Abstract
Purpose of Review
Trauma constitutes a social and a clinical problem. The CT protocol to be adopted in polytrauma patients is still not standardized across institutions. A variety of protocols can be found in the available literature, which differ from each other in timing acquisition and number of phases.
Recent Findings
Even if multiple recent studies are investigating the role of split bolus technique, multiphasic protocol has been shown to be associated with early detection and adequate characterization of vascular injuries, so it should be still considered as the “best” CT protocol for the assessment of high-energy trauma patients.
Summary
The article provides a review on the currently available literature on the CT protocols adopted in polytraumatized patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trauma constitutes a social and a clinical problem: social, as it represents one of the most common causes of death or permanent disability in the population below 40 years [1••, 2••, 3, 4], and clinical, as patients may manifest a variety of presentations. Yet, there remains no consensus on the best diagnostic protocols to be utilized for trauma imaging amongst institutions [5••].
In the emergency setting, radiologists play a crucial role in the diagnosis and management of trauma patients, being members of the Trauma Team [6], with an adequate training in CT trauma studies [7, 8•]. Together with other trauma specialists, including emergency physicians, surgeons, anesthesiologists, and ancillary staff, radiologists have the responsibility to care for trauma patients providing important detailed information for timely management [8•].
The mechanism of trauma and not only the clinical presentation should guide the imaging work-up, distinguishing between two categories: minor trauma and major (poly-)trauma, and remembering that the probability of survival following the traumatic event, depend both on the mechanism of the trauma itself, but also on the timing and modalities of patient management, especially in the first hour following the event, commonly referred as the “golden hour” [2••].
In minor trauma, most hospitals in Europe adopt head CT (when needed), chest and skeletal radiographs, and ultrasonography (US) as the first-line imaging techniques. In the majority of Europe, US is performed by a consultant radiologist working 24/7 in the emergency department, for assessment of free fluid in the peritoneal, pericardial, and pleural spaces [8•], although, this may not be the case in other areas as Emergency Physicians or Trauma Surgeons tend to perform FAST/eFAST scans in the emergency department. Moreover, US may reach good sensitivity and specificity for the identification of intra-abdominal solid organ injuries, and in case of doubtful findings it can be integrated by intravenous contrast medium (contrast-enhanced US -CEUS-) if needed [8•, 9,10,11, 12•, 13]. However, US may be limited by patient habitus, lack of patient cooperation and reflex bowel distention (ileus) and not ensure a sufficient assessment of the retroperitoneum. This approach reduces the number of CT scans and the associated radiation dose exposure, but increases the total examination time and number of false negatives. While the risk of under diagnosis may be acceptable in minor trauma patients, considering the low incidence of significant conditions, this is not acceptable in major trauma patients, in whom the trauma mechanism exposes the patient to potentially unstable injuries, that need to be promptly ruled out.
Major trauma can be defined as a pathophysiological event consisting of: a sudden deceleration, impact or compression [14, 15] at speeds more than 65 km/h in car accidents, more than 45 km/h in motorcycle trauma [16], a fall from a height greater than 3 m, or a crush injury by heavy objects [17, 18].
In this category of patients, multi-detector CT (MDCT) should be considered the reference imaging standard, with a sensitivity of 95% and a negative predictive value approaching 100% to depict injuries [19,20,21,22]. Furthermore, CT allows the detection of otherwise undiagnosed injuries in 22% of cases [23], and additional findings that may change the management in up to 34% of patients [24, 25, 26•]. There is still not sufficient evidence to demonstrate a beneficial effect on survival by employing fast and detailed diagnostic work-up by immediate total-body CT, however, time to treatment is reported to be shorter for polytrauma patients who underwent diagnostic assessment with total-body CT scanning [26•, 27•].
Indeed, the current technical progress makes it possible to CT image the patient in a very short time, allowing to get a prompt and detailed diagnosis of parenchymal and vascular injuries, active bleeding as well as bone injuries, in few minutes. This contributed to the change of the “classic paradigm” of invasive laparotomy, to a non-operative management whenever possible, thus reducing mortality and morbidity [7, 8•, 28, 29]. Given its high negative predictive value, MDCT permits the early discharge of the patient, saving the costs of unnecessary hospitalization, when it is appropriate to do so [26•, 30].
