Abstract
This paper discusses the real meaning of the results from cryogenic calorimetry. A new concept of heat input, denominated as effective heat input, is proposed, based on the fact that what is important for welding engineering is the heat that determines the cooling rates and governs the metallurgical transformations. A descriptive model is proposed to explain how heat flows inside a plate to demonstrate that part of the heat leaves the plate before diffusing into it. Experiments using a liquid nitrogen calorimeter, fully automated to minimize intrinsic errors, were carried out to verify the difference in the outcomes when either bead size or plate thickness is a variable in the test. It was concluded that other intrinsic errors persist and the test, as conceived at the moment, can be applied only on comparative manner if all conditions are the same. However, a promising parameter described here as net heat input maybe taken from the test, which would be independent of variables as bead size and plate thickness and more useful for heat flow and metallurgical estimations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The importance of heat input in welding is widely known. Representing the energy delivered to the workpiece, the heat input governs the bead formation (operational and economical aspects), the resultant distortion/residual stress, and the metallurgical transformations. Due its relevance, scholars, researchers, and engineers in the field of welding have been developing work for correlating heat input with the results obtained in the weldments. Welding standards, recommendations, and procedures usually determine limits that the predicted heat input must fit. However, even considering the large knowledge on this matter, built along the years, there are three pitfalls related to the application of heat input in welding. The first one is related to the definition of heat input, the second one concerns its measurement, and the third drawback is a matter of conception.
Even though the meaning of the wording is clear and defined by official sources, such as from the American Welding Society’s [2], where it is stated that “heat input” is related to the energy supplied to the workpiece, there are several published works where heat input is considered simply as the product of the mean current and the mean voltage divided by the travel speed. In this case, the losses of the welding energy to the environment (surrounding atmosphere, unmelted electrode, contact tip, shielding gas, etc.) by radiation, convection, and/or conduction are neglected. Some other workers try to be more precise in the expression of heat input by introducing an efficiency factor (η thermal).
In addition to the difficulty of determining this efficiency factor, the way the welding energy is calculated is a matter not always considered in the scientific work in this area. The literature shows differences of opinion between authors regarding the method that should be used (arithmetical average power, effective power, and instantaneous power). Bosworth [3] has found that the differences of applying the different methods on the final value can reach 30 %. Joseph et al. [11], using a calorimetry, stated that the only measure of welding energy which is reasonably well correlated to current variations is based on the instantaneous power. Nascimento et al. [16] analyzed all the methods mentioned above and the respective consequences on the heat input and thermal efficiency calculations. They showed that the arithmetic mean power method can be applied in a few cases, in which there is no variation in current and voltage (like in spray transfer gas metal arc welding), but it is safer to use the instantaneous power method (more laborious, yet generic). More recently, Melfi [15] shows that method to calculate the heat input, using instantaneous energy, was added in the 2010 edition of the ASME Boiler and Pressure Vessel Code: Section IX (item QW409.1) for waveform-controlled welding.
In relation to measurement of the thermal efficiency factor (consequently, the heat input), a review of the technical literature shows that some debate has taken place concerning the most appropriate method to obtain a correct measurement. One of the most used techniques is the water calorimeter, as described by Lu and Kou [13, 14], which allows a continuous cooling with continuous water flow through the root side of the weld (the water temperature during and after welding is monitored and the input heat is related to it). Bosworth [3] and Essers and Walter [6] also used calorimeters where the monitoring of the water temperature was the main parameter to determine the heat involved in the weldments. Similar principle was used by Cantin and Francis [4], who used as heat absorber a block of electrical conductor grade aluminum, over which it was attached the plate to be welded (all inside an isolated box). Pépe et al. [17] improved Cantin and Francis’s calorimeter by using a movable lid to close the upper surface of the isolated box just behind the arc movement. Another class of calorimeter used to measure heat efficiency was described and applied by Giedt et al. [8], Fuerschbach and Knorovsky [7], and DuPont and Marder [5]. This calorimeter consists of a special box (in which the hot workpiece is placed inside) and works based on the Seebeck principle (voltage is produced proportionally to the heat flux through the calorimeter walls, i.e., gradient layer principle). Soderstrom et al. [20] used another interesting calorimeter for the measurement of droplet heat content.
