cadmium) and Al (Aluminium), Cd (Cadmium) and Zn (Zinc), Fe (Iron) and Al (Aluminium), with correlation coefficient value of −0.918, −0.992 and − 0.833.Also ANOVA results shows significant relationship (p < .05) between the parameters studied.
Element enrichment factors (EFc)
Element enrichment factors (EFc), is used to assess the degree of anthropogenic influence on element load in the soil, and also to differentiate between elements originating from the natural and anthropogenic activities. Result of EFc assessment is shown in Table 4.
An enrichment factor (EFc) greater than unity, is an indication that the investigated metal is more abundant in the dumpsite relative to that found in the control station/site. The enrichment factor of the soil in the vicinity of the dumpsite was quite high for all the metals as compared with the control, suggesting a very serious anthropogenic influence which is as a result of all kinds of assorted wastes ranging from domestic, commercial and industrial wastes which the landfill receives. The mean accumulation factors of trace heavy metals in the soil samples were higher than unity indicating that the investigated metals were more abundant in the dumpsite relative to that found in the control station/site. Their order of abundance in the dumpsite area due to anthropogenic influence is; Pb > Zn > Cr > Cr > Cd > Al > Fe.
The transfer factor (TF)
The TF of the metals is presented in Table 5. The transfer factor is an indication of the plant species ability or tendency to uptake a certain element from the soil. If the ratio is less than one(<1), it shows the plants has low uptake of the element from the soil and the metal will pose no risk to the consumers. If the ratio is 1, the plants will be a potential health risk to the consumers but if the ratio is greater than 1 then the population will experience health risk, [40]. Hence, if the value of the transfer factor is higher for plants, more elements would be accumulated by them [41].The metal with the highest transfer factor value recorded was Cd with the values ranging from 0.02, in water leaves to 1.3 in bitter-leaves followed by Cr which was only detected in bitter-leaf with the value 1.004.Similar results were reported [42] where some plants happens to be hyperaccumulators, while the plants excluded Fe, Al and Zn from their uptake. Bioaccumulation of Cd in the plants samples was high and its consumption could lead to hypertension, arthritis, diabetes, anemia, cancer, cardiovascular disease, cirrhosis, reduced fertility; hypoglycemia, headaches, osteoporosis, kidney disease, and strokes as a result of Cd poisoning. Pb was not detected in all the plants samples, indicating that the studied plants from the vicinity of the dumpsite did not have any contamination by Pb and therefore, no fear of Pb related toxicity. Zn, Fe, and Al showed considerably low Tf values in the order of Zn < Fe < Al with the average transfer factor values of 0.011, 0.021, and 0.053 respectively in all the plants samples. and this is considered low. Their low Tf values may be due to their strong adsorption onto the organic matter which renders it less bioavailable to plants.
Previous studies have indicated that the uptake of metals by plants differs from one metal to another, from one plant species to another and from one dumpsite to the other [5, 42]. Generally most of the heavy metals are more available to plants under acidic conditions than under alkaline conditions [43], hence the high level of these metals in the plants at the studied area might be due to direct deposition, foliar absorption and the translocation from roots to the upper part of the plant. The high transfer factor (Tf) of Cd to plants in the studied area could be attributed to the slightly acidic nature of the soil. Pb was not detected in all the plant samples hence there was no influence of the Pb concentration in the soil on the plants of the area, and this could be due to the low bioavailability of the metal in the soil of the areas. Pb binds more readily to organic matter [24, 44].
Ecological risk indices
The ecological risk assessment result is presented in Table 6. The contamination factors values obtained indicate low contamination state by Lead, Chromium and Zinc; considerable contamination with Cadmium (Cd), and very high contamination with iron (Fe). The overall degree of contamination (Cd) value of 442.131 and modified degree of contamination (mCd) value of 88.42 also indicated very high and ultra-high degree of contamination of the soils by the studied metals. Their order of contamination is Fe > Cd > Zn > Pb > Cr. This finding agrees with the previous findings on the area by Ajah et al. [14], who reported moderate contamination of the study area during the raining season and extreme level of soil pollution by heavy metals during the dry season.