CT Protocols
The CT protocol to be adopted in trauma patients is still not standardized across institutions. A variety of protocols can be found in the available literature, which differ from each other in timing acquisition and number of phases. This is related with the continuous attempts to find a good compromise between reduction of radiation exposure for usually young patients, and adequate imaging quality (Table 1). But, in an era in which we moved to non-operative management of polytraumatized patients, also other aspects, beyond the imaging quality need to be considered when choosing the best protocol. Indeed, the real capability to detect and to characterize in detail the injuries, particularly vascular injuries, guides the choice among the different therapeutic options. By adopting this approach, emergency radiologists can assume a leading role to ensure timely and accurate diagnosis of all trauma related injuries.
Monophasic CT Protocols
The monophasic protocol consists in a single MDCT acquisition after the intravenous (IV) administration of the contrast medium (CM), extended from the neck to the pelvis, and preceded by an unenhanced scan of the head. The protocols described in the literature vary by the rate of injection and the acquisition delay: 100 mL of contrast medium administered at an infusion rate of 4 mL/s with acquisition 60 s after the end of the injection [31] or 120 mL at 2 mL/s followed by 60 mL of physiological solution at the same infusion rate with acquisition at 85 s since the start of the injection [32] are the most commonly adopted, but others are also described with further differences in the infusion rate of the contrast media [33•, 34,35,36]. In the authors’ opinion, MDCT studies performed with these protocols may be sub-optimal, not allowing an adequate identification and characterization of vascular injuries which may be present at the time of imaging and masked by the timing of the acquisition, such as pseudoaneurysms, arterial injuries and dissections. Indeed, these injuries are depicted during the dedicated arterial phase of acquisition [1••, 5••].
Multiphasic CT Protocol
This protocol basically includes a non-contrast scan of the head, followed by an arterial and a venous phase, with a single bolus and two separate acquisitions. The post-contrast scans are acquired from the circle of Willis to the symphysis pubis. The patient should be positioned, when possible, with abducted upper limbs, to reduce the radiation dose and to obtain a higher image quality of the thoraco-abdominal organs [1••, 2••, 33•, 37,38,39,40]. The IV CM (80–130 mL iodinated contrast medium, according to the patient’s weight), at a high concentration (370–400 mg I/mL), is injected at 3.5–5 mL/s, and followed by a 40 mL saline chaser at the same flow rate, to obtain optimal vessel depiction. Automated bolus tracking identifies the arterial phase; a region of interest (ROI) is placed on the aortic arch, and arterial phase scanning starts when an attenuation threshold of 100 Hounsfield Unit (HU) is reached; depending on the speed of acquisition of the scanner it may be necessary to wait few additional seconds. The portal venous phase is performed at a 60- to 70-s delay from the beginning of the injection. As in Europe, a consultant radiologist is always present in the CT suite 24/7, she/he supervises, modifies the standard CT protocol if needed, and provides a first reading for immediate and appropriate patient management (i.e., tension pneumothorax, shattered spleen or kidney, etc). However, this approach is not uniformly applied in different countries, so the European Society of Emergency Radiology has conducted a survey to gather information about the current organization and practice of emergency radiology in Europe that will be published soon. In selected patients, an additional late phase of the abdomen and pelvis at 3–5 min may be required to differentiate arterial bleeding from lower-pressure venous bleeding, or at 5–20 min to depict urinary extravasation in patients with kidney injuries [41,42,43]. Furthermore, to exclude bladder injuries, irrespective of the presence or absence of pelvic fractures, it is necessary to obtain a dedicated CT cystogram. This should be obtained, in some authors’ opinion, after the contrast-enhanced CT acquisitions [8•, 44, 45••], by active distention of the bladder with diluted iodinate cm through a urethral catheter (about 300–350 mL of 5% diluted contrast media) [46,47,48,49], as passive distention of the bladder during the “excretory phase” usually does not permit an overall assessment of bladder injuries [50, 51]. In patients with penetrating or gunshot injuries, a CT cystogram is particularly advised, as well as the eventual use of oral/rectal contrast administration, to exclude bladder and/or bowel perforation [52•, 53, 54]. If there is clinical suspicion of lower extremity fractures at risk for vascular injury, the CT scans are extended to the feet and at least arterial and portal venous phases are required to correctly characterize vascular injuries. The arterial phase allow to detect if a vessel injury is arterial in origin, whereas the following portal venous phase allow to establish the entity of the bleeding, orienting the management: conservative, endovascular or surgical [1••, 55, 56].
