Abstract
Many places around the world are faced with environmental challenges such as unacceptable waste disposal and pollution of water resources. Heavy metal pollution of soil, water, and plants, and their health effect on people is a persistent social issue. Numerous researches have highlighted the health risks of residents in close proximity to open dumpsites. Dumpsites are sources of heavy metal impurity and toxicity to the surrounding environment. This study aimed to investigate two sites (site A and control site B) for their physicochemical properties and concentrations of heavy metals in the soil, water and plants in and around these sites. Concentrations of four heavy metals, namely: Cu, Hg, Mn and Pb in the plant, water, and soil samples were measured using atomic absorption spectrophotometry (AAS) after sample digestion. Soil pH for both sites ranged from slightly acidic (6.67) to neutral (7.09), which falls within the normal range suggested by WHO. The physicochemical parameters of soil and water were found to be within the normal range, except electrical conductivity in water which was above the permissible limits recommended by WHO. Dumpsite soils (site A) had higher heavy metal concentrations compared to site B. A noteworthy observation was the fact that Hg concentrations were the only one found to exceed the maximum permissible levels in soil (ranging from 1.08 to 8.45 mg/kg), Acacia karroo (1.71–13.27 mg/kg), and water (11.42–55.04 mg/L) while Mn only exceeded this limit in water samples (ranging between 0.023 and 1.106 mg/L). High Hg concentration in this study is a thing of concern.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
15.1 Introduction
One of the most valuable nation’s assets is soil and it is the characteristic resource that determines the region’s wealth. In soil heavy metals pollution changes the composition and the activity of microbial soil communities. The bioactivity, richness and microbial diversity decrease with the increase in the concentration of heavy metals [1]. The soil is the main sink for heavy metals released into the environment by five mentioned anthropogenic activities [2]. It is also the major source of metals in plants and vegetables since they absorb pollutants in their soluble form through roots and transfer them to other parts such as seeds and leaves [3].
Around the world, the heavy metals and metalloids cause a serious problem in the environment [4]. Heavy metals are natural components of the Earth’s crust and are determined to have a density higher than 5 g/cm3. The most common metals are Cu, Cd, Fe, Zn, and Ni. Metals like Fe are important for a living when present in a small required amount, while other elements like Hg are dangerous even at the small amount due to their certain physical and chemical properties [5]. They are non-biodegradable, persistent [6], and dangerous because they have a tendency to bio-accumulate and enter the food chain through plants and animals [7].
Metals are introduced into the soil from different sources such as the use of fertilizers containing phosphates, pesticides, vehicle emissions, industrial waste, lead-acid batteries, and from atmospheric deposition of metal and metalloids bearing particles [4]. The solubility and bioavailability of metal ions in the soil usually change due to the influence of factors such as soil pH, organic matter content and clay content [8].
Humans and animals are exposed directly and indirectly to these heavy metals through three primary routes, which are: ingestion, inhalation and skin absorption [9]. Uncontrolled and unmanaged dumpsites exist in many developing countries because they remain the cheapest method of waste disposal.
These open dumpsites can cause several years of human health risk [10]. Dumpsites produce health hazards due to the unpleasant smell generated by degrading activities of micro-organisms on organic waste. The unrestricted burning of municipal solid waste also results in serious environmental pollution, due to the release of thick, noxious smoke that pollutes the air [11].
Water pollution occurs as the result of human activities such as releases of effluents on water. A previous investigation revealed that many rural communities in Eastern Cape and across South Africa especially among the poor rural population with limited investments in infrastructure suffer from lack of supply of healthy water and sanitation facilities [12].
Acacia karroo is an average large, single-stemmed and deciduous tree with a rounded crown, often broader than tall and it grows up to 12 meters high [13]. It belongs to the plant family of Fabaceae, and subfamily of Mimosoideae. This plant is known in many cultures, commonly known as Sweet thorn (English), Soetdoring (Afrikaans), Mookana (North Sotho), Mooka (Tswana), or umuNga (Zulu or Xhosa) [14]. The species consists of paired, greyish to white thorns, finely textured dark green leaves, and produces yellow pompoms flowers with sweet smelling [13]. Unlike other most pioneer plants, Acacia karroo fixes nitrogen through the symbiotic association with nitrogen-fixing microorganisms which increase the soil’s fertility of the surrounding soil [15]. The aim of this study was to evaluate the influence of anthropogenic activities on the metal levels and physicochemical properties of soil, water and plants found in and around Alice landfill in order to document potential environmental hazards and possible risk to public health due to these activities.
15.2 Materials and Methods
15.2.1 Study Area and Site Description
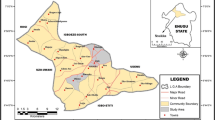
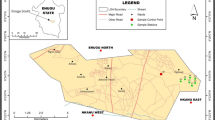
Alice landfill site (site A) where the study samples were collected is located in Alice Township under Raymond Mhlaba Municipality in South Africa, and it is about ≈4 km from the Fort Hare University East campus in Alice, where the second site (site B) which is the control site is located. Site A covered about 2 ha of land and the entire dumpsite was full of natural vegetation which is indicative of either abundance or deficiency of certain minerals.
Site A was divided into three portions: portion A (east side) had lots of broken glasses; portion B (south side) was dominated by rusted tins and broken glasses with evidence of burnt waste, and portion C was outside the fence of the dumpsite and was only occupied by natural vegetation. However, site B is not a dumpsite but an undisturbed piece of land with its soil surface covered by natural vegetation and located near a mountain. The municipal water dam is located at about 200 m from the dumpsite and is close to a cattle kraal (Fig. 15.1).