Pollution load index (PLI) gives a composite value to ascertain the overall status of the soil. The PLI value of 130.859 obtained for the area was high. The high value of the PLI is as a result of the very high contamination factor of Cd in the study area with the Cf-value of 11.290. Geo-contamination (Igeo) levels of individual metals in the soils samples varied. When compared with their background values, it is seen that the soil samples were uncontaminated with Pb, Zn, and Cr; uncontaminated-moderately contaminated with Cd and moderately-heavily contaminated with Fe. This result further agrees with those from Ajah, [14]. The Ecological Risk index Assessment of Heavy Metal (Eir) value for all the elements studied except for Cd were less than 40, indicating a low ecological risk. The Eir value for Cd was 338.93 while the sum of the other meals Eir was 1.252. Apart from Cd, other heavy metals under investigation in the soil posed low ecological risk.
Potential health risk assessment
The result of the health risk assessment of the intake of heavy metals by the residents in the study area through the consumption of scent leaves (Ocimum gratissimum), bitter leaves (venora amygdaline), water leaves (talinium triangulare), and maize (Zea mays)is presented in Table 7. The daily intake rate of Ocimum gratissimum, venora amygdaline, talinium triangulare, and Zea mays were found from the vegetable consumption survey of the target population to be 0.002, 0.042, 0.042 and 0.032 kg/person/day respectively for people of average weight of 60 kg [3] while the oral reference does for Cd and Zn were 0.001 and 0.3 respectively [17].The EDI values for Cd, in scent leaves, bitter leaves, water leaves, and maize were; 0.00029, 0.0067, 0.0011 and 0.0047 kg/person/day respectively, and the EDI values for Zn were; 0.00040, 0.0071, 0.0099 and 0.0099 kg/person/day respectively. The target hazard quotient (THQ) values for Cd in the plants samples were 0.2, 6.73, 1.07, and 4.71 for Ocimum gratissimum, venora amygdaline, talinium triangulare, and Zea mays respectively, all above one except for the THQ value for Ocimum gratissimum, indicating that people consuming the plants cultivated on the vicinity of the dumpsite may experience adverse effects due to Cd toxicity. The THQ values for Zn were all below one suggesting that the studied crops are of no potential health risk to the target population due to the presence of Zn in the plants samples [3, 45].The Total chronic hazard index in this study is equal to 12.662 (THI =12.662) and according to Lemly [46]; THI = 1.1–10 refers to moderate hazard while THI > 10 refers to high hazard. The hazardous quotient for Cadmium (Cd) in the plants samples were in the order; Ocimum gratissimum < talinium triangulare < maize < venora amygdaline. Cadmium is a toxic element because it can be absorbed via the alimentary tract, penetrate through placenta during pregnancy, and damage membranes and DNA. Once in the human body, it may remain in the metabolism from 16 to 33 years and is connected to several health problems, such as renal damages and abnormal urinary excretion of proteins [47]. Decrease in bone calcium concentrations and increase of urinary excretion of calcium have also been attributed to exposure to Cd, eventually causing death. It also affects reproduction and endocrine systems of women [48].
Conclusion
The rate of increase in heavy metal pollution in the study area is alarming. The results obtained for the four plants samples (Ocimum gratissimum, venonia amygdalinare, Talinum triangulare, and Zea mays) under investigations for the six (6) heavy metals shows that they are all, by far above the FAO and WHO acceptable limits. The population is likely to experience a potential human health risk of Cd. This therefore implies that the consumption of these plants is risky as this will lead to a lot of health problems. As regular consumption of these plants will expose the consumers to heavy metal toxicity as the years go by.
References
Adeniji K. Ogu VI. Sustainable Physical Development in Nigeria: Institute of Social and Economic Research Publications; 1998.
Ahmed G, Uddin MK, Khan GM, Rahman MS, Chowdhury DA. Distribution of trace metal pollutants in surface water system connected to effluent disposal points of Dhaka export processing zone (DEPZ), Bangladesh: a statistical approach. J Nat Sci Sustainable Technol. 2009;3:293–304.
Ekere NR, Oparanozie TI, Ogbuefi-Chima FI, Ihedioha JN, Ayogu JI. Assessment of some heavy metals in facial cosmetic products. J Chem Pharm Res. 2014;6(8):561–4.
Ogbonna DN, Benjamin LK, Patrick OY. Some Physico-chemical and heavy metal levels in soils of waste dumpsites in Port Harcourt municipality and environs. J Appl Sci Environ Manage. 2009;13(4):65–70.
Amusan AA, Ige DV, Olawale R. Characteristics of soil and crops uptake of metal in municipal waste dumpsites in Nigeria. J Hum Ecol. 2005;17:167–71.
Jena V, Dixit S, Gupta S. Risk assessment of heavy metal toxicity through edible vegetables from industrial area of Chhattisgarh. Int J Res Environ Sci Technol. 2012;2:124–7.
Guerra F, Trevizarri AR, Muraoka T, Marcante NC, Canniatti-Brazaca SG. Heavy metals in vegetables and potential risk for humanhealth Sci. Agric. 2012;69(1):54–60.
Association of Official Analytical Chemists (AOAC). Official methods of Analysis 15th ed.Virgina, USA;1990.
Canadian Council of ministers of the Environment ( CCME). Canadian soil quality guidelines for the protection of environmental and human health. Summary of a protocol for the Derivation of Environmental and Human Health Soil Quality Guidelines. In: Canadian Environmental quality guidelines, Chapter 7, Canadian Council of Ministers of the Environment, Winnipeg; 1999.
Hakanson L. An ecological risk index for aquatic pollution control—a sediment ecological approach. Water Res. 1980;14:975–1001.
Abrahim GMS, Parker RJ. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki estuary, Auckland. New Zealand Environmental Monitoring Assessment. 2008;136:227–38.
Tomlinson DC, Wilson JG, Harris CR, Jeffrey DW. Problems in assessment of heavy metals in the estuaries and the formation of pollution index. Helgoland Marine Res. 1980;33:566–75.
Muller G. Index of geoaccumulation in sediments of the Rhine River. Geojournal. 1969;2(3):108–18.
Ajah KC, Ademiluyi J, Nnaji CC. Spatiality, seasonality and ecological risks of heavy metals in the vicinity of a degenerate municipal central dumpsite in Enugu, Nigeria. J Environ Health Sci Eng 2015; 13 (15): https://doi.org/10.1186/s40201-015-0168-0.
Heide S. Trace element accumulation in selected bioindicators exposed to emissions along the industrial facilities of Danube lowland. Turk J Chem. 2002;26:815–23.
United States Environmental Protection Agency (USEPA). Risk assessment guidance for superfund Human Health Evaluation Manual (Part A) Interim Final Vol. I United States Environmental Protection Agency Washington DC. 1989.
USEPA. Risk-based concentration table. Washington DC: United State Environmental Protection Agency; 2010.
USEPA, (2013). Reference dose: description and use in health risk assessments, background documents 1A, integrated risk information system (IRIS): united state Environmental Protection Agency: Washington DC 15 march http//www epa.gov/rfd.htm. 2013.
World Health Organization (WHO) Heavy metals- environmental aspects. Environmental Health Criteria No. 85: World Health Organization: Geneva, 1989.
Getachew D, Habtamu D. Heavy Metal Pollution of Soil around Solid Waste Dumping Sitesand Its Impact on adjacent Community: the case of Shashemane Open Landfill, Ethiopia J Environ Earth Sci 2015; 5(15), http://www.iiste.org
Obasi EI, Akubugwo O, Ugbogu C, Otuchristian G. Assessment of Physico-chemical properties and heavy metals bioavailability in dumpsites along Enugu-port Harcourt expressways, south-east, Nigeria. Asian J Appl Sci. 2012;5:342–56.
Ovieasogie PO, Ndiokwere CL. Fractionation of lead and cadmium in refuse dump soil treated with cassava milling effluent. J Agric Environ. 2008;3:9–21.
Gray CW, McLren RG, Roberta AH, Condron LM. Sorption and desorption of cadmium from some New Zealand soils: effects of pH and contact time. Aust J Soil Res. 1998;36:199–216.
Tukura BW, Kagbu JA, Gimba CE. Correlation analyses of organically bound trace metals and sediment in Kubani dam, Zaria, Nigeria. J Environ Sci Technol. 2011;4:437–43.
Amos-Tautua B. Assessment of some heavy metals and physicochemical properties in surface soils of municipal open waste dumpsite in Yenagoa. Nigeria Afr J Environ Sci Technol. 2014;8(1):41–7. https://doi.org/10.5897/AJEST2013.1621.
Ogundiran MB. Osibanjo O. Heavy metal concentrations in soils and accumulation in plants growing in a deserted slag dumpsite in Nigeria African J Biotechnol. 2008;7(17):3053–60.
Al A-T, Helal MID. Mobilization of Pb, Zn, cu and cd, in polluted soil. PakJ Biol Sci A. 2004;7:1972–80.
World Health Organization,(WHO). Permissible Limit of heavy metals in soils and plants. International Programme on Chemical Safety. Environmental Health Criteria 165. Geneva, 1996.