Heat losses that occur during welding and in the lapsed time between finishing the weld and introducing the sample in the calorimeter seems to be a setback shared by most of the calorimetric techniques. To minimize this problem, Haelsig et al. [9] presented another approach of water calorimeter in which the metal plate is positioned at a certain angle in the calorimetric vessel. Parallel to the movement of the welding torch, the water level in the calorimetric vessel is constantly increased. With this design, the heat from the heated portion of the metal is immediately transferred to the circulating water.
Cryogenic calorimeters have also been used by several researchers in welding [10, 11, 17, 21] with good results and with the advantage of reducing the time of the experiments. The plate is rapidly inserted into a Dewar containing liquid nitrogen after welding and the amount of liquid boiled off is measured. But, even though a reasonably accurate procedure has been stated for this method, some intrinsic problems exist in the methodology. Pépe et al. [17] has shown that both the delay time between completing welding and inserting the specimen into the liquid nitrogen and the weld bead length influences the measured quantity. They indicate that the heat input reduces with increasing delay time or bead length (a drop of approximately 10 % in the heat efficiency from delays changing from 5 to 100 s and from welds with 5 and 25 s of duration). The reduction in efficiency is caused by conduction from the sample into the jig, as well as convection from the sample after and during welding. These authors claim that for calculating the actual process efficiency it is necessary to subtract the errors due to both the welding and delay times.
Pépe et al. [17] also showed that different parameters (consequently, different welding energies) change the heat efficiency of a process. A reduction in efficiency was met for increasing welding energy, in agreement to the results of Bosworth [3]. One can suppose that if Pépe et al. used the same procedure and test plate sizes, a higher welding energy could represent bigger losses to the environment before digging the plate into the cryogenic vessel. DuPont and Marder [5], using the Seebeck calorimeter, found that the arc efficiency did not vary significantly with a process over the range of currents investigated. Pépe et al. further demonstrated that welding in a groove increased the process efficiency for around 5 %, since much of the radiation heat losses were absorbed by the side walls. In summary, heat efficiency and heat input measured by liquid nitrogen calorimeter seems to depend not only of the process, as simplified in a great number of papers, but they depend on the test parameters, welding process parameters, test plate size and geometry, etc.
A further concern on cryogenic calorimetry measurements is the concept of heat input. As widely known in the welding field, the heat input governs the metallurgical transformations. Different peak temperatures and cooling rates can be imposed in a region of the welded workpiece if the delivery of heat is different. As summarized above, even some test variables can change the values of the heat input obtained in the cryogenic calorimetry. In fact, the best wording to define the results from cryogenic calorimetry would be “absorbed heat” rather than heat input, because there is no evidence that all the measured heat will influence the metallurgical transformations. If the heat leaves the plate by the surfaces (edges, top, and root sides) before diffusing into the plate, this quantity should not be taken in account. If the bead is long during the measurements, the heat that is lost to the atmosphere during the welding through the surface, before digging the plate in the liquid nitrogen, can be significant, but it is not accounted as heat input. On the other hand, part of the heat that would go out by the root surface before diffusing is counted as heat inside the plate in the test. Thus, the absorbed heat measured in the test will be dependent on the bead length and plate dimension (thickness, width, and length) and shape. But it is not sure that absorbed heat would always represent the consequent cooling rates, which is of major importance to control, as one would expect from the words heat input.
The aspects referred above lead to the conclusion that the cryogenic calorimeter test can be used only for comparative purpose, as long as all test parameters and welding conditions are the same. To solve this limitation, the means of transforming the absorbed heat in an equivalent heat input regardless the test variables is a matter under development by the authors’ team. However, before reaching successful results in this direction (in the attempt of minimizing all potential intrinsic errors mentioned above), a more systematic assessment on the effect of some test variables on the absorbed heat measured in the test is needed. Thus, the objective of this work is to undertake cryogenic calorimetric measurements in different conditions to study the effect of bead length and plate thickness, but minimizing the influence of some random test variables to prevent disguising the effect of these two factors.
2 A descriptive model for effective heat input in arc welding
Before presenting the experimental procedure, it is important to define more strictly the concept of heat input. The authors have proposed in another publication [18] the scheme presented in Fig. 1 for describing the heat input concept of moving heat source. The explanation is based on the heat balance (in and out) in a plate under welding and on the consequence of this balance on the cooling rate. In summary, the welding energy (consumed electrical power to maintain the arc) is the driven force of the system. Taking into account in this summary only the transfer of energy from the arc into the plate, this heat transfer would happen mainly by three means.
Schematic representation of the input and output of heat in a plate during moving arc welding proposed by Scotti et al. [18] (italics heat losses from the plate that do not influence near bead cooling rates, dotted lines heat leaving, full lines heat arriving)
The first way happens at the connection arc-plate (heat generated at the anodic or cathodic layer), where heat is transferred by conduction to the plate (forming the pool). The higher the current, the more heat is transferred to the plate surface. It is reasonable to say that more than 80 % of the total voltage of an arc is consumed at the connection arc-plate and arc-electrode. Scotti et al [19], working with short-circuiting and Ar + O2 + N2 shielding gases found that the voltage drop in the arc connections to the anode and cathode represents almost the total voltage drop (the arc column represented only 3.5–4 % of the total arc voltage, which includes electrode and droplet falls). Lenivkin et al. [12] found a cathode drop of 13.2 V, an anode drop of 7.25 V and an arc column electric field of 2.9 V/mm when GMA welding were performed using carbon steel wire shielded by N2 (12.4 % for a 1 mm arc and 29.8 % for a 3 mm arc). Thus, this quantity is the predominant one on heat transfer from the arc to the plate, but hardly one can predict a fixed percentage that goes to the plate (since it depends on a great number of variables, including the shielding gas composition).
The second way of heat transfer to the plate represents the heat carried by the droplets, transferring from the electrode to the weld pool. Soderstrom et al. [20] measured this amount as from 20 to 30 % of the total welding energy. However, part of the heat carried by the droplets is lost to the environment due to spattering and metal evaporation.
In the third way, the energy from the plasma column is delivered to the plate, mainly into the pool adjacent area. Radiation from the arc column is a way to transfer heat to the plate, especially outside of the coupling between the arc column and the plate (due to the high temperature inside of this region, the heat is transferred by conduction, as seen above). But, the plasma jet also carries energy (enthalpy) and transfer it by convection (through the phenomenon of advection and diffusion), forced or natural, to the plate. However, part of the heat content of the plasma column losses to the environment (straight or indirectly), by radiation (mainly) and convection (there is a difference of velocity between the gas flow and the plasma jet, heating the frontier plasma column-shielding gas). Fortunately, a significant fraction of this heat loss from the arc column goes to the plate (not considered, then, losses). This happens by forced convection, since the corresponding surrounding layer of heated non-ionized gas also carries energy (enthalpy).
Once the heat is transferred to the plate, most part of it will diffuse inside the plate, but part of it is released back to the atmosphere by convection through the still heated surface of the bead already solidified (volume close behind the arc). The diffused heat will both melt a fraction of metal (a greater amount of metal will melt if, depending on the heat concentration and intensity and metal diffusivity coefficient, the heat builds up locally before diffusing into the plate) and only diffuse into the plate (conduction), the latter not affecting directly the fusion phenomenon. The amount of heat that was used to melt the metal (sensitive heat from room temperature to melting point + fusion latent heat + sensitive heat from melting point to boiling point, related to both droplets and fused region) is eventually transferred to the plate by conduction. But part of this heat in the weld pool is lost to the environment by radiation and natural convection, through the high temperature surface of the bead under solidification (just behind the arc). Other fraction of the heat in the weld pool is also lost to the environment, either before or during welding, because both droplet and weld bead while liquid undergo evaporation and mass loss (spattering). DuPont and Marder [5] in their paper review cite that evaporation and radiation from the liquid pool during welding of iron have been estimated to be on the order of 30–10 W, respectively. Even considering the losses by convention, these authors cite that the total heat loss during welding is approximately 125 W, which represents approximately 1 % of the total arc power (amount neglectable in calorimetry, according to them).
The greatest part of the heat that diffuses into the plate will distribute inside the plate (sidewards), cooling down the hottest parts, closer to the bead, and heating up the coldest part, father to the bead, until the thermal equilibrium is reached on the whole plate. The remaining surface losses (natural convection) close the equilibrium are not important anymore as far as metallurgical aspects is concerned, since the temperature is already below the transformation temperatures of most materials. On the other hand, cooling rate resulting from the heat conduction process is the governing factor to the metallurgical transformations. However, depending on the plate thickness, heat goes also through the thickness and reaches the root face. Depending on the plate and thickness and the diffusivity coefficient, the losses of heat can take place by three means: (a) natural convection losses with no importance at low temperature if the plate is thick enough to not have the surface significantly heated (for a given arc energy); (b) radiation and convection losses at high temperature from the root bead pool and convection losses (solidified root bead) if the plant is thin enough to have the opposite surface undergoing melting temperature; (c) natural convection at median temperature if the plate thickness is intermediate.
The importance of knowing how the heat is delivered to the plate and how it is distributed into the plate bulk has the purpose of bring over a discussion on the meaning of “absorbed heat” and “effective heat input”. The authors propose to denominate absorbed heat as the heat quantity measured in cryogenic calorimetry test, for instance. To avoid confusion in terminologies, they prefer to leave the term heat input to mean in a generic way the amount of heat entering a plate (bulk heat input), regardless if either it can leave the plate by the surfaces or not be measured by calorimetry. However, they propose a third term, effective heat input, to describe the heat input which really affects the cooling rate, i.e., the bulk heat which diffuses really into the plate through the heat-affected zone (HAZ) of a bead, excluding heat that leaves the plate to the atmosphere before diffusing into the HAZ (effective heat input should not be confused with effective heat efficiency, the latter related to the share of heat input used to melt the plate). As seen in Fig. 1 model, heat losses written in italic does not affect the cooling rate. It means that, for the same absorbed heat measured in a calorimetry test, the effective heat input can be different and, consequently, so the cooling rates experimented by different parts of the metal. The higher the effective heat, the slower the cooling in the HAZ (it is not the purpose of this work to measure neither heat input nor effective heat input, but only absorbed heat).
3 Experimental methodology
The principle of the liquid nitrogen (LN2) calorimeter test is to quantify the heat absorbed by a plate during welding by submerging the plate in a Dewar flask of liquid nitrogen. The total internal heat of the plate after welding is possible to be determined by knowing the nitrogen latent heat evaporation, the plate mass and the difference between the mass of the Dewar before and after the just-welded specimen to have been dropped into the bath. If the internal heating of the plate with the bead at room temperature is also measured (LN2 mass difference before and after the specimen is dropped again into bath), the absorbed heat by the welded plate due to the weld (heat carried by the droplets, heat generated at the connection arc-plate and heat transferred from the plasma-gas enthalpy) is determined. Recalling, this absorbed heat is generally called heat input by the welding community. However, in fact, part of the heat that is input into the plate during the welding leaves the plate before the specimen is dig into the Dewar flask, reason for the authors to prefer the use of the term absorbed heat
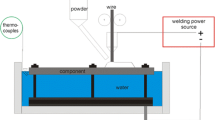
In this work, it was used a fully automated cryogenic calorimeter, as illustrated in Fig. 2 (more details is given in [1]). In summary, the test plate is hold by two claws, with the minimal contact interference. In this device, the only action of the operator is to turn on the welding power. Synchronized with the welding power, the torch movement starts and it travels up to a determined distance (adjusted by a microswitch), corresponding to the desired bead length. When the weld reaches the end, the right side claw opens at the same time that the left side one turns 90° to position the specimen over the liquid nitrogen flask and to release it into the container (this takes 3.64 ± 0.20 s). The difference of mass is automatically measured by an electronic scale under the flask (the natural LN2 evaporation rate at room temperature in this device is very low, 2.96 g/min). A computerized data acquisition system and a dedicated program measure at the same time the welding parameters (current and voltage, at the rate of 2 kHz) and the LN2 mass variation (at the rate of 10 Hz). With this approach, it is possible to determine with precision and high repeatability the welding time and the nitrogen mass loss, parameters needed to calculate the absorbed heat per unit of weld length and the thermal efficiency. The importance of this rig is the possibility of avoiding random errors (by repeating one operation three times in a row, the absorbed heat for a given condition was 46.8 ± 0.9 kJ). But, the intrinsic error of the test cannot be avoided, as seen below.
Schematic representation of the automated cryogenic calorimeter: 1 torch, 2 pneumatic system, 3 plate, 4 calorimeter flask, 5 scale [1]
3.1 Recognition of intrinsic errors
The cryogenic calorimeter presents intrinsic measurement errors. Errors due to different delay times (between the end of the weld and the piece all inside the liquid nitrogen) and bead lengths have already been mentioned in the introduction. The first one will always exist. But the source of this error is still wider. It is not only a matter of elapsed time, but also of trajectory and speed. Heat losses will increase if the time is longer and the movement is faster during the trajectory of the plate to the flask. As this last point is not commented in the literature, an experiment was carried to assess the variation of measured absorbed heat when the specimens are drop into the flask from different heights (see Fig. 3). Table 1 shows significant differences in the test outcome (around 6.4 % from the fastest distance and the shortest one). It means that if this test is carried manually, the results cannot be the same. As future work, the estimation of this intrinsic error should be done.
Concerning the error source due to bead length, to make welds with long sizes would sound a good approach to minimize errors; the share of errors due at the end of the welding (overstated values, since the heat has not dissipated by the bead/plate surface yet) would become insignificant as the bead increases. However, the share of error due to the welding starting (understated, since the heat would have left the plate before digging it the liquid nitrogen) is also inevitable; the longer the weld, the bigger the error.
3.2 Validation of the descriptive model for effective heat input
A series of measurements of absorbed heat was carried out using the above mentioned full automated calorimeter. The welding condition was kept the same in all runs (short-circuit MIG/MAG bead-on-plate welding, using a 1.2-mm AWS ER70S-6 wire, with a CTWD of 12 mm and wire feed speed of 3.6 m/min, shielded by Ar + 25%CO2 and traveling at 34 cm/min). Initially, two plate thicknesses (3.2 and 9.5 mm) and five different bead lengths (from approximately 10–150 mm long) were employed on test plates of plain carbon steel (200 × 100 mm). The width of the plate was made wider enough to prevent from other intrinsic error, matter of further investigation. Table 2 presents the data from monitoring each run and the consequent results from the measurement and calculations from the calorimetry.
Figure 4 is a graphical representation of the effect the plate thickness and weld bead length over the absorbed heat. As seen, despite using the same welding energy, the absorbed energy was lower for increasing bead length and smaller thickness (the difference reaches values as high as 15 %). These results are in disagreement with DuPont and Maders [5], for whom the heat losses by radiation, convection, and evaporation are neglectable. On the contrary, the intrinsic errors can lead to significant differences.
Absorbed heat per length from welds at different lengths on thinner and thicker plates (welding and test conditions presented at the footnote of Table 2)
The reliability of the results can be confirmed by some facts. One can observe that if an extrapolation to a null weld length is applied to both fitting curves, the absorbed heat would be the same, around 540 J/mm. This approach of extrapolating the results for hypothetical beads with no lengths indicates the capacity of a process to input heat into the plate; as the process and parameters were the same in all experiments, this capacity should be the same, regardless the thickness.
Another fact that supports the high reliability of the results is that the thinner plates absorbed less heat than the thicker plates, in agreement to the model of item 2 (if the plate is thin, there higher losses to the environment by radiation and convection from the root pass before the heat is diffused into the plate). In addition, the longer the bead, the less the absorbed heat. These results are also in full agreement to the model presented in item 2. If the bead is long, there is more time to loss heat to the environment before digging the plate into the flask with liquid nitrogen.
Then, the same tests with the same welding conditions were performed for a larger range of plate thicknesses (adding to previous tests 4.75, 6.3, and 7.95 mm plate thicknesses), aiming to verify if the absorbed heat behavior would remain constant. Figure 5 shows the complete graphical representation of the results. It is interesting to note that if the tendencies are extrapolated to bead length = 0, they reach the same point (the potential heat before entering the plate surface). A good repeatability between the earlier tests and present ones can be mentioned (for 3.2 and 9.56 mm thick plate).
Absorbed heat per length from welds at different lengths for different plate thicknesses (welding and test conditions presented at the footnote of Table 2)
The extrapolated value at time equals 0 would represent the actual heat input that the process delivers to the plate (before diffusing into the plate) by a moving heat source. This would be a third terminology proposed by the authors, namely net heat input. This value should be used to calculate cooling rates, isotherms, etc. (considering that rate of diffusion throughout the workpiece is not uniform, since the whole plate is not in uniform temperature, since the whole plate is not in uniform temperature, this aspects can be taken into to account in calculations by using as entrance the net heat input value, rather from the usual heat input one). But it is important to comment that to do so, the geometry of the heat source must be considered at the same time to reach precise calculations. In addition, it is also important to point out that with this approach, net heat input is determined by the automated cryogenic calorimetry with minimized random errors and free from two intrinsic errors, namely bead length and plate thickness, although other intrinsic errors, such as specimen fall distance and time and even specimens width, still persist and need to be estimated to reach a more precise determination (to be dealt in a near future).
These results suggest a potential application for the liquid nitrogen colorimeter, but further tests (different joint configuration, current level, etc.) should be done to reach the conclusion of the possibility for determining the maximum capacity of a process to input heat in a workpiece.
3.3 The verification of the concept of effective heat input
Figure 6 shows macrographies of weld transverse sections in which the same welding parameters were used in both weld. It can be noticed that in the thin plate the bead is greater and the heat affected zone is larger with coarser grains. It seems to be a result of a lower cooling rate for the thin plate. Despite of the lower absorbed heat (Table 2), as a consequence of more looses by radiation from the back of the weld, the effective heat input (which has a real metallurgical impact) was higher for the thin plate at the same welding conditions.
This behavior would be expected, because the heat conduction flux is bidirectional in thin plate case, in contrast to the three-directional flux in a thicker plate; it is easier for the heat to be diffused into the plate (thick plate) than to be lost for environment (thin plate). However, one should expected much less difference if it is considered that the absorbed heat was lower (since it was used the same process and same welding parameters). The effective heat input is the cause of the smaller differences.
4 Conclusions
-
The liquid nitrogen cryogenic calorimeter is an adequate method for measuring the absorbed heat by a plate welded by different process and with different parameters and conditions, since the variation in results meet theoretical explanations
-
The automation of a cryogenic calorimeter devise increases remarkably the reliability of the test concerning repeatability of results, but not avoiding intrinsic errors
-
Even if the test is applied only for comparison purposes (as the researchers should be aware of at the moment), the users must be conscious of the intrinsic sources of errors (plate thickness and shape, bead length, elapsed time, and trajectory between the weld ending and plate digging) to use the results
-
The proposed model for effective heat input in arc welding helps the user to explain the difference in results, always based on the share of heat which is lost or not between the welding starting and the plate inside the liquid nitrogen flask
-
The concept of effective heat input must be applied in welding in replacement of heat input to better use of absorbed heat taken from cryogenic calorimeters
-
One potential use of the cryogenic calorimeter would be to measure the maximum capacity of inputting heat of a process/welding condition (net heat input)
References
Arevalo HH (2011) Development and assessment of experimental rig for calorimetry via liquid nitrogen and continuous flow (water) in welding processes. MSc Dissertation, Federal University of Uberlândia, Brazil, 145p (in Portuguese)
AWS (2001) Standard welding: terms and definitions, standard AWS A3.0:2001
Bosworth MR (1991) Effective heat input in pulsed gas metal arc welding with solid wire electrodes, Weld J 70(5):111s–117s
Cantin GMD, Francis JA (2005) Arc power and efficiency in gas tungsten arc welding of aluminum. Sci Technol Weld Join 10(2):200–210
DuPont JN, Marder AR (1995) Thermal efficiency of arc welding processes. Weld J 74(12):406s–416s
Essers W, Walter R (1981) Heat transfer and penetration mechanisms with GMAW and plasma-GMA welding. Weld J 60(2):37s–42s
Fuerschbach PW, Knorovsky GA (1991) A study of melting efficiency in plasma arc and gas tungsten arc welding. Weld J 70(11):287s–297s
Giedt WH, Tallerico LN, Fuerschbach PW (1989) GTA welding efficiency: calorimetric and temperature field measurements. Weld J 68(1):28s–32s
Haelsig A, Kusch M, Mayr P (2011) A new technology for designation the efficiency of gas shielded arc welding gives new findings , IIW Doc XII-2031-11 Commission XII meeting, IIW Annual Assembly 2011, Chennai
Hsu C, Soltis P (2002) Heat input comparison of STT vs. short-circuiting and pulsed GMAW vs. CV processes, In: 6th International Conference Trends in Welding Research. ASM International, Pine Mountain, pp 369–374
Joseph A, Harwig D, Farson DF, Richardson R (2003) Measurement and calculation of arc power and heat transfer efficiency in pulsed gas metal arc welding. Sci Technol Weld Join 8(6):400–406
Lenivkin VA, Diurgerov NG, Sagirov KN (1989) Technological properties of the MIG/MAG welding arc, 1st edn. Mashinostroenie, Moscow (in Russian)
Lu MJ, Kou S (1989) Power inputs in gas metal arc welding of aluminum—part 1. Weld J 68(9):382s–388s
Lu MJ, Kou S (1989) Power inputs in gas metal arc welding of aluminum—part 2. Weld J 68(11):452s–456s
Melfi T (2010) New code requirements for calculating heat input. Weld J 89(6):61–65
Nascimento AS, Batista MA, Nascimento VC, Scotti A (2007) Assessment of electrical power calculation methods in arc welding and the consequences on the joint geometric, thermal and metallurgical predictions. Soldagem e Inspeção 12(2):97–106 (in Portuguese)
Pépe N, Egerland S, Colegrove PA, Yapp D, Leonhartsberger A, Scotti A (2011) Measuring the process efficiency of controlled gas metal arc welding processes. Sci Technol Weld Join 16(5):412–417
Scotti A, Liskevych O, Reis RP (2012) A descriptive model of the heat flux in arc welding aiming the effective heat input Concept. Soldagem & Inspeção (ISSN 0104–9224 printed/ISSN 1980–6973 online) (in Portuguese)
Scotti A, Ponomarev V, Costa AV (2006) A methodology for voltage drop determination in GMA welding: arcs with short-circuiting metal transfer. Eur Phys J Appl Phys. doi:10.1051/epjap:2006060
Soderstrom EJ, Scott KM, Mendez PF (2011) Calorimetric measurement of droplet temperature in GMAW. Weld J AWS 90(1):1s–8s
Watkins AD (1989) Heat transfer efficiency in gas metal arc welding. Thesis for the Degree of Master of Science, College of Graduate Studies, University of Idaho April
Acknowledgments
The authors wish to thank Federal University of Uberlândia (UFU) for infrastructure utilized during the development of this work and FAPEMIG and CNPq, a Minas Gerais state and federal, respectively, agencies for research development, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Doc. IIW-2352, recommended for publication by Commission XII “Arc Welding Processes and Production Systems” and by Study Group SG-212 “The Physics of Welding”.
Rights and permissions
About this article
Cite this article
Liskevych, O., Quintino, L., Vilarinho, L.O. et al. Intrinsic errors on cryogenic calorimetry applied to arc welding. Weld World 57, 349–357 (2013). https://doi.org/10.1007/s40194-013-0035-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-013-0035-5