15.2.2 Plant, Soil and Water Sample Collections
To determine the influence of dumped materials in the study site on the accumulation of heavy metals in plant tissues, leaf samples of Acacia karroo growing on the dumpsite (site A), were collected randomly with a stainless-steel knife. Plant samples were deposited in the herbarium of the Department of Botany, University of Fort Hare and identified by Professor A. Maroyi and voucher specimen with specimen number NG/01 [16]. Plant parts were separated, pre-cleaned with distilled water and dried to a constant mass at 80 °C in an oven. Dried samples were crushed into a fine powder using a laboratory grinder.
Milled samples were sieved through a 2 mm mesh to obtain a fine powder and were stored in a plastic bag [17].
The soil samples were collected two times a week for a period of six weeks. Samples were collected using a stainless-steel auger at the depth of 0–30 cm from each of the 3 portions (A, B and C) of site A to have a total of three samples for site A.
The fourth composite sample was collected from the control site B. The collected dry soil samples were placed in clean labelled polyethylene bags and then transported to the laboratory for further analysis.
Water samples were collected from the dam once a week for six weeks. Sterilized plastic bottles were rinsed with distilled water, dried and were used to collect water samples randomly below the water surface.
The samples were acidified using nitric acid to prevent further microbial activities and kept in an ice bag at 4 °C [18].
15.2.3 Determination of the Physicochemical Properties of Soil
Using a mortar and pestle, soil samples were crushed and sieved in a 2 mm mesh to remove coarse debris to obtain representative samples [19].
15.2.3.1 Moisture Content
Soil samples were added to a beaker of known mass and the combined mass of soil and beaker was measured. The samples were oven dried at 105 °C for 24 h until constant mass was obtained. Samples were cooled at room temperature and transferred into a clean plastic bag for further analysis [19].
15.2.3.1.1 Calculation
15.2.3.2 pH
In a 100 ml beaker of known mass, 20 ml of calcium chloride solution was added to 20 g of sieved soil sample; the resulting mixture was stirred and allowed to stand for 30 min with occasional stirring. The determination of pH was done by immersing a hand-held glass electrode pH meter into the partially settled solution, making sure the electrode does not touch the walls of the beaker [20].
15.2.3.3 Organic Carbon (SOC)
A 1.0 g of soil sample was weighed into a 250 ml conical flask, 10 ml of K2Cr2O7 was added and the flask was gently swirled to dissolve the soil. Then 20 ml of conc. H2SO4 was quickly added and mixed thoroughly and the flask was allowed to stand on a fume hood for 30 min to cool down. Thereafter, 200 ml of water was added and the formed suspension was filtered using a filter paper. About 3–5 drops of ferroin indicator were added and the solution was titrated with FeSO4. The end point was approached when the solution changed from dark green to blue to reddish brown [21].
15.2.3.3.1 Calculation
where M = concentration of FeSO4, V1 = volume of blank, V2 = volume of FeSO4, 0.39 = constant and 1.724 = constant.
15.2.3.4 Electrical Conductivity (Salinity)
A 10 ml of well-mixed water sample was added into a measuring cylinder. The previously prepared soil sample was added to the water until the contents of the container increased by 5 ml to bring the volume to 15 ml. More water was added to the mixture to bring the total volume to 30 ml. The content was shaken intermittently for 5 min and allowed to settle for 5 min. The EC probe was dipped into the solution to measure the electrical conductivity [22].
15.2.4 Determination of the Physicochemical Properties of Water
Analytical methods for water physicochemical properties which are temperature, pH, and electrical conductivity were measured at the collection site (dam that is ≈200 m from the dumpsite) using multi-parameter water quality instrument [18, 23].
15.2.4.1 Alkalinity
Two or three drops of phenolphthalein were added into 100 ml of collected water samples. The appearance of a pink color in water indicates alkalinity. The solution was titrated with 0.02 N H2SO4 until a clear solution was obtained [18].
15.2.4.2 Total Hardness
50 ml of water sample was mixed with 1–2 ml buffer of pH 10 and a few drops of Eriochrome black-T indicator. The mixture was then titrated with 0.01 M EDTA till the wine-red solution changed to blue [18].
15.2.4.2.1 Calculation
where C = ml of EDTA for titration, D = mg of CaCO3 equivalent to 1 ml of ethylenediamine-tetra-acetic acid (EDTA).
15.2.4.3 Total Dissolved Solids (TDS)
The evaporating dish and filter paper were washed, dried and weighed. Then 50 ml of the well-mixed water sample was pipetted and filtered using Whatman filter paper. The filtered sample was transferred into an evaporating dish and dried in an oven at 180 °C. The cooled dried sample in the evaporating dish was measured to obtain mass [23].
15.2.4.3.1 Calculation
where A = mass of dried residue + dish (mg), B = is the mass of the dish.
15.2.5 Determination of Heavy Metals in the Plant, Soil and Water Samples
Acacia karroo samples: 2 g was weighed into a clean crucible, burnt to ashes at 450 °C and was then cooled in a desiccator. The ash was dissolved in 5 ml of 20% HCl, placed in a 100 ml volumetric flask and the solution was made up to the mark using deionized water [7].