Umoh SD, Etim EE. Determination of heavy metal contents from dumpsites within Ikot Ekpene, Akwa Ibom state. Nigeria Using Atomic Absorption Spectrophotometer The Int J Eng Sci. 2013;2(2):123–9.
Norrish K. The Geochemistry and mineralogy of trace elements. In Nicholas DJD, Egan AR (eds): Trace elements in Soil – Plant – Animal system. New York. Academic press, 1975.
European Union (EU). Council directive on the protection of environment and in particular of the soil, when sewage sludge is used in agriculture. Official Journal European Community, L181, Annex 1A, 6–12. 1986.
Njoku PC, Ayoka AO. Evaluation of heavy metal pollutants from soils at municipal solid waste deposit inOwerri, Imo state. Nigeria; J Chem Soc Nigeria. 2007;32(1):57–60.
Kalagbor IA, Barisere V, Barivule G, Barile S, Bassey C. Investigation of the presence of some heavy metals in four edible vegetables, bitter leaf (Vernonia amygdalina), scent leaf (Ocimum gratissimum), water leaf (Talinum triangulare) and fluted pumpkin (Telfairia occidentalis) from a cottage farm in Port Harcourt. Res J Environ Earth Sci. 2014;6(1):18–24.
WHO/FAO. Fruits and vegetables for health. Report of Joint FAO/WHO Workshop. Kobe, Japan, September 1–3, 2004,
Alina K-P. Effect of organic wastes on the extractability of cadmium, copper, nickel and zinc in soil. Geoderma. 2004;122:297–303.
Peverill KI, Sparrow LA, Reuter DJ. Soil analysis: an interpretation manual, CSIRO Australia.Phanerochaete chrysosporium under lead stress. Chemosphere. 1999;81:1091–7.
Ayeni OO, Ndakidemi PA, Snyman RG, Odendaal JP. Metal contamination of soils collected from four different sites along the lower Diep River, Cape Town. South Africa Int J Phy Sci. 2010;5(13):2045–51.
Divrikli U, Horzum N, Soylak M, Elci L. Trace heavy metal contents of some spices and herbal plants from western Anatolia Turkey. Int J Food Sci Technol. 2006;41:712–6.
Odukoya OO, Arowolo TA, Bamgbose O. Pb, Zn and cu levels in tree barks as indicator of atmospheric pollution. Environ Int. 2000;26:11–6.
Sajjad K, Robina F, Shagufta S, Mohammad AK, Sadique M. Health risk assessment of heavy metals for population via consumption of vegetables. World Appl Sci J. 2009;6(12):1602–6.
Saglam C. Heavy metal accumulation in the edible parts of some cultivated plants and media samples from a vol- canic region in southern Turkey. Ekoloji. 2013;22(86):1–8.
Agyarko K, Darte E. Berlinger K metal levels in some refuse dumpsite soils and plantsin Ghana. J Plant Soil Environ. 2010;56(5):244–51.
Hess R, Schmid B. Zinc supplement overdose can have toxic effects. J Pediat Haematol/Oncol. 2002;24:582–4.
Adekola FA, Abdus-Salama N, Baleb RB, Oladejia IO. Sequential extraction of trace metals and particle size distribution studies of Kainji lake sediment, Nigeria. Chem Speciat Bioavailab. 2010;22:43–9.
Mokgolele M, Likuku SA. Preliminary investigation of transfer of metals from soil to vegetables: case study of Spinacia oleracea L. Afri J Environ Sci and Tech. 2016;10(9):307–13.
Lemly AD. Evaluation of the hazard quotient method for risk assessment of selenium. Ecotox Environ Saf. 1996;35:156–62.
Castro-Gonzalez MI, Mendez-Armenta M. Heavy metals: implications associated to fish consumption. Environ Toxicol Pharmacol. 2008;26(3):263–71.
Apostoli P, Catalani S. Metal ions affecting reproduction and development. Metal ions Life Sci. 2011;8:263–303.
Acknowledgements
The authors graciously recognize the assistance rendered by our Laboratory Assistants and Attendants in areas of sample pretreatment and collections respectively. However, they wish to remain anonymous.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ekere, N.R., Ugbor, M.C.J., Ihedioha, J.N. et al. Ecological and potential health risk assessment of heavy metals in soils and food crops grown in abandoned urban open waste dumpsite. J Environ Health Sci Engineer 18, 711–721 (2020). https://doi.org/10.1007/s40201-020-00497-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-020-00497-6