Abstract
Purpose of Review
Sinonasal squamous cell carcinoma (SNSCC) is a rare disease with considerable histologic diversity. Currently, there is a poor understanding of the etiology and pathogenesis of SNSCC. Here, we review recent literature to summarize what is known regarding (1) the etiology of SNSCC, (2) the role of Human Papilloma Virus (HPV) in SNSCC, and (2) the molecular underpinnings of SNSCC.
Recent Findings
1. High risk HPV appears to play a role in the pathogenesis of a subset of SNSCCs. SNSCCs with high risk HPV have improved survival compared with those without HPV and occur in patients who are younger, similar to HPV mediated oropharyngeal cancer. 2. A subset of inverted papillomas have transcriptionally active low-risk HPV and have a higher risk of transformation, while low risk HPV negative inverted papillomas frequently have EGFR mutations.
Summary
SNSCC is a diverse disease with likely multiple etiologies including carcinogen, irritant exposure, and HPV. While not definitively proven, evidence supports a role for high-risk HPV in a subset of SNSCC, and low-risk HPV in a subset of inverted papillomas which transform to SNSCC. In-depth molecular and genomic studies are needed in SNSCC to better understand the genomic underpinnings and oncogenic drivers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Squamous cell carcinoma (SCC) of the nasal and paranasal sinuses (SNSCC) is the most common histologic subtype of all sinonasal tumors, making up more than 50% of cases [1, 2]. SNSCCs arise from mucosal sites throughout the paranasal sinuses with contemporary literature supporting the most common originating site to be the nasal cavity, followed by maxillary sinus [3] (Table 1). The incidence of SNSCC in males is 0.52 cases per 100,000 patients, and females 0.23 cases per 100,000 patients, with a male to female incidence ratio of 1.85–2.26:1 [1, 2, 4,5,6]. While the incidence of SNSCC is decreasing, 5-year overall survival (OS) rates have not changed appreciably over the last three decades, hovering around 50% [1,2,3,4]. This is in part due to the advanced stage of disease at diagnosis and high rate of local recurrence [7•, 8]. When outcomes are sub-stratified by tumor site, patients with nasal cavity SCC have improved 5-year relative survival (RS) (74.5%) compared with patients with maxillary sinus SCC (35%) and ethmoid sinus SCC (33%) (Table 1). Frontal and sphenoid sinus SCC carry the worst prognosis with 5 year survival of − 30% [3, 9]. Worsened survival in these subsites, compared with nasal SCC, may be related to stage of presentation, difficulty accessing the tumors due to proximity to vital structures or, as discussed below, etiologic variability. Across all tumors, increased age, T and N classification is associated with worse overall survival (OS) [2, 9]. Smoking status is associated with worse outcomes in SNSCC, with current smokers having a decreased 5-year OS compared with reformed smokers [10]. Worse outcomes are also seen in patients with poor performance status and African American patients, similar to the HNSCC overall [9]. The incidence of SNSCC arising as a second primary in head and neck cancer patients is low (0.2%) [11]. Below, we will review current opinions on the etiology and pathogenesis of SNSCC, with an emphasis on emerging data assessing the role of human papilloma virus (HPV) in SNSCC.
Etiology
Carcinogen Exposure
Occupational hazards can explain some of the etiologic risk for SNSCC, as well as the male predominance [1, 4, 12]. For example, professionals with multi-year histories of working with wood, which is a designated human carcinogen, and more specifically softwood dust, have up to 20-times increased risk of developing SNSCC compared with the general population and compared with other sinonasal tumors [13,14,15,16,17,18,19]. Occupational exposure to several industrial compounds and chemical substances, such as leather dust, glues, formaldehyde, chrome, nickel, arsenic and welding fumes, and various compounds used in the textile industry, have been attributed to tumorigenesis in up to 30% of SNSCC [4, 15,16,17, 20,21,22]. Limited reports have also been published of SNSCC in hairdressers and rubber workers [23].
Historic case-control studies have analyzed the role of smoking in the development of SNSCC [24,25,26,27,28]. All suggest that smoking poses an increased risk for the development of SNSCC. Presence of a dose–response relationship in most studies and the decrease in risk associated with time since quitting, support the existence of a causal association [17]. Importantly, evidence suggests that smoking tobacco can increase the risk of SNSCC two to threefold which is significantly less than for many other tobacco-associated cancers [24, 29]. There is further evidence to support secondhand tobacco smoke exposure as a risk factor [18].
Viral Oncogenesis
HPV is well established as a causative etiology in a HNSCC [30]. Up to 80% of oropharyngeal SCC (OPSCC) are HPV mediated and − 5% of other upper aerodigestive tract subsites as well [31, 32]. When evaluating tonsillar tissue subsites (lingual and palatine tonsils), up to 92% of cases are HPV mediated [32]. Importantly, HPV mediated OPSCC (HPVmOPSCC) is not only etiologically distinct from non-HPVmOPSCC but also epidemiologically and molecularly. For example, HPVmOPSCC is increasing in incidence, carries a significantly improved prognosis and lacks many of the driver mutations seen in non-HPVmOPSCC, such a TP53 [33]. The role of HPV in SNSCC is not established; however, emerging epidemiologic and molecular literature supports a potential role for HPV in a subset of SNSCC [34, 35].