For accurate vascular and parenchymal evaluation, a slice thickness ranging from 0.5 to 3 mm, and preferably with a spacing of 0.5–1.5 mm, is recommended. Post-processing with three-dimensional (3D) multiplanar reconstructions (MPR) and volume rendering reconstructions is helpful for identifying injuries of the vessels and sites of active bleeding, as well as for searching for osseous injuries which can be missed on the axial images [42, 55,56,57,58,59] (Fig. 1). The availability of multiple acquisition phases in polytraumatized patients is also useful to overcome motion artifacts.
Enhanced-CT of a 54 years old male patient underwent major trauma: arterial phase in axial plane, MIP reconstruction (a), arterial phase in coronal plane (b) and portal venous phase in coronal plane (c). Note the absence of hemoperitoneum and the presence of two pseudoaneurysms at the inferior pole of the spleen (a, b arrows) only evident in the arterial phase of study. These lesions cannot be depicted in the portal venous phase (c)
A controversial topic is the use of an unenhanced thoraco-abdominal MDCT phase, as there are published guidelines stating there is no need for unenhanced imaging of the upper abdomen [36, 60], while there are publications suggesting the added value of such an examination [61, 62], as the unenhanced initial acquisition may be useful to promptly detect hyperdense clot (i.e. the “sentinel clot sign”: areas of higher attenuation near an injury site, likely to indicate the source of bleeding), to improve the detection of intramural vascular hematomas, to easily differentiate calcifications from spots of active bleeding or to simply evaluate the presence of intravascular prosthesis [42, 56, 63, 64]. However, CT dual-energy acquisitions with new scanners may offer the ability to answer these questions by generating virtual unenhanced images from the acquired post-contrast images [65].
Multiphasic protocol should be considered the “optimal” CT protocol to be adopted in high-energy trauma settings and in their follow-up [65], as the goal is the early detection and precise characterization of injuries that may affect the patient’s treatment and prognosis, with a high degree of sensitivity and specificity, such as vascular injuries which may require immediate intervention [66,67,68,69]. This CT approach is commonly applied in most European trauma centers and also in the USA, whereas in other countries, as United Kingdom, the split-bolus technique is preferred. In other European centers the choice of the CT protocol for trauma is still not uniformed and may vary basing on individual center choices and on the patient’s age.
CT angiography should be the first-line investigation for all patients with suspected vascular trauma without clear clinical conditions mandating immediate surgery [56, 70].
In this sense, the scan extension, from circle of Willis to the symphysis pubis, is particularly important to avoid underestimation of any kind of vascular injury, including cerebrovascular injuries, the incidence of which ranges between 1 and 3.3% of all major trauma patients [71]. The overall reported sensitivity of CT angiography for vascular cervical trauma is of 41–100%, specificity of 86–100%, and negative predictive values of 90–100% [58].
This kind of injuries may be dangerously underestimated when neck is not included in the scan, sensibly reducing the patient outcome. Indeed, early diagnosis and treatment is associated with a reduction in the rate of post-traumatic stroke [72••].
Furthermore, CT angiography is highly sensitive (86–100%) and specific (40–100%) in detecting blunt aortic injury with high positive predictive values (7–100%) and negative predictive values (93.9–100%) when compared to conventional angiography [56]. The detection and characterization of vascular injuries is more relevant and of greater clinical significance than purely detecting an abdominal solid organ injury [67] considering that the vast majority of patients are now treated non-operatively [28, 29]. Patients with arterial vascular injuries can be safely sent to the interventional radiology department for arterial stent placing (e.g., for neck vessels or aortic injuries) or embolization when appropriate (e.g., contained arterial injuries or active arterial bleeding), limiting surgery in only a small percentage of patients (e.g., with a shattered spleen or kidney, and when hemorrhage cannot otherwise be controlled). When venous injuries occur, the estimation of the bleeding entity modify the management from conservative in slight bleeding, to operative in conspicuous bleeding. In most patients, this approach has saved time and lives, significantly reducing morbidity, death from sepsis and other complications, and financial costs [73] (Fig. 1).