Soil samples: 20 ml HNO3 was measured and added to 10 g of the prepared soil sample in a 250 ml conical flask. The content in the flask was placed on a hot plate until the solution was reduced to 5 ml, after which 20 ml of distilled water was added until the suspension was reduced to 10 ml. When digestion was complete, the residue was allowed to cool to room temperature and was filtered using Whatman filter paper and the resulting solution was made up to 50 ml in a volumetric flask with distilled water [5].
Water samples: 100 ml of water sample was measured and placed in a conical flask containing 10 ml of conc. HNO3. The mixture was heated gently and evaporated on a hot plate to a volume of 20 ml. The heating was continued with the addition of necessary conc. HNO3 until digestion was complete as shown by a light colored clear solution [18].
An atomic absorption spectrophotometer (AAS) was used to analyse copper (Cu), lead (Pb), mercury (Hg) and manganese (Mn) in the digested samples [3].
15.2.5.1 Statistical Analysis
The data were analyzed using the Microsoft office Excel and Statistical Package for the Social Sciences (SPSS). The data were subjected to analysis of variance (ANOVA) and significant difference between means was determined at p ≤ 0.05.
15.3 Results and Discussion
15.3.1 Physicochemical Properties of Soil Samples
The results of the physicochemical properties of the soil samples in the dumpsite and the control site are presented in Table 15.1.
pH: The mean hydrogen ion concentration (pH) in the dumpsite (site A) ranged from 6.79 to 7.09, while in the control site (site B), the mean pH was 6.67 (see Table 15.1). All these pH values are classified as neutral pH range for soil, as pH range of 6.6–7.3 is indicative of a neutral pH. Soil pH measures soil acidity or basicity, and directly influences nutrient bioavailability.
For most plants, the optimum range of soil pH for healthy plant growth is 7.0. Some of the nutrients availability such as nitrogen, phosphorus, and potassium are generally lowered as soil pH decreases [24]. The results of this study indicated that all collected soil samples from both sites are within the standard pH range set by the WHO. They also show that soil pH slightly differs among the portions of dumpsite A.
The ANOVA results show that there was no statistical difference between two portions of site A (A and B), while portion C of site A and site B were significantly different compared to portions A and B of site A. The difference in pH may be attributed to the wet climate which has great potential to encourage soil acidity due to organic matter decomposition which produces hydrogen ions [25, 26]. The movement and the availability of macro and micronutrients in the soil are affected by pH and other soil components. The solubility of metallic contents of the soil increases as soil pH drops. These soluble ions become freely available in different fractions to increase the acidity of the soil environment. An acidic soil environment can ultimately alter the microbial population of the soil and therefore shift microbial growth in favour of the fungi species with a potential for enhanced micro and macronutrient solubility and mobility [25].
Soil Moisture Content: The results in Table 15.1 showed that the average soil moisture contents in site A ranged from 11.07 to 13.48% while the average of site B was 10.16%. Soil moisture content is the amount of water present in the soil. The low levels of moisture in both sites may be due to the nature of the topography of these sites. Sites A and B were both located on hill slopes with site B hillier than site A. There was no significant difference noted between all portions of site A and site B. It is expected that this type of topography will encourage quick run-off of rainwater during rainy days before the soil could absorb enough water and hence, the resulting dry soils. The high temperature on sampling days is also a factor contributing to the high rate of evaporation of soil moisture and the resultant dry soil observed in this study. The combined effect of these two factors in addition to the natural vegetation occupying the soil surface with their water demand for growth and transpiration process is also seen as contributing factors [27] to the low moisture contents in the sites investigated.
Soil Organic Carbon (SOC) and Soil Organic Matter (SOM): SOC shows variation between sampling portions and ranged from 1.10 to 2.01% (see Table 15.1). Site A-portion A recorded the lowest SOC while site B located outside dumpsite A had the highest SOC. As expected, the SOM content of the soil samples followed the same trend as the SOC recorded above and is found to be low for site A while at optimum level at site B (Table 15.1). The ANOVA analysis shows a significant difference between site A and site B for SOC, while for SOM portion B and portion C of site A correlated significantly with the control site (site B).
SOC of about 2% (3.4% SOM) is widely suggested by several workers as the threshold level for sustaining soil quality, below which deterioration may occur [28, 29]. The most significant factors controlling SOC dynamics are temperature and precipitation [30]. Overall, the percentage of organic carbon in site B is optimum while that of site A is low. Low organic carbon and organic matter content in site A may be attributed to warm temperatures which decreased SOC due to increased microbial decomposition rate [31] of the organic materials in the dumpsite. Microbial activities also generate heat during decomposition process to further hasten the breakdown of organic materials in wastes. Decomposition is a biological process that includes the physical breakdown and biochemical transformation of complex organic molecules of dead material into simpler organic and inorganic molecules [32].
High decomposition rate aided by moisture and high temperature leads to an increase in the level of organic matter in the soil and subsequently, increasing the rate of metal ions absorption. Municipal wastes are known to have a high concentration of metals and this is because SOM has a large sorption capacity towards metals [33]. However, any form of human intervention influences the activity of soil organisms. When soil is disturbed through cultivation, tillage, burning of vegetation and so on, the soil generally contains lower levels of SOM than comparable lands under natural vegetation [34] which may be one of the reasons for the high SOM and SOC obtained in this study for the control site B compared to site A. Return of plant residues or organic wastes to the soil stimulates high microbial activity and increases SOM decomposition.
Electrical conductivity (EC): The EC of collected soil samples from site A were generally higher than that of site B (see Table 15.1). For instance, the EC of site A-portion A was more than four times higher than site B. Similarly, the EC in site A-portions B and C was also more than three times the EC in site B. In general, the values of EC in this investigation are well below the salinity threshold for soil. Soil EC is a major indicator of soil salinity. It is a measure of the number of salts in the soil but does not indicate the specific salt or ions that might be present. EC is a good indicator of the presence of salts like sodium, potassium, chloride or sulfate [35]. Saline soils are those with salt levels (EC) above 4 dS/m [36]. In the present study, the soil EC was found to be lower than the saline level. However, a good soil EC level for crop production will be somewhere between 200 and 1200 µS/cm (1.2 mS/cm). The difference between the means is statistically significant between site A and control site B. Soil EC that is lower than 200 µS/cm has insufficiently available nutrients for the plants and could be an indication of a sterile soil with little microbial activity.
On the other hand, EC above 1200 µS/cm may be an indication of too much dissolved salts (e.g. fertilizer) in the soil or a developing salinity problem from lack of drainage [37]. Although site B had the highest SOC and SOM, the soil EC value shows that the site has insufficiently available nutrients.
15.3.2 Physicochemical Properties of Water Samples
The physicochemical properties of the sampled water are presented in Table 15.2.
pH: Generally, the pH dynamics of the water samples was erratic and changes with every sample collection. For instance, while the pH of water changed from slightly acidic to slightly alkaline between week one and week two, it dropped significantly to acidic state in the third week followed by a slight increase during the fourth week. Thereafter, the pH of the sampled water progressively dropped to 5.41. The pH of drinking water recommended by WHO is 6.5–8.5 [35]. The pH of water is a good indicator of its quality and pollution extent. The pH values for all the collected water samples are within allowable limits for surface water [38], except for samples collected between week five and six which are both acidic with a mean value below the recommended standard range as shown in Table 15.2.
The pH of water samples collected in week six (5.41) was more strongly acidic than that of week five (6.39), and this might be caused by burning of waste and fossil fuels which releases carbon dioxide (CO2) in air. When CO2 dissolves in water, it creates weak acid such as carbonic acid. ANOVA results indicate that pH of samples was significantly different. Acidic water can pose a threat to aquatic life and human health [39].
At low pH, the acidic nature of the water may liberate toxic heavy metals like Ag, Al, Cd, Co, Cu, Hg, Mg, Ni, Pb and Zn [40].
Water Temperature: This also varied with every sampling, progressively reducing from 25.9 °C for week one to 14.05 °C in week six (Table 15.2). Variations in water temperature may be associated with wind force, inland water flow, the influx of the rainwater and atmospheric temperature [41]. Water temperature may vary with season and size of the water body, it may also vary on a daily basis. Water temperature is often lowest during the night and early morning and increased from mid to late afternoon [42], thus explaining the temperature variation observed in this study during the cause of water collection for a consecutive 6 week period.
Electrical conductivity (EC): As shown in Table 15.2, the EC values ranged from 21.65 to 53.85 mS/cm, which are well above healthy level and considered not suitable for irrigation, human and livestock consumption. The WHO normal range for electrical conductivity of water is 400–600 µS/cm [35]. In all the tested water samples, the values obtained for EC were higher than these permissible limits. There was a statistical significant difference noted between the collected samples. High EC level in the water may be indicative of the high amount of dissolved salts such as Ca, Cl and Mg present in soil water as ions. Also, salinity may be influenced by natural phenomena, like evapotranspiration and rainfall [43]. Evaporation of water from the dam surface due to high temperatures can concentrate the dissolved solids in the remaining water [44] while rainfall causes dilution.
Total Water Hardness: Water hardness ranges between 50.0 and 58.0 mg/L (Table 15.2). Water hardness is caused by a variety of dissolved multivalent metallic ions, predominantly calcium and magnesium cations. In this study, the water can be categorized as soft since hardness falls below 60 mg/L of dissolved ions [45, 46] and is, therefore, suitable for use as potable water. The analysis from ANOVA indicates significant difference between the samples. However, depending on pH and alkalinity, hardness above 200 mg/L can result in scale deposition, particularly on heating. On the other hand, soft waters with a hardness of less than 100 mg/L have a low buffering capacity and may be more corrosive to water pipes [47].
Total Dissolved Solids (TDS): The TDS of water ranged between 420 and 720 mg/L (Table 15.2). These values fall within the approved WHO standard for drinking water and that of South African guidelines for domestic water use which is in the range of 450–1000 mg/L [48]. The TDS results indicate the presence of solutes (inorganic salts and little organic materials) in water [47, 49], and may be attributed to the prevailing high rainfall, which causes significant dilution [50] of the dam. The ANOVA results show that there was statistical significant difference for TDS in water during the six weeks.
15.3.3 Concentration of Heavy Metals in Plant, Soil and Water Samples
Results of the concentrations of heavy metals in the soils, water, and Acacia karroo samples investigated in this study are presented in Table 15.3, Figs. 15.2 and 15.3 respectively.
Copper (Cu): Generally, values obtained for Cu in soil samples of site A were significantly higher than that of site B (Table 15.3). Site A-portion B recorded the highest deposit of Cu with a significant (p < 0.05) increase in Cu deposit compared with site A-portion C and site B.