HPV Prevalence in SNSCC
Recent retrospective studies and meta-analyses suggest that − 30% of SNSCC have HPV present, regardless of detection method [36, 37]. Firstly, it is important to recognize that different HPV detection methods (for example, In Situ Hybridization (ISH), DNA PCR, RNA PCR) have different analytic sensitivities. Briefly, HPV detection methods can be divided to:
- (i)
Nucleic acids hybridization assays such as Southern blot, Dot Blot hybridization, and ISH. These techniques use labeled nuclei acid hybridization assays to detect HPV in samples. Although the specificity of this method is high, sensitivity can be lower, and less than that seen in PCR analysis [38]. However, ISH is more specific for HPV infection than p16 immunohistochemical staining (IHC) [39].
- (ii)
Immunohistochemical staining (IHC). p16 protein is a surrogate marker of transcriptionally active HPV infection has been found to be highly sensitive yet less specific [40].
- (iii)
Nucleic acids amplification assays such as Polymerase chain reaction (PCR). These assays commonly have primers designed to amplify a region of HPV DNA or mRNA and have high sensitivity [41, 42].
- (iv)
Signal-amplification assays such as Hybrid Capture 2 (hc2) and the Cervista HPV. These assays use a non-radioactive signal-amplification method based on the hybridization of the target HPV-DNA to labeled RNA probes in solution [41, 42].
HPV DNA is detected across many head and neck subsites by PCR; however, the detection of HPV DNA does not indicate causation as an oncogenic driver. Methods to assess transcriptional activity are assumed to more accurately assess if HPV may potentially be driving tumorigenesis, as opposed to a bystander infection, for example, the detection of HR-HPV E6 or E7 mRNA by RT-PCR, among other techniques. Combined detection techniques are also advocated for determining a potentially causative viral infection, such as detection of both 70% nuclear and cytoplasmic p16 expression and HR-HPV DNA by PCR or ISH (or RNA by RT-PCR or ISH) [43, 44].
Prevalence of HPV Genotypes in SNSCC
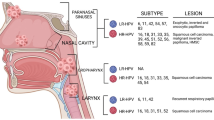
Fifteen oncogenic HPV genotypes are associated with mucosal tumors and include 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68,73, and 82 [45]. Several studies have assessed HPV genotypes in SNSCC and found HPV-16 to be the most common, similar to other HPVmHNSCCs [34, 37, 45,46,47,48,49]. Less commonly identified genotypes are HPV-18, 31, and 33, again paralleling HPVmOPSCC [34, 37, 45, 50] Rare cases of HPV 35, 39, 45, and 82 have also been reported [45]. Table 2 summarizes recent literature examining the prevalence of HPV in SNSCC, as well as the detection methodology. HPV prevalence also appears to vary by anatomic subsite within the sinonasal cavity with the nasal cavity having the highest prevalence (Table 3) [36].
Epidemiology and Demographics of HPV-Associated SNSCC
Numerous studies have examined of the epidemiology of SNSCC with and without HPV present in the tissue. These studies lack standardization for how HPV presence is detected making interpretation challenging. Overall, HPV-positive cases are associated with younger age patients (58.0 vs 63.7 years) similar to HPVmOPSCC [56]. However, unlike HPVmOPSCC, HPV positive SNSCC is more often found in patients who smoke, or are reformed smokers [49]. HPV positive SNSCC are less likely to be well-differentiated compared with SNSCC without HPV present [36]. Importantly, HPV positive SNSCC patients appear to have higher 5 years OS compared with HPV negative SNSCCs (68.1–80% vs 31–51.5%) [36, 46]. In a meta-analysis, Syrjänen et al. stratified the prevalence of HPV in SNSCC by the geographic origin and found the highest summary effect size (64.8%) is derived from studies reported in China, followed by those conducted in Europe 39.4%, and USA/Canada 32.9%, and Asia 22.7% (Table 3)[37]. These results suggest SNSCC may differ in etiology by geography, similar to nasopharyngeal carcinoma [36, 37]. In a study published this year, Oliver at al reviewed the NCDB for SNSCC cases with HPV testing, finding again, that HPV is associated with younger age and improved OS [57].
HPV and SNSCC Histologic Subtypes
A portion of the heterogeneity reported in HPV prevalence in SNSCC may be explained by evaluation of different SNSCC subtypes. The presence of transcriptionally active HR-HPV varies for the various SCC subtypes in the sinonasal tract and is more frequently detected in nonkeratinizing SCC (NKSCC) compared with keratinizing (KSCC) (35–50% vs 4–25%, respectively) but is lower than in non-keratinizing OPSCC [36, 43, 46, 49, 58, 59]. Other SNSCC variants show even higher transcriptionally active HR-HPV prevalence, such as basaloid 46–56.5%; papillary 42–80% [36, 43] and adenosquamous carcinomas 66–83% [34, 43].