Regarding the abdominal vascular injuries, several studies demonstrated that the acquisition of two separate post-contrast CT phases (arterial and portal venous) increases the sensitivity and the accuracy both in splenic than in liver vascular injury detection [5••, 74,75,76], with a reported significant difference in the sensitivity of arterial phase in comparison with portal venous phase of 70% vs 17% for the detection of intrasplenic pseudoaneurysm [74]. In data reported by Uyeda et al. [76], 46% of patients with contained vascular injuries were identified only during the arterial phase of image acquisition; similarly, Melikian et al. found dual-phase CT was more sensitive (80.0% vs. 37.5%, P = 0.016) and more accurate (76.2% vs. 37.5%) than single phase imaging for diagnosing splenic vascular injury [75]. About liver vascular injuries, our previous published data [5] similarly shown that in 71.5% of patients it was possible to detect contained vascular injuries exclusively in the arterial phase of the CT study, and adequately characterize active bleeding from an arterial origin in 76.9% of cases. This has important management implications.
MDCT Assessment of Bleeding in Trauma
Bleeding represent the most common cause of preventable death in trauma patients, being responsible of about 40% of death [77].
In blunt trauma patients, bleeding is mainly related with stretching mechanisms on the vessel wall. These kind of injuries may be clinically silent until patients conditions become critical with hemorrhagic shock [1••].
So, one of the main goal of CT in trauma is to identify, quantify and characterize the bleeding or the presence of vascular injuries at risk for bleeding, to ensure the best patient’s treatment.
Active bleeding is seen as the presence of extravasated contrast agent, and it may be classified into three main categories according to its size and morphology: a spot (punctiform self-limiting bleeding), a jet (linear bleeding with no significant morphological change), or pooling (active extravasation of contrast media, with significant change in its shape and volume over multiple phases of acquisition) [78].
Multiplanar Reformations (MPR) and Maximum Intensity Projection (MIP) can help in revealing the bleeding vessel of origin and the severity of hemorrhage.
To properly detect active bleeding, a rapid rate of IV CM injection, and high iodine concentration are suggested. This is as the degree of arterial vessel contrast enhancement is directly affected by the contrast medium delivery rate and from their concentration, so a faster delivery increases the magnitude of the aortic enhancement [79].
If an active bleeding is firstly seen in the arterial CT phase, it can be defined as arterial in origin, but it should be also considered that arterial active bleeding can be seen in the portal venous phase in patients with hemodynamic alterations, or due to arterial spasm that limits the bleeding.
From a therapeutic point of view, the importance of the active bleeding depends on its severity and origin, as not all the active bleeding need to be operatively managed in an emergency setting. Slight active bleeding, especially if intraparenchymal and of venous origin, may be self-limiting and conservatively managed [5••]. If only a single phase is acquired, it is not possible to adequately evaluate and separate clinically relevant arterial bleeding from other less relevant sources of bleeding.
Contained Vascular Injuries
The use of a MDCT multiphasic protocol permits the differentiation of contained bleeding injuries from actively bleeding injuries [5••, 80••].
Contained vascular injuries (i.e., intimal tear, intramural hematoma, pseudoaneurysms and arteriovenous fistulas) can generally be safely treated non-operatively, with stent-grafts or by embolization; conversely, if untreated, these injuries may increase in extension, in volume and occasionally in number, and can rupture causing active bleeding [1••, 5••, 80••]. In patients with contained vessel injury, MPRs and MIP images can be useful to provide crucial information to the interventional radiologist and/or surgeon, which is necessary for planning therapeutic intervention.
The main drawback of the multiphasic MDCT protocol for trauma is related to the radiation dose as a consequence of the multiple acquisition phases. Indeed, the reported radiation dose reduction between split-bolus whole-body CT and multiphasic CT protocol is between 31.9 and 68.1% [72••, 81].
However, given the greatly increased morbidity and mortality associated with vascular injuries, and the possibility that, especially young trauma patients are able to compensate until sudden shock occurs, the additional radiation dose should not discourage acquisition of multiple phase images [82]. In this sense, the introduction of iterative reconstructive techniques into CT imaging may led to a decrease in whole-body CT dose, from 15 to 20 to 5–10 mSv [83] as well as the adoption of dual-energy CT, that may lead to radiation doses lower than those for single-energy CT, also considering that virtual non-contrast images can be retrospectively created from post-contrast dual-energy CT acquisitions [65].
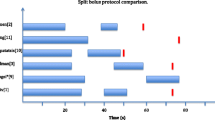
Split Bolus CT Protocol
Due to the larger adoption of MDCT in trauma patients, usually involving young population, and the associated non-negligible radiation dose, efforts have been made to try to reduce the radiation dose. In this sense, several authors are studying the adoption of a split-bolus MDCT protocol [31, 34, 36, 64, 71, 81, 84,85,86,87]. This CT protocol consists in a single pass through the CT gantry after iv injection of two or three boluses (arterial and portal venous) of CM given sequentially, with a time delay or saline bolus between [33•, 72••, 81]. The sequential contrast boluses result in a single acquisition, reflecting the combination of arterial and portal venous phases (and potentially a urinary excretory phase) [72••].