The highest deposit of Cu (25.19 ppm) at dumpsite A was more than nine times higher than the concentration in site B (2.67 ppm) while the lowest Cu deposit at site A was more than four times the concentration recorded in site B. Despite the high deposit of Cu in dumpsite A, there was no detectable trace of Cu in the water and Acacia karroo samples (Figs. 15.2 and 15.3).
WHO recommended 10 mg/kg and 2 mg/L as the maximum permissible limits of Cu for plants and drinking water respectively [51], while the permissible limit recommended by Dutch standard for soil is 36 mg/kg [52]. However, Cu concentration in soils of both study sites was much lower than the approved standard. In human beings, a high concentration of Cu causes metal fumes fever, hair and skin decolorations, dermatitis, respiratory tract diseases, and some other fatal diseases [53].
Mercury (Hg): Mean concentration of mercury (Hg) was significantly higher (p < 0.05) in all the portions of site A compared to site B (Table 15.3). The deposit of this metal was higher in site A-portion A than portion B by about 21%, and with a significant (p < 0.05) 7.8 times more Hg deposit compared with site B. In addition, a significant high concentration of Hg was recorded in the water and Acacia karroo samples (Figs. 15.2 and 15.3). The mean concentrations of Hg for all collected soil, water, and Acacia karroo samples were found to be extremely above the approved limit of WHO. For instance, the permissible limit of Hg recommended by WHO for drinking water is 0.006 mg/L [45] which is much lower than the concentration of Hg found in the dam water (11.420–55.035 mg/L) evaluated in this study. WHO approved 1.0 and 0.03 mg/kg respectively as the maximum permissible limits of Hg for soil and plants [54], whereas in South Africa, the soil permissible limit for Hg is 0.93 mg/kg [55]. In our study, however, Hg concentrations ranged from 1.08 to 8.45 mg/kg in the dumpsites soils and 1.71–13.27 mg/kg in Acacia karroo samples. High concentration of Hg in the soil, water, and plant studied in the current investigation may be due to dumping and burning of industrial (municipal and medicinal) wastes which have been produced with mercury on the dumpsites. Exposure to disinfectants (like mercurochrome), antifungal agents, toiletries, creams and organometallics may increase Hg concentration, hence waste generated from these products may be a contributing factor to the high values obtained for these samples [56].
Also, the emissions from coal-using power plants nearby might have contributed significantly to the problem of high Hg levels in the soils of site A and the control site B. The Acacia karroo plants growing in site A must have absorbed a significant amount of Hg from the soil and transported this to other parts of the plants. Run-off of Hg-contaminated rainwater during rainfall from the dumpsite to the dam may have also elevated the concentration of Hg in the dam water. Mercury is a naturally occurring metallic element found in trace amount in water, soil, and air, and is a widespread environmental toxicant and pollutant which induces severe alterations in the body tissues and causes a wide range of adverse health effects [57, 58]. All forms of mercury are toxic and their effects include gastrointestinal toxicity, neurotoxicity, and nephrotoxicity [58].
Manganese (Mn): This metal occurs naturally in water, soil, and plants. It is an essential trace heavy metal required by plants and animals for normal growth. High concentration of Mn causes hazardous effects on the lungs and brains of humans [59]. Mn deposit in soil was significantly (p < 0.05) higher at site A, recording about twice the amount found in the site B (Table 15.3). Traces of Mn were also detected in the dam water and tissue of the Acacia karroo species (Figs. 15.2 and 15.3). Maximum permissible limit of Mn in plants recommended by WHO is 500 mg/kg and toxicity occurs in plants if this level is exceeded [60, 61]. WHO recommended 0.4 mg/L [47] in drinking water while 1 to 45 mg/kg was approved for soil [51].
Mean Mn concentrations for water samples in this study ranged from 0.023 ± 0.020 to 1.106 ± 0.780 mg/L and were all above the normal permissible limit, except for the samples collected in the first week (0.023 ± 0.020 mg/L). On the other hand, Acacia karroo samples recorded mean concentrations of Mn (0.14 ± 0.10 to 0.55 ± 0.39 mg/kg) which are below the normal standard. Likewise, the mean concentrations of Mn in all evaluated soil samples fall within the normal range set by the WHO. Land disposal of manganese-containing wastes is the principal source of manganese releases to the soil [61]. High values of Mn in the water samples may be as a result of a natural occurrence of this metal in surface water and groundwater, erosion of contaminated soils into the water as well as human activities which might have caused Mn contamination [62]. High concentration levels of Mn indicate that the potability of the dam water has been markedly impaired.
Lead (Pb): Deposition of Pb in the soil samples followed a similar trend observed for Cu where Pb concentration in site A-portion B was significantly (p < 0.05) higher than site A-portion C and site B, recording about 5.3 times more Pb deposit than site B (Table 15.3). Traces of Pb were also found in the dam water and plant tissue (Figs. 15.2 and 15.3). Dutch permissible limit for Pb in the soil is 85 mg/kg while WHO limits of this metal for plants and drinking water are 2.0 mg/kg [52] and 0.01 mg/L [47] respectively. High Pb concentration in the dumpsites soils compared to control site B may be due to large deposits of used batteries, used plastics (polyethylene) materials, lubrication oils and automobile exhaust fumes. Nevertheless, the lead concentrations in all the soil samples irrespective of the site were lower than the approved limit. Pb concentrations in both water and Acacia karroo were also found to be below the standard approved range. In water samples Pb concentration ranged from 0.0011 ± 0.0010 to 0.0044 ± 0.0030 mg/L while 0.003 ± 0.002 to 0.009 ± 0.010 mg/kg were the Pb concentration range obtained for Acacia karroo samples.
Lead has no biological importance [59] and is known for its harmful effects on humans as well as causing chronic neurological disorders, especially in foetus and children [63]. Lead poison causes a condition called plumbism or saturnism in a human body [64]. Although lead occurs naturally in the environment, anthropogenic activities such as fossil fuels burning, mining, and manufacturing contribute to its release in high concentrations to the environment [58].
Heavy metal concentration in plants can be caused by several factors which include soil metal concentrations, soil pH, cation exchange capacity, organic matter content, types and varieties of plants, and plant age. It is generally accepted that the metal concentration in soil is the dominant factor [60, 65]. Heavy metal availability can also be directly affected by the plant itself [66].
15.4 Conclusions
Generally, landfill site had a higher concentration of metals than control site probably due to wastes dump. The concentration of Hg and Mn in water samples were above the normal limits permitted by WHO and Hg was beyond the approved limits in both soil and plant samples. At the moment, heavy metal concentrations in the dumpsite are not of a serious environmental problem according to WHO standard except Hg. Continuous accumulation of metals should be avoided to prevent threat to human health and the ecosystem in future. Specifically, the accumulation of Pb and Hg should be of concern because the US Government Agency for Toxic Substances and Disease Registry ranked these two metals among the three most toxic elements with arsenic ranked first [67].
They are capable of causing serious adverse effects to human health [67, 68], hence Pb and Hg should be well monitored. This study shows the need to sort wastes dumped at the dumpsite and to encourage recycling of the materials. Surrounding dams must be monitored to deliver less expensive quality municipal water. Modern waste disposal facilities for appropriate waste processing are important as well as community education to avoid indiscriminate disposal of wastes.
References
Xie Y, Fan J, Zhu W, Amombo E, Lou Y, Chen L, Fu J (2016) Effect of heavy metals pollution on soil microbial diversity and bermudagrass genetic variation. Front Plant Sci 7:755
Osunkiyesi AA, Taiwo AG, Olawunmi OA, Akindele OI, Sobo AA (2014) Environmental impact of heavy metal contaminants and micronutrients in soil samples of metal dumpsites in Abeokuta, Ogun State, Nigeria. IOSR J Appl Chem 7:52–55
Meng M, Li B, Shao JJ, Wang T, He B, Shi JB, Ye ZH, Jiang GB (2014) Accumulation of total mercury and methylmercury in rice plants collected from different mining areas in China. Environ Pollut 184:179–186
Kalantari MR, Shokrzadeh M, Ebadi AG, Mohammadizadeh C, Choudhary MI (2006) Soil pollution by heavy metals and remediation (Mazandaran-Iran). J Appl Sci 6:2110–2116
Ukpong EC, Antigha RE, Moses EO (2013) Assessment of heavy metals content in soils and plants around waste dumpsites in Uyo Metropolis, Akwa Ibom State. IJES 2:75–86
Nda-Umar UI, Nathaniel GS, Mann A, Yisa J (2012) Assessment of heavy metal species in some decomposed municipal solid wastes in Bida, Niger State, Nigeria. Adv Anal Chem 2:6–9
Ebong GA, Akpan MM, Mkpenie VN (2008) Heavy metal contents of municipal and rural dumpsite soils and rate of accumulation by Carica papaya and Talinum triangulare in Uyo, Nigeria. J Chem 5:281–290
Mitsios IK, Golia EE, Tsadilas CD (2005) Heavy metal concentrations in soils and irrigation waters in Thessaly region, Central Greece. Commun Soil Sci Plan 36:487–501
Azeez JO, Hassan OA, Egunjobi PO (2011) Soil contamination at dumpsites implication of soil heavy metals distribution in municipal solid waste disposal system: a case study of Abeokuta, Southwestern Nigeria. Soil Sediment Contam 20:370–386
Aucott M (2006) The fate of heavy metals in landfills: a review. New York Academy of Sciences, New York, USA
Njagi JM (2013) Assessment of heavy metal concentration in the environment and perceived health risks by the community around Kadhodeki dumpsite, Nairobi Country. Doctoral Dissertation, Kenyatta University, Kenya
Momba MNB, Tyafa Z, Makala N, Brouckaert BM, Obi CL (2006) Safe drinking water still a dream in rural areas of South Africa. Case study: the Eastern Cape Province. Water SA 32:715–720
Kotz JC, Treichel PM, Weaver GC (2005) Chemistry and chemical reactivity. Brooks & Cole, Belmont, USA
Taylor CL, Barker NP (2012) Species limits in Vachellia (Acacia) karroo (Mimosoideae: Leguminosae): evidence from automated ISSR DNA “fingerprinting”. S Afr J Bot 83:36–43
Baroni L, Cenci L, Tettamanti M, Berati M (2007) Evaluating the environmental impact of various dietary patterns combined with different food production systems. Eur J Clin Nutr 61:279–286
Opaluwa OD, Aremu MO, Ogbo LO, Abiola KA, Odiba IE, Abubakar MM, Nweze NO (2012) Heavy metal concentrations in soils, plant leaves and crops grown around dump sites in Lafia Metropolis, Nasarawa State, Nigeria. Adv Appl Sci Res 3:780–784
Zhang H, Cui B, Xiao R, Zhao H (2010) Heavy metals in water, soils, and plants in riparian wetlands in the Pearl River Estuary, South China. Procedia Environ Sci 2:1344–1354
Tsade HK (2016) Atomic absorption spectroscopic determination of heavy metal concentrations in Kulufo River, Arbaminch, Gamo Gofa Ethiopia. J Environ Anal Chem 3:177
Parajuli PB, Duffy S (2013) Evaluation of soil organic carbon and soil moisture content from agricultural fields in Mississippi. Open J Soil Sci 3:81–90
Eckert D, Sims JT (2011) Recommended soil pH and lime requirement tests. In: Recommended soil testing procedures for the Northeastern United States. Northeast Regional Bulletin 493:19–26
Vlab.amrita.edu. (2013) Soil analysis—Determination of available organic carbon in the soil. Retrieved from http://vlab.amrita.edu/?sub%E2%80%89=%E2%80%892&brch%E2%80%89=%E2%80%89294&sim%E2%80%89=%E2%80%891552&cnt%E2%80%89=%E2%80%891. Accessed 25 May 2016
Motsara MR, Roy RN (2008) Guide to laboratory establishment for plant nutrient analysis, vol 19. Food and Agriculture Organization of the United Nations, Rome, Italy
Sam KK (2013) Determination of total dissolved solids (TDS) in water and wastewater. Inclusive Science and Engineering. Available at: http://www.inclusive-science-engineering.com/determination-total-dissolved-solids-tds-water-wastewater/. Accessed 26 Aug 2016
Nduwumuremyi A (2013) Soil acidification and lime quality: sources of soil acidity, effects on plant nutrients, the efficiency of lime and liming requirements. J Agr Allied Sci 2:26–34
Olaniran AO, Balgobind A, Pillay B (2013) Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci 14:10197–10228
Oloyede-Kosoko SOA, Idowu AO, Ayoni OO (2015) Evaluation of water quality with World Health Organization and Nigeria Industrial Standards using geographic information system. World Sci News 24:18–42
Zhou W, Hui D, Shen W (2014) Effects of soil moisture on the temperature sensitivity of soil heterotrophic respiration: a laboratory incubation study. PLoSOne 9:e92531
Sato JH, Figueiredo CCD, Marchão RL, Madari BE, Benedito LEC, Busato JG, Souza DMD (2014) Methods of soil organic carbon determination in Brazilian savannah soils. Sci Agr 71:302–308
Musinguzi P, Tenywa JS, Ebanyat P, Tenywa MM, Mubiru DN, Basamba TA, Leip A (2013) Soil organic carbon thresholds and nitrogen management in tropical agroecosystems: concepts and prospects. J Sustain Dev 6:31–43
Deb S, Bhadoria PBS, Mandal B, Rakshit A, Singh HB (2015) Soil organic carbon: towards better soil health, productivity, and climate change mitigation. Clim Change Environ Sustainability 3:26–34
Hopkins FM, Filley TR, Gleixner G, Lange M, Top SM, Trumbore SE (2014) Increased below ground carbon inputs and warming promote loss of soil organic carbon through complementary microbial responses. Soil Biol Biochem 76:57–69
Ranjan R, Divya M, Bavitha M (2015) The importance of soil food web for a healthy environment and sustainable development. Int J Appl Res 1:15–20
Wild B, Schnecker J, Alves RJE, Barsukov P, Bárta J, Čapek P, Gentsch N, Gittel A, Guggenberger G, Lashchinskiy N, Mikutta R (2014) Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in Arctic permafrost soil. Soil Biol Biochem 75:143–151
Xiao S, Zhang W, Ye Y, Zhao J, Wang K (2017) Soil aggregate mediates the impacts of land uses on organic carbon, total nitrogen, and microbial activity in a Karst ecosystem. Sci Rep 7:41402
Ullman JL (2013) Soil salinity in agricultural systems: the basics. Agricultural & Biological Engineering University of Florida. Available at: http://hos.ufl.edu/sites/default/files/faculty/gdliu/ullman.pdf. Accessed on 21 April 2018
Ray RW, Nyle CB (2016) The nature and properties of soils, 15th edn. Pearson, USA
Verma JK, Sharma A, Paramanick KK (2015) To evaluate the values of electrical conductivity and growth parameters of apple saplings in nursery fields. Int J Appl Sci Eng Res 4:321–332
Al-Badaii F, Shuhaimi-Othman M, Gasim MB (2013) Water quality assessment of the Semenyih River, Selangor, Malaysia. J Chem Article ID 871056
Postel S, Richter B (2012) Rivers for life: managing water for people and nature. Island Press, USA
Turhanen PA, Vepsäläinen JJ, Peräniemi S (2015) Advanced material and approach for metal ions removal from aqueous solutions. Sci Rep 5:8992
Sallam GA, Elsayed EA (2015) Estimating the impact of temperature and relative humidity change on the water quality of Lake Manzala Egypt. J Nat Resourc Dev 5:76–87
Middel A, Häb K, Brazel AJ, Martin CA, Guhathakurta S (2014) Impact of urban form and design on mid-afternoon microclimate in Phoenix local climate zones. Landscape Urban Plan 1:16–28
Mwangi FN (2014) Land use practices and their impact on the water quality of the Upper Kuils River (Western Cape Province, South Africa). Doctoral dissertation, University of Western Cape
Rehman HU, Akbar NU, Gul I, Gul N, Akhwan S, Sajed M, Khan P, Khan MA, Hamidullah SB, Wahab A (2015) Impacts of some physicochemical parameters of water and soil collected from Panjkora River, Pakistan. Global Veterinaria 15:57–61
Mgombezi D, Rao V, Vuai SH, Singh SK (2017) An investigation on effectiveness of cactus materials (Opuntia Spp.) as adsorbents for hard water treatment. Int J Sci Technol Res 6:239–244
USGS (2017) USGS water-quality information: water hardness and alkalinity. US Geological Survey Office of Water Quality. Available at: https://water.usgs.gov/owq/hardness-alkalinity.html. Accessed 28 Feb 2017
Kumar RRR, Series SC (2012) Water, soil, and sediment characterization: Sharavathi river basin, Western Ghats. Envis Technical Report, Environmental Information System (ENVIS) Centre for Ecological Sciences, Indian Institute of Science, Bangalore, India
Fisher-Jeffes LN, Armitage NP, Carden K (2017) The viability of domestic rainwater harvesting in the residential areas of the Liesbeek River Catchment, Cape Town. Water SA 43:81–90
Saana SBBM, Fosu SA, Sebiawu GE, Jackson N, Karikari T (2016) Assessment of the quality of groundwater for drinking purposes in the Upper West and Northern regions of Ghana. Springerplus 5:2001
Edokpayi JN, Odiyo JO, Msagati TA, Potgieter N (2015) Temporal variations in physicochemical and microbiological characteristics of Mvudi River, South Africa. Int J Environ Res Pub Health 12:4128–4140
Okeyode IC, Rufai AA (2011) Determination of elemental composition of soil samples from some selected dumpsites in Abeokuta, Ogun State, Nigeria, using atomic absorption spectrophotometer. Int J Basic Appl Sci 11:55–70
Ogundele DT, Adio AA, Oludele OE (2015) Heavy metal concentrations in plants and soil along heavy traffic roads in North Central Nigeria. J Environ Anal Toxicol 5:1
Shah A, Niaz A, Ullah N, Rehman A, Akhlaq M, Zakir M, Suleman Khan M (2013) Comparative study of heavy metals in soil and selected medicinal plants. J Chem Article ID 621265
Anyalogbu EAA, Anadi CC, Nweje-Anyalowu PC, Nnoli MC (2017) Use of waterleaf (Talinum triangulare) in the remediation of soil exposed to heavy metals: a green technology approach. World J Pharm Life Sci 3:48–53
Kamunda C, Mathuthu M, Madhuku M (2016) Health risk assessment of heavy metals in soils from the Witwatersrand gold mining basin, South Africa. Int J Environ Res Pub Health 13:663
Pagrut NS (2012) Heavy metal toxicity in canines with special reference to reproductive status. Doctoral dissertation, MAFSU, Nagpur, India
Asiamah G (2014) Heavy metal concentration in the Owere River at Konongo. Master dissertation, Kwame Nkrumah University of Science and Technology, Ghana
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Luch A (ed) Molecular, Clinical and Environmental Toxicology. Experientia Supplementum, vol 101. Springer, Basel, Switzerland
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity mechanism and health effects of some heavy metals. Interdisc Toxicol 7:60–72
Khan MU, Malik RN, Muhammad S (2013) Human health risk from heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere 93:2230–2238
Trollip DL, Hughes JC, Titshall LW (2013) Sources of manganese in the residue from a water treatment plant. Water SA 39:265–270
World Health Organization (2011) Manganese in drinking-water: background document for development of WHO guidelines for drinking-water quality. Switzerland, Geneva
Neal AP, Guilarte TR (2013) Mechanisms of lead and manganese neurotoxicity. Toxicol Res 2:99–114
Garcés JBG, Artuz RIM (2012) Lead poisoning due to bullets lodged in the human body. Colomb Med 43:230–234
Naser HM, Sultana S, Mahmud NU, Gomes R, Noor S (2011) Heavy metal levels in vegetables with growth stage and plant species variations. Bangladesh J Agric Res 36:563–574
Hajar EWI, Sulaiman AZB, Sakinah AM (2014) Assessment of heavy metals tolerance in leaves, stems, and flowers of Stevia rebaudiana plant. Procedia Environ Sci 20:386–393
Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Pub Health 47:74–83
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdisc Toxicol 5:47–58
Acknowledgements
The authors are grateful to Govan Mbeki Research and Development Centre (GMRDC), University of Fort Hare, Directorate of Research and Development, Walter Sisulu University and University Research Foundation, Cape Peninsula University of Technology, NRF-Sasol Inzalo Foundation for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Maphuhla, N.G., Oyedeji, A.O., Lewu, F.B., Oyedeji, O.O., Lewu, M.N. (2019). Physicochemical Properties and Heavy Metals Accumulation in the Plant, Soil and Water from Municipal Landfill in Alice, South Africa. In: Ramasami, P., Gupta Bhowon, M., Jhaumeer Laulloo, S., Li Kam Wah, H. (eds) Chemistry for a Clean and Healthy Planet. ICPAC 2018. Springer, Cham. https://doi.org/10.1007/978-3-030-20283-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-20283-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20282-8
Online ISBN: 978-3-030-20283-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)