HPV in Sinonasal Papillomas
Sinonasal papillomas (SP) are benign epithelial neoplasms arising in the sinonasal tract and are histopathologically divided into three subtypes: (i) Inverted (ISP) 62%, (ii) exophytic (ESP) 32%, and (iii) oncocytic (OSP) 6% [60].
Inverted Sinonasal Papilloma
While ISPs are fundamentally benign, malignant transformation rates are reported as 1.9–11% [43, 60, 61]. The majority of carcinomas associated with ISP are present at the time of diagnosis (64%) while 36% are identified after initial treatment [61]. Most cases of carcinoma associated with ISP are SCC (75%), but rarely other tumor types such as sinonasal undifferentiated carcinoma or verrucous carcinoma have been described [61]. HPV in ISPs has been detected by different methods (DB—Dot Blot hybridization, SB—Southern Blot hybridization, ISH and PCR) with Low-risk HPV (LR-HPV) (6 and 11) being 2.8 times as frequent as HR-HPV (16 and 18) in ISP and ISP-associated SCC (ISPSCC) [62]. However, HR-HPV is more frequent in cases with high-grade dysplasia and carcinoma [54•, 63]. A recent meta-analysis suggests that HPV-18 may be specifically associated with ISPSCC [64]. When examining the literature surrounding HPV and ISPs, there is enormous variability with reports using similar detection approaches reporting vastly different HPV detection rates. For example, HR- HPV mRNA was detected by ISH in 100% of ISPs by Stoddard et al. (n = 19) while Rooper at al reported 0% detection [65, 66]. Interestingly, Udager et al. have demonstrated that many ISPs (88%) and SNSCC arising from ISPs (77%), possess EGFR mutations while non ISP related SNSCCs do not harbor EGFR mutations [67]. Further, the same group reported that all ISPs (n = 58) and ISPSCCs (n = 22) demonstrate either an EGFR mutation or HPV infection, and that HPV and EGFR mutation are mutually exclusive in all cases of ISPSCC. All paired ISP and ISPSCC samples demonstrated concordant HPV status, and EGFR genotypes and ISP progression to SNSCC were significantly associated with the presence of HPV infection and the absence of an EGFR mutation [54•]. In a study published this year, Mehrad et al. extended these findings, demonstrating that a subset of ISPs has transcriptionally active LR-HPV and that these lack EGFR mutations and have a higher risk of transformation. Similarly, LR-HPV negative ISPs frequently have EGFR mutations, further supporting the concept that EGFR mutations and LR-HPV infection are mutually exclusive [68••].
Oncocytic Sinonasal Papilloma
Malignant transformation occurs in 4–17% of OSPs, most of these being SCC [69]; however, mucoepidermoid, small cell, adenocarcinoma, and sinonasal undifferentiated carcinomas have also been described [70, 71]. In WHO 2017, and additional literature, HPV has not been found to be associated with OSP [70, 72, 73]. However, recent studies and meta-analysis found 22.5% of OSPs cases are HPV positive [62, 63]. Interestingly, a recent study identified KRAS alterations in OSP (n = 51) and OSP-associated SCC (n = 5) suggesting it is indeed a precursor lesion to SCC yet is biologically distinct from other SNSCCs [69].
Exophytic Sinonasal Papilloma
ESPs may also be etiologically related to HPV. In a large meta-analysis, ESPs were associated with HPV in 63.5% of cases, predominantly with low-risk HPV (6 and 11), and rarely with types 16 and 57 [62]. HPV status has not been shown to correlate with carcinoma development [73]. Malignant change in ESP is extremely rare [74]. In summary, while the majority of SNCCs arise de novo, a subset arises from sinonasal papillomas. HPV DNA has clearly been demonstrated to be present in some papillomas; however, the role of HPV in this transformation process remains unclear.
HPV-Related Multiphenotypic Sinonasal Carcinoma
HPV related multiphenotypic sinonasal carcinoma with adenoid cystic–like features (HMSC) is a newly described entity under the category of NKSCC in the latest WHO classification [75]. HMSC has a high predisposition for local recurrence (− 38%), has a female predominance and typically affects the nasal cavity (89%) with or without paranasal sinus involvement [76]. HMSC is associated with strong and diffuse p16 staining in all cases and Ki-67 staining in 40–90% [77, 78]. HMSC is by definition associated with high-risk HPV infection, with HPV-33 and HPV-35 being the most common genotypes [78,79,80,81,82] and less frequently HPV 56, 16 and 82 [78, 82, 83].
Pathogenesis
Unlike the remainder of mucosal HNSCCs (oral, oropharynx, larynx), in which the genomic landscape is well described, in large part, through The Cancer Genome Atlas (TCGA), the pathogenesis and genomic underpinnings of SNSCC remains poorly defined.
SNSCC Genomics
The genomic landscape of SNSCC is poorly defined. Here, we summarize what is known as follows:
- (i)
Somatic mutations: TP53 mutations have been described in a significant portion of SNSCCs, particularly in those tumors associated with exposure to wood dust (70%) [14, 33]. Similar to other HNSCCs, patients with TP53 altered SNSCC appears to have worse OS (43.8% vs 84.1% 3-year OS) [33]. Mutations in KRAS and HRAS have been found in a small subset of SNSCCs and as noted above, KRAS alterations are particularly common in OSP associated SCC [69, 84, 85].
- (ii)
Copy number alterations: Amplification of FGFR1 has been reported in 20% of SNSCC and in 33% carcinomas associated with ISP [65] while SOX2 amplification has been demonstrated in 37% of SNSCC cases and was associated with significantly higher rate of tumor recurrences [86].
- (iii)
Expression changes: p53 expression alterations have been reported in around 50% of SNSCCs [8, 87, 88]. Several studies have demonstrated EGFR overexpression in 40–89% of SNSCCs which was associated with significantly shorter disease-free survival and worse local recurrence rate [33, 88, 89]. Overexpression of HER2 (ErbB2) has also been detected in a subset of SNSCCs [88, 89]. Finally, overexpression of VEGFR-encoding gene has been reported in − 50% of SNSCCs [62, 90].
- (i)
Genome instability: A possible factor involved in pathogenesis of SNSCCs is microsatellite instability (MSI). DNA mismatch repair deficiency has been reported to occur in 21% of SNSCC [91].
- (ii)
Chromosomal aberrations: SNSCCs appear to have a number of chromosomal alterations [92]. One study identified losses occurring at 9p21, 13q14, 17p13, 17q21, and 18q11 with frequent gains observed on 8q24, 11q13, 17q12, 19p13, and 20q11–q13 [92] and resultant amplification of 7p12(EGFR), 11p13(CD44), 11q13(CCND1 and EMS1), and 17q21 (ERBB2)[92], with some of these common to HNSCC [93, 94].
SNSCC Immune Microenvironment
Immune checkpoint inhibitors (anti-PD-1) have dramatically altered treatment paradigms for advanced HNSCC; however, initial clinical trials leading to their approval did not include SNSCC [95][96]. In HNSCC, tumors expressing PD-L1 have improved response rates compared with non-PD-L1 positive tumors [96]. Riobello et al. evaluated the prevalence of PD-L1 expression in 96 SNSCC tumors, observing that 17%, 30%, and 50% of tumors have > 50%, 5%, and 1% membranous PD-L1 staining of tumor cells, respectively [7•]. Our institutional experience (n = 11 patients) with advanced SNSCC treated with anti-PD-1 therapy is that response rates are at least equivalent to other HNSCCs (unpublished). Considering the poor survival of patients with recurrent or metastatic SNSCC, studies are needed to evaluate the efficacy of anti-PD-1 treatment.
Conclusions
SNSCC is a complex disease with likely numerous etiologic subtypes and processes driving tumorigenesis. The molecular underpinnings, etiology, and pathogenesis of SNSCC is significantly understudied and remains poorly understood compared with other HNSCCs. Current literature supports a role for HPV in SNSCC, yet the size of this contribution and effects on outcomes require significantly more dissection. High-quality studies that aim to rigorously interrogate SNSCC molecular etiology and pathogenesis are greatly needed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012. https://doi.org/10.1002/hed.21830.
Jain S, Li Y, Kuan EC, Tajudeen BA, Batra PS, Batra S. Prognostic factors in paranasal sinus squamous cell carcinoma and adenocarcinoma: a SEER database analysis. J Neurol Surgery, Part B Skull Base. 2019;80:258–63.
Dutta R, Dubal PM, Svider PF, Liu JK, Baredes S, Eloy JA. Sinonasal malignancies: a population-based analysis of site-specific incidence and survival. Laryngoscope. 2015. https://doi.org/10.1002/lary.25465.
Sanghvi S, Khan MN, Patel NR, Yeldandi S, Baredes S, Eloy JA. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124:76–83.
Danesh-Sani SA, Sarafraz A, Chamani M, Derakhshandeh H. Paranasal sinuses malignancies: A 12-year review of clinical characteristics. Med Oral Patol Oral Cir Bucal. 2016. https://doi.org/10.4317/medoral.21170.
Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001. https://doi.org/10.1002/1097-0142(20011215)92:12<3012::AID-CNCR10131>3.0.CO;2-E.
• Riobello C, Vivanco B, Reda S, López-Hernández A, García-Inclán C, Potes-Ares S, et al. Programmed death ligand-1 expression as immunotherapeutic target in sinonasal cancer. Head Neck. 2018;40:818–27 Findings from this study suggest that proportion of patients with SNSCC may benefit from therapy with immune checkpoint inhibitors.
Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol. 2014;11:460–72.
Robin TP, Jones BL, Gordon OM, Phan A, Abbott D, McDermott JD, et al. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer. 2017. https://doi.org/10.1002/cncr.30686.
Russo AL, Adams JA, Weyman EA, Busse PM, Goldberg SI, Varvares M, et al. Long-term outcomes after proton beam therapy for sinonasal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2016;95:368–76.
Wolpoe ME, Goldenberg D, Koch WM. Squamous cell carcinoma of the sinonasal cavity arising as a second primary in individuals with head and neck cancer. Laryngoscope. 2006;116:696–9.
Kuijpens JLP, Louwman MWJ, Peters R, Janssens GORJ, Burdorf A, Coebergh JWW. Trends in sinonasal cancer in the Netherlands: more squamous cell cancer, less adenocarcinoma: A population-based study 1973–2009. Eur J Cancer. 2012. https://doi.org/10.1016/j.ejca.2012.05.003.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans., International Agency for Research on Cancer. Wood dust and formaldehyde. World Health Organization, International Agency for Research on Cancer. 1995.
Holmila R, Bornholdt J, Heikkilä P, et al. Mutations in TP53 tumor suppressor gene in wood dust-related sinonasal cancer. Int J Cancer. 2010. https://doi.org/10.1002/ijc.25064.
Siew SS, Kauppinen T, Kyyrönen P, Heikkilä P, Pukkala E. Occupational exposure to wood dust and formaldehyde and risk of nasal, nasopharyngeal, and lung cancer among Finnish men. Cancer Manag Res. 2012. https://doi.org/10.2147/CMAR.S30684.
Luce D, Leclerc A, Morcet J-F, Casal-Lareo A, Gérin M, Brugère J, et al. Occupational risk factors for sinonasal cancer: a case-control study in France. Am J Ind Med. 1992. https://doi.org/10.1002/ajim.4700210206.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012.
Fukuda K, Shibata A. Exposure-response relationships between woodworking, smoking or passive smoking, and squamous cell neoplasms of the maxillary sinus. Cancer Causes Control. 1990;1:165–8.
Binazzi A, Ferrante P, Marinaccio A. Occupational exposure and sinonasal cancer: a systematic review and meta-analysis. BMC Cancer. 2015. https://doi.org/10.1186/s12885-015-1042-2.
D’Errico A, Pasian S, Baratti A, Zanelli R, Alfonzo S, Gilardi L, et al. A case-control study on occupational risk factors for sino-nasal cancer. Occup Environ Med. 2009;66:448–55.
IARC World Health Organization International Agency For Research On Cancer Iarc Monographs on the evaluation of carcinogenic risks to humans. Tobacco smoke and involuntary smoking. Tob. Smoke Involunary Smok. 2004; Volume 83.
Hoppe BS, Stegman LD, Zelefsky MJ, Rosenzweig KE, Wolden SL, Patel SG, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting-the MSKCC experience. Int J Radiat Oncol Biol Phys. 2007. https://doi.org/10.1016/j.ijrobp.2006.09.023.
Leclerc A, Luce D, Demers PA, et al. Sinonasal cancer and occupation. Results from the reanalysis of twelve case-control studies. Am J Ind Med. 1997;31:153–65.
Brinton LA, Blot WJ, Becker JA, Winn DM, Browder JP, Farmer JC, et al. A case-control study of cancers of the nasal cavity and paranasal sinuses. Am J Epidemiol. 1984. https://doi.org/10.1093/oxfordjournals.aje.a113812.
Hayes RB, Kardaun JWPF, De Bruyn A. Tobacco use and sinonasal cancer: a case-control study. Br J Cancer. 1987. https://doi.org/10.1038/bjc.1987.303.
Strader CH, Vaughan TL, Stergachis A. Use of nasal preparations and the incidence of sinonasal cancer. J Epidemiol Community Health. 1988. https://doi.org/10.1136/jech.42.3.243.
Zheng W, Blot WJ, Shu X-O, Diamond EL, Gao Y-T, Ji B-T, et al. A population-based case-control study of cancers of the nasal cavity and paranasal sinuses in Shanghai. Int J Cancer. 1992. https://doi.org/10.1002/ijc.2910520410.
T Mannetje A, Kogevinas M, Luce D, et al. Sinonasal cancer, occupation, and tobacco smoking in European women and men. Am J Ind Med. 1999. https://doi.org/10.1002/(SICI)1097-0274(199907)36:1<101::AID-AJIM14>3.0.CO;2-A.
Youlden DR, Cramb SM, Peters S, Porceddu SV, Møller H, Fritschi L, et al. International comparisons of the incidence and mortality of sinonasal cancer. Cancer Epidemiol. 2013;37:770–9.
Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010. https://doi.org/10.1056/NEJMoa0912217.
Stein AP, Saha S, Kraninger JL, Swick AD, Yu M, Lambert PF, et al. Prevalence of human papillomavirus in oropharyngeal cancer. Cancer J (United States). 2015;21:138–46.
Gelwan E, Malm IJ, Khararjian A, Fakhry C, Bishop JA, Westra WH. Nonuniform distribution of high-risk human papillomavirus in squamous cell carcinomas of the oropharynx: rethinking the anatomic boundaries of oral and oropharyngeal carcinoma from an oncologic HPV perspective. Am J Surg Pathol. 2017. https://doi.org/10.1097/PAS.0000000000000929.
Doescher J, Piontek G, Wirth M, Bettstetter M, Schlegel J, Haller B, et al. Epstein-Barr virus infection is strictly associated with the metastatic spread of sinonasal squamous-cell carcinomas. Oral Oncol. 2015;51:929–34.
Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–92.
Lewis JS, Westra WH, Thompson LDR, et al. The sinonasal tract: another potential “hot spot” for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol. 2014. https://doi.org/10.1007/s12105-013-0514-4.
Kılıç SSSSSSS, Kılıç SSSSSSS, Kim ES, Baredes S, Mahmoud O, Gray ST, et al. Significance of human papillomavirus positivity in sinonasal squamous cell carcinoma. Int Forum Allergy Rhinol. 2017;7:980–9.
Syrjänen K, Syrjänen S. Detection of human papillomavirus in sinonasal carcinoma: systematic review and meta-analysis. Hum Pathol. 2013;44:983–91.
Smeets SJ, Hesselink AT, Speel EJM, Haesevoets A, Snijders PJF, Pawlita M, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007. https://doi.org/10.1002/ijc.22980.
Bishop JA, Ma XJ, Wang H, Luo Y, Illei PB, Begum S, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–82.
Westra WH. Detection of human papillomavirus in clinical samples. Otolaryngol Clin N Am. 2012. https://doi.org/10.1016/j.otc.2012.04.001.
Abreu ALP, Souza RP, Gimenes F, Consolaro MEL. A review of methods for detect human Papillomavirus infection. Virol J. 2012. https://doi.org/10.1186/1743-422X-9-262.
Brink AATP, Snijders PJF, Meijer CJLM. HPV detection methods. IOS Press; 2007.
Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60–7.
Lewis JS, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas guideline from the college of American pathologists. Arch Pathol Lab Med. 2018;142:559–97.
Chowdhury N, Alvi S, Kimura K, Tawfik O, Manna P, Beahm D, et al. Outcomes of HPV-related nasal squamous cell carcinoma. Laryngoscope. 2017;127:1600–3.
Alos L, Moyano S, Nadal A, Alobid I, Blanch JL, Ayala E, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009. https://doi.org/10.1002/cncr.24309.
Yamashita Y, Hasegawa M, Deng Z, et al. Human papillomavirus infection and immunohistochemical expression of cell cycle proteins pRb, p53, and p16INK4a in sinonasal diseases. Infect Agent Cancer. 2015;10:1–8.
Ambreen B, Reyaz TA, Sheikh J, Imtiyaz H, Summyia F, Ruby R. Histopathological study of lesions of nose and paranasal sinuses and association of Human Papilloma Virus (HPV) with sinonasal papillomas and squamous cell carcinoma in the. Int J Med Res Heal Sci. 2016;5:7–16.
Laco J, Sieglová K, Vošmiková H, et al. The presence of high-risk human papillomavirus (HPV) E6/E7 mRNA transcripts in a subset of sinonasal carcinomas is evidence of involvement of HPV in its etiopathogenesis. Virchows Arch. 2015;467:405–15.
Beigh A, Rashi R, Junaid S, Khuroo MS, Farook S. Human Papilloma Virus (HPV) in sinonasal papillomas and squamous cell carcinomas: a PCR-based study of 60 cases. Gulf J Oncolog. 2018
Chung CH, Guthrie VB, Masica DL, et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann Oncol. 2015. https://doi.org/10.1093/annonc/mdv109.
Becker C, Kayser G, Pfeiffer J. Squamous cell cancer of the nasal cavity: new insights and implications for diagnosis and treatment. Head Neck. 2016. https://doi.org/10.1002/hed.24391.
Sahnane N, Ottini G, Turri-Zanoni M, et al. Comprehensive analysis of HPV infection, EGFR exon 20 mutations and LINE1 hypomethylation as risk factors for malignant transformation of sinonasal-inverted papilloma to squamous cell carcinoma. Int J Cancer. 2019;144:1313–20.
• Udager AM, McHugh JB, Goudsmit CM, Weigelin HC, Lim MS, Elenitoba-Johnson KSJ, et al. Human papillomavirus (HPV) and somatic EGFR mutations are essential, mutually exclusive oncogenic mechanisms for inverted sinonasal papillomas and associated sinonasal squamous cell carcinomas. Ann Oncol. 2018;29:466–71 Findings from this study shed light on the potential mechanism in pathogenesis of IPs and IPSCC.
Bulane A, Goedhals D, Seedat RY, Goedhals J, Burt F. Human papillomavirus DNA in head and neck squamous cell carcinomas in the Free State. South Africa J Med Virol. 2019. https://doi.org/10.1002/jmv.25556.
Liederbach E, Kyrillos A, Wang CH, Liu JC, Sturgis EM, Bhayani MK. The national landscape of human papillomavirus-associated oropharynx squamous cell carcinoma. Int J Cancer. 2017. https://doi.org/10.1002/ijc.30442.
Oliver JR, Lieberman SM, Tam MM, Liu CZ, Li Z, Hu KS, Morris LGT, Givi B. Human papillomavirus and survival of patients with sinonasal squamous cell carcinoma. Cancer cncr.32679. 2019.
Larque AB, Hakim S, Ordi J, Nadal A, Diaz A, Del Pino M, et al. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod Pathol. 2014;27:343–51.
Thompson LDR, Franchi A. New tumor entities in the 4th edition of the World Health Organization classification of head and neck tumors: nasal cavity, paranasal sinuses and skull base. Virchows Arch. 2018. https://doi.org/10.1007/s00428-017-2116-0.
Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol. 2002;15:279–97.
Nudell J, Chiosea S, Thompson LDR. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014. https://doi.org/10.1007/s12105-014-0527-7.
Syrjänen K, Syrjänen S. Detection of human papillomavirus in sinonasal papillomas: systematic review and meta-analysis. Laryngoscope. 2013;123:181–92.
Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of schneiderian inverted papillomas: an analytic overview of the evidence. Head Neck Pathol. 2008. https://doi.org/10.1007/s12105-008-0048-3.
Zhao RW, Guo ZQ, Zhang RX. Human papillomavirus infection and the malignant transformation of sinonasal inverted papilloma: a meta-analysis. J Clin Virol. 2016;79:36–43.
Stoddard DG, Keeney MG, Gao G, Smith DI, García JJ, O’Brien EK. Transcriptional activity of HPV in inverted papilloma demonstrated by in situ hybridization for E6/E7 mRNA. Otolaryngol Head Neck Surg. 2015. https://doi.org/10.1177/0194599815571285.
Rooper LM, Bishop JA, Westra WH. Transcriptionally active high-risk human papillomavirus is not a common etiologic agent in the malignant transformation of inverted schneiderian papillomas. Head Neck Pathol. 2017;11:346–53.
Udager AM, Rolland DCM, McHugh JB, et al. High-frequency targetable EGFR mutations in sinonasal squamous cell carcinomas arising from inverted sinonasal papilloma. Cancer Res. 2015;75:2600–6.
•• Mehrad M, Stelow EB, Bishop JA, Wang X, Haynes W, Oliver D, Chernock RD, Lewis JS. Transcriptionally active HPV and targetable EGFR mutations in sinonasal inverted papilloma. Am J Surg Pathol. 2019; 1. This manuscript demonstrates that a subset of ISPs has transcriptionally active LR-HPV and that these lack EGFR mutations and have a higher risk of transformation. Similarly, LR-HPV negative ISPs frequently have EGFR mutations, further supporting the concept that EGFR mutations and LR-HPV infection are mutually exclusive.
Udager AM, McHugh JB, Betz BL, et al. Activating KRAS mutations are characteristic of oncocytic sinonasal papilloma and associated sinonasal squamous cell carcinoma. J Pathol. 2016;239:394–8.
Zhang L, Hu C, Zheng X, et al. Oncocytic Schneiderian papilloma-associated adenocarcinoma and KRAS mutation: A case report. Medicine (Baltimore). 2018. https://doi.org/10.1097/MD.0000000000011025.
Vorasubin N, Vira D, Suh JD, Bhuta S, Wang MB. Schneiderian papillomas: Comparative review of exophytic, oncocytic, and inverted types. Am J Rhinol Allergy. 2013. https://doi.org/10.2500/ajra.2013.27.3904.
Gaffey MJ, Frierson HF, Weiss LM, Barber CM, Baber GB, Stoler MH. Human papillomavirus and Epstein-Barr virus in sinonasal Schneiderian papillomas: an in situ hybridization and polymerase chain reaction study. Am J Clin Pathol. 1996. https://doi.org/10.1093/ajcp/106.4.475.
El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumors. Fourth edition - WHO - OMS -. In: IARC, Lyon. p WHO classification of tumors of the oral cavity; 2017.
Batsakis JG, Suarez P. Schneiderian papillomas and carcinomas: a review. Adv Anat Pathol. 2001. https://doi.org/10.1097/00125480-200,103,000-00001.
Stelow EB, Bishop JA. Update from the 4th Edition of the World Health Organization classification of head and neck tumors: tumors of the nasal cavity, paranasal sinuses and skull base. Head Neck Pathol. 2017. https://doi.org/10.1007/s12105-017-0791-4.
Bishop JA, Westra WH. Human papillomavirus-related multiphenotypic sinonasal carcinoma: An emerging tumor type with a unique microscopic appearance and a paradoxical clinical behaviour. Oral Oncol. 2018. https://doi.org/10.1016/j.oraloncology.2018.10.011.
Liu J, Li X, Zhang Y, Xing L, Liu H-G. Human papillomavirus-related squamous cell carcinomas of the oropharynx and sinonasal tract in 156 Chinese patients. 2016.
Rupp NJ, Camenisch U, Seidl K, Rushing EJ, Anderegg N, Broglie MA, et al. HPV-related multiphenotypic sinonasal carcinoma: four cases that expand the morpho-molecular spectrum and include occupational data. Head Neck Pathol. 2019. https://doi.org/10.1007/s12105-019-01079-1.
Bishop JA, Ogawa T, Stelow EB, Moskaluk CA, Koch WM, Pai SI, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013. https://doi.org/10.1097/PAS.0b013e31827b1cd6.
Hwang SJ, Ok S, Lee HM, Lee E, Park IH. Human papillomavirus-related carcinoma with adenoid cystic-like features of the inferior turbinate: a case report. Auris Nasus Larynx. 2015. https://doi.org/10.1016/j.anl.2014.07.005.
Bishop JA, Andreasen S, Hang J-F, et al. HPV-related multiphenotypic sinonasal carcinoma hpv-related carcinoma with adenoid cystic carcinoma-like features. 2017.
Andreasen S, Bishop JA, Hansen TV, Westra WH, Bilde A, von Buchwald C, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features of the sinonasal tract: clinical and morphological characterization of six new cases. Histopathology. 2017;70:880–8.
Hang JF, Hsieh MS, Li WY, Chen JY, Lin SY, Liu SH, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a series of five cases expanding the pathological spectrum. Histopathology. 2017. https://doi.org/10.1111/his.13301.
Bornholdt J, Hansen J, Steiniche T, et al. K-ras mutations in sinonasal cancers in relation to wood dust exposure. BMC Cancer. 2008. https://doi.org/10.1186/1471-2407-8-53.
López F, García Inclán C, Pérez-Escuredo J, Álvarez Marcos C, Scola B, Suárez C, et al. KRAS and BRAF mutations in sinonasal cancer. Oral Oncol. 2012. https://doi.org/10.1016/j.oraloncology.2012.02.018.
Schröck A, Göke F, Wagner P, et al. Sex determining region Y-box 2 (SOX2) amplification is an independent indicator of disease recurrence in sinonasal cancer. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0059201.
Holmila R, Cyr D, Luce D, et al. COX-2 and p53 in human sinonasal cancer: COX-2 expression is associated with adenocarcinoma histology and wood-dust exposure. Int J Cancer. 2008. https://doi.org/10.1002/ijc.23360.
Takahashi Y, Bell D, Agarwal G, Roberts D, Xie TX, El-Naggar A, et al. Comprehensive assessment of prognostic markers for sinonasal squamous cell carcinoma. Head Neck. 2014. https://doi.org/10.1002/hed.23423.
López F, Llorente JL, Oviedo CM, Vivanco B, Marcos CÁ, García-Inclán C, et al. Gene amplification and protein overexpression of EGFR and ERBB2 in sinonasal squamous cell carcinoma. Cancer. 2012;118:1818–26.
Bandoh N, Hayashi T, Takahara M, Kishibe K, Ogino T, Katayama A, et al. VEGF and bFGF expression and microvessel density of maxillary sinus squamous cell carcinoma in relation to p53 status, spontaneous apoptosis and prognosis. Cancer Lett. 2004. https://doi.org/10.1016/j.canlet.2003.11.031.
Martínez JG, Pérez-Escuredo J, López F, Suárez C, Álvarez-Marcos C, Llorente JL, et al. Microsatellite instability analysis of sinonasal carcinomas. Otolaryngol Head Neck Surg. 2009. https://doi.org/10.1016/j.otohns.2008.10.038.
López F, Llorente JL, García-Inclán C, Alonso-Guervós M, Cuesta-Albalad MP, Fresno MF, et al. Genomic profiling of sinonasal squamous cell carcinoma. Head Neck. 2011. https://doi.org/10.1002/hed.21417.
Snijders AM, Schmidt BL, Fridlyand J, Dekker N, Pinkel D, Jordan RCK, et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 2005. https://doi.org/10.1038/sj.onc.1208601.
Järvinen AK, Autio R, Kilpinen S, Saarela M, Leivo I, Grénman R, et al. High-resolution copy number and gene expression microarray analyses of head and neck squamous cell carcinoma cell lines of tongue and larynx. Genes Chromosom Cancer. 2008. https://doi.org/10.1002/gcc.20551.
Cohen EEW, Bell RB, Bifulco CB, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019. https://doi.org/10.1186/s40425-019-0662-5.
Burtness B, Harrington KJ, Greil R, et al.. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomized, open-label, phase 3 study. Lancet. London; 2019. https://doi.org/10.1016/S0140-6736(19)32591-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Katya Elgart and Daniel L. Faden declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Skull Base Surgery
Rights and permissions
About this article
Cite this article
Elgart, K., Faden, D.L. Sinonasal Squamous Cell Carcinoma: Etiology, Pathogenesis, and the Role of Human Papilloma Virus. Curr Otorhinolaryngol Rep 8, 111–119 (2020). https://doi.org/10.1007/s40136-020-00279-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-020-00279-6