Among the more recent studies on this topic, Hakim et al. [88] compared the image quality of conventional arterial and portal venous phase CT with two biphasic injection protocols in poly-trauma patients consisting in the injection of 65 mL contrast medium at a rate of 1.5 mL/s; after completion at 43 s, a second 65 mL contrast bolus was started at a rate of 3.5 mL/s followed by a single spiral acquisition, at approximately 60 or 70 s. The authors found comparable image quality with less radiation and reduction in acquisition time.
Marovic et al. [84] in a study focused on splenic injuries in a cohort of 36 split-bolus trauma CT examinations, found that splenic image quality was diagnostic in all cases, however, in comparison with Digital Subtraction Angiography (DSA) in the diagnosis of active arterial hemorrhage and splenic pseudoaneurysm, split-bolus protocol had significantly lower sensitivity, of 50.0% and 38.9% respectively.
Godt et al. [71] compared the image quality and injury findings of a portal venous phase CT with those of a triple-split-bolus CT protocol as follow: first bolus consisting in 20 mL of intravenous contrast medium followed by a 30-mL saline chase, both at a flow rate of 3 mL/s. At least 5 min after, the second bolus of 100 mL contrast media is injected at a flow rate of 5 mL/s, followed by a 45-mL saline chase (flow rate 6 mL/s). After a delay of 32 s, the third bolus of 55 mL contrast medium is administered followed by a 55-mL saline chase, both injected at a rate of 5 mL/s. Hereafter, the CT scan was initiated by manual bolus tracking with the region of interest (ROI) in the descending aorta. They found the triple-split-bolus CT protocol achieved better contrast enhancement, equal performance in organ injury diagnosis, and similar image quality compared to the portal venous CT protocol, however, no vascular injuries were detected in the study population.
All the studies investigating images quality for abdominal split-bolus single-pass CT found an adequate images quality with higher parenchymal enhancement and lower arterial enhancement than conventional CT protocol. The reason for the higher enhancement of parenchymal organs is probably related to the higher amount of contrast medium and, consequently, of the iodine dose applied in all split-bolus protocols [71]. This lead to the suggestion in these protocol to adopt contrast media with lower iodine concentration (300–360 mg I/mL) [88] or differently set an adequate windows setting.
There are no doubts that the split-bolus single-pass approach is superior to the single bolus single-pass CT, and that the parenchymal enhancement adopting split-bolus protocol is adequate, but the main problem remains the identification and characterization of vascular injuries. For their depiction, a greater contrast between arterial vessels and the surrounding parenchyma is needed, and further a subsequent venous phase to evaluate the stability or the presence of active bleeding, as this significantly affects therapeutic management. So, the main limitation of this technique remains the possible inadequate detection and characterization of vascular injuries and the possible difficulties in distinguishing vascular injuries from pre-existing parenchymal finding. There are still no studies that adequately explored this point; for this reason, several authors, Hakim et al. [88], Godt et al. [71], and Leung et al. [81], prefer to avoid potential difficulties in differentiating parenchymal and vascular injuries and contrast leakage from ureteral or bladder injury, adopting, in seriously injured patients, the conventional single-bolus dual-phase CT protocol.
Conclusion
Due to the high sensitivity and specificity of MDCT for injuries detection and characterization, and short execution time, in the last years a rapid increase in the number of whole-body CT examinations in trauma patients has been observed. So, different CT protocols were investigated attempting to reduce the radiation exposure in a predominantly young population. However, currently, the only protocol ensuring a complete characterization on injuries modifying patient’s management is still the multiphasic CT study. Therefore, efforts must be made in adequate patient selection and technological advancement rather than in the reduction of scans potentially useful in identifying parenchymal and vascular damage.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance and •• Of major importance
••Iacobellis F, Ierardi AM, Mazzei MA, Biasina AM, Carrafiello G, Nicola R, Scaglione M. Dual-phase CT for the assessment of acute vascular injuries in high-energy blunt trauma: the imaging findings and management implications. BJR 2016;89(1061):20150952. It is a review on the role of dual-phase MDCT in the identification and management of traumatic acute vascular injuries, with correlative surgical and interventional findings.
••Schueller G, Scaglione M, Linsenmaier U, Schueller- Weidekamm C, Andreoli C, De Vargas Macciucca M, Gualdi G. The key role of the radiologist in the management of polytrauma patients: indications for MDCT imaging in emergency radiology. Radiol Med 2015;120:641–54. In the article are discussed the indications for MDCT in the polytrauma setting.
Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–93.
Stone TJ, Norbet C, Rhoades P, Bhalla S, Menias CO. Computed tomography of adult blunt abdominal and pelvic trauma: implications for treatment and interventions. Semin Roentgenol. 2014;49:186–201.
••Iacobellis F, Scaglione M, Brillantino A, Scuderi MG, Giurazza F, Grassi R, Noschese G, Niola R, Al Zuhir NYS, Romano L. The additional value of the arterial phase in the CT assessment of liver vascular injuries after high-energy blunt trauma. Emerg Radiol. 2019 Dec;26(6):647-654. The study evaluate the value of the arterial phase in the CT assessment of vascular injuries of the liver.
Miele V, Andreoli C, Grassi R. The management of emergency radiology: key facts. Eur J Radiol. 2006;59(3):311–4 Epub 2006 Jun 27 PubMed PMID: 16806785.
Pinto A, Reginelli A, Pinto F, Lo Re G, Midiri F, Muzj C, Romano L, Brunese L. Errors in imaging patients in the emergency setting. Br J Radiol. 2016;89(1061):20150914.
•Scaglione M, Laccetti E, Picascia R, Altiero M, Iacobellis F, Elameer M, Grassi R. Errors in Imaging of Abdominal and Pelvic Trauma. Book Chapter in: Errors in Emergency and Trauma Radiology Ed. Patlas MN, Katz DS, Scaglione M, Springer 2019. The article reviewed the cause of errors in imaging of abdominal and pelvic trauma.
Piccolo CL, Trinci M, Pinto A, Brunese L, Miele V. Role of contrast-enhanced ultrasound (CEUS) in the diagnosis and management of traumatic splenic injuries. J Ultrasound. 2018;21(4):315–27.
Miele V, Piccolo CL, Galluzzo M, Ianniello S, Sessa B, Trinci M. Contrast-enhanced ultrasound (CEUS) in blunt abdominal trauma. Br J Radiol. 2016;89(1061):20150823.
Pinto F, Valentino M, Romanini L, Basilico R, Miele V. The role of CEUS in the assessment of haemodynamically stable patients with blunt abdominal trauma. Radiol Med. 2015;120(1):3–11. https://doi.org/10.1007/s11547-014-0455-3.
•Sessa B, Trinci M, Ianniello S, Menichini G, Galluzzo M, Miele V. Blunt abdominal trauma: role of contrast-enhanced ultrasound (CEUS) in the detection and staging of abdominal traumatic lesions compared to US and CE-MDCT. Radiol Med. 2015 Feb;120(2):180-9. The article evaluate the accuracy of contrast-enhanced ultrasound (CEUS) in the detection and grading of abdominal traumatic lesions in patients with low-energy isolated abdominal trauma in comparison with baseline ultrasound (US) and contrast-enhanced multidetector computed tomography (CE-MDCT), concluding that in patients with low-energy isolated abdominal trauma US should be replaced by CEUS as the first-line approach, whereas CE-MDCT must always be performed in CEUS-positive patients to exclude active bleeding and urinomas.
Pinto F, Miele V, Scaglione M, Pinto A. The use of contrast-enhanced ultrasound in blunt abdominal trauma: advantages and limitations. Acta Radiol. 2014;55(7):776–84.
Palas J, Matos AP, Mascarenhas V, Heredia V, Ramalho M. Multidetector computer tomography: evaluation of blunt chest trauma in adults. Radiol Res Pract. 2014;2014:864369.
Scaglione M, Pinto A, Pedrosa I, Sparano A, Romano L. Multi-detector row computed tomography and blunt chest trauma. Eur J Radiol. 2008;65:377–88.
Commitee on Trauma of the American College of Surgeons. Resources for optimal care of the injured patient. Chicago: ACS; 2014.
Scaglione M, Andreoli A. TCMD nel trauma ad elevata energia. Mailand: Springer; 2012.
van Vugt R, Deunk J, Brink M, Dekker HM, Kool DR, van Vugt AB, Edwards MJ. Influence of routine computed tomography on predicted survival from blunt thoracoabdominal trauma. Eur J Trauma Emerg Surg. 2011;37:185–90.
Linsenmaier U, Kanz KG, Rieger J, Rock C, Pfeifer KJ, Reiser M. Structured radiologic diagnosis in polytrauma. Radiologe. 2002;42(7):533–40.
Philipp MO, Kubin K, Hörmann M, Metz VM. Radiological emergency room management with emphasis on multidetector-row CT. Eur J Radiol. 2003;48(1):2–4.
Huber-Wagner S, Lefering R, Qvick LM, Körner M, Kay MV, Pfeifer KJ, Reiser M, Mutschler W, Kanz KG, Working Group on Polytrauma of the German Trauma Society. Effect of whole-body CT during trauma resuscitation on survival: a retrospective, multicentre study. Lancet. 2009;373(9673):1455–61.
Gunn ML, Lehnert BE, Lungren RS, Narparla CB, Mitsumori L, Gross JA, Starnes B. Minimal aortic injury of the thoracic aorta: imaging appearances and outcome. Emerg Radiol. 2014;21:227–33.
Pfeifer R, Pape HC. Missed injuries in trauma patients: a literature review. Patient Safety Surg. 2008;2:20.
Deunk J, Brink M, Dekker HM, Kool DR, Blickman JG, van Vugt AB, Edwards MJ. Routine versus selective multidetectorrow computed tomography (MDCT) in blunt trauma patients: level of agreement on the influence of additional findings on management. J Trauma. 2009;67(5):1080–6.
Deunk J, Dekker HM, Brink M, Vugt VR, Edwards MJ, Vugt VAB. The value of indicated computed tomography scan of the chest and abdomen in addition to the conventional radiologic work-up for blunt trauma patients. J Trauma. 2007;63:757–63.
•Long B, April MD, Summers S, Koyfman A. Whole body CT versus selective radiological imaging strategy in trauma: an evidence-based clinical review. Am J Emerg Med. 2017 Sep;35(9):1356-1362. The article evaluate the literature concerning mortality effect, emergency department (ED) length of stay, radiation, and incidental findings associated with whole body CT approach in trauma patient.
•Treskes K, Saltzherr TP, Edwards MJR, Beuker BJA, Den Hartog D, Hohmann J, Luitse JS, Beenen LFM, Hollmann MW, Dijkgraaf MGW, Goslings JC; REACT-2 study group. Emergency Bleeding Control Interventions After Immediate Total-Body CT Scans in Trauma Patients. World J Surg. 2019 Feb;43(2):490-496. The aim of this study was to assess whether an initial trauma assessment with immediate total-body CT is associated with lower mortality in patients requiring emergency bleeding control interventions. A potentially clinically relevant absolute risk reduction of 11.2% in comparison with standard workup was observed.
Brillantino A, Iacobellis F, Robustelli U, Villamaina E, Maglione F, Colletti O, De Palma M, Paladino F, Noschese G. Non operative management of blunt splenic trauma: a prospective evaluation of a standardized treatment protocol. Eur J Trauma Emerg Surg. 2016;42(5):593–8 Epub 2015 Sep 28 PubMed PMID: 26416401.
Brillantino A, Iacobellis F, Festa P, Mottola A, Acampora C, Corvino F, Del Giudice S, Lanza M, Armellino M, Niola R, Romano L, Castriconi M, De Palma M, Noschese G. Non-operative management of blunt liver trauma: safety, efficacy and complications of a standardized treatment protocol. Bull Emerg Trauma. 2019;7(1):49–54.
Mirvis SE, Shanmuganathan K. The 2008 RadioGraphics monograph issue: emergency imaging in adults. Radiographics. 2008;28(6):1539–40.
Beenen LF, Sierink JC, Kolkman S, Nio CY, Saltzherr TP, Dijkgraaf MG, Goslings JC. Split bolus technique in polytrauma: a prospective study on scan protocols for trauma analysis. Acta Radiol. 2014;56(7):809–73.
Eichler K, Marzi I, Wyen H, Zangos S, Mack MG, Vogl TJ. Multidetector computed tomography (MDCT): simple CT protocol for trauma patient. Clin Imaging. 2015;39(1):110–5.
•Gunn ML, Kool DR, Lehnert BE. Improving Outcomes in the Patient with Polytrauma: A Review of the Role of Whole-Body Computed Tomography. Radiol Clin North Am. 2015;53(4): 639-56, vii. The article provides an overview of the current concepts surrounding WBCT in trauma.
Loupatatzis C, Schindera S, Gralla J, Hoppe H, Bittner J, Schröder R, Srivastav S, Bonel HM. Whole-body computed tomography for multiple traumas using a triphasic injection protocol. Eur Radiol. 2008;18(6):1206–14.
Sedlic A, Chingkoe CM, Tso DK, Galea-Soler S, Nicolaou S. Rapid imaging protocol in trauma: a whole-body dual-source CT scan. Emerg Radiol. 2013;20(5):401–8.
Nguyen D, Platon A, Shanmuganathan K, Mirvis SE, Becker CD, Poletti PA. Evaluation of a single-pass continuous wholebody, 16-MDCT protocol for patients with polytrauma. Am J Roentgenol. 2009;192(1):3–10.
Soto JA, Anderson SW. Multidetector CT of blunt abdominal trauma. Radiology. 2012;265(3):678–93.
Uyeda JW, Anderson SW, Sakai O, Soto JA. CT angiography in trauma. Radiol Clin North Am. 2010;48:423–38.
Sica G, Guida F, Bocchini G, Codella U, Mainenti PP, Tanga M, Scaglione M. Errors in imaging assessment of polytrauma patients. Semin Ultrasound CT MR. 2012;33(4):337–46.
Liguori C, Gagliardi N, Saturnino PP, Pinto A, Romano L. Multidetector computed tomography of pharyngo-esophageal perforations. Semin Ultrasound CT MR. 2016;37(1):10–5.
West OC, Anderson J, Lee JS, et al. Patterns of diag- nostic error in trauma abdominal CT. Emerg Radiol. 2002;9:195–200.
Scaglione M, Iaselli F, Sica G, Feragalli B, Nicola R. Errors in imaging of traumatic injuries. Abdom Imaging. 2015;40:2091–8.
Stuhlfaut JW, Anderson SW, Soto JA. Blunt abdominal trauma. Current imaging techniques CT findings in patients with solid organ, bowel and mesenteric injury. Semin Ultrasound CT MRI. 2007;28:115–29.
Chan DP, Abujudeh HH, Cushing GL Jr, et al. CT cystography with multiplanar reformation for suspected bladder rupture: experience in 234 cases. Am J Roentgenol. 2006;187:1296–302.
••Joshi G, Kim EY, Hanna TN, Siegel CL, Menias CO. CT cystography for suspicion of traumatic urinary bladder injury: indications, technique, findings, and pitfalls in diagnosis: radioGraphics fundamentals | online presentation. Radiographics. 2018;38(1):92–3. The indications, procedural technique and findings of CT cystography in the acute traumatic setting are described.
Vaccaro JP, Brody JM. CT cystography in the evaluation of major bladder trauma. Radiographics. 2000;20:1373–81.
Quagliano PV, Delair SM, Malhotra AK. Diagnosis of blunt bladder injury: a prospective comparative study of computed tomography cystography and conventional retrograde cystography. J Trauma. 2006;61:410–21.
Deck AJ, Shaves S, Talner L, Porter JR. Computerized tomography cystography for the diagnosis of traumatic bladder rupture. J Urol. 2000;164:43–6.
Peng MY, Parisky YR, Cornwell EE, Radin R, Bragin S. CT cystography versus conventional cystography in evaluation of bladder injury. Am J Roentgenol. 1999;173:1269–72.
American College of Radiology. ACR Appropriateness Criteria® suspected lower urinary tract trauma. 2013.; https://www.guidelinecentral.com/summaries/acr-appropriateness-criteria-suspected-lower-urinary- tract-trauma/.
Mee SL, McAninch JW, Federle MP. Computerized tomography in bladder rupture: diagnostic limitations. J Urol. 1987;137:207–9.
•Dreizin D, Munera F. Multidetector CT for penetrating torso trauma: state of the art. Radiology. 2015;277(2):338–55. This article review the multi- detector CT role and technique in penetrating torso trauma.
Gross JA, Lehnert BE, Linnau KF, Voelzke BB, Sandstrom CK. Imaging of urinary system trauma. Radiol Clin North Am. 2015;53(4):773–88.
Pinto A, Russo A, Reginelli A, et al. Gunshot wounds: ballistics and imaging findings. Semin Ultrasound CT MR. 2019;40(1):25–35.
Monazzam S, Goodell PB, Salcedo ES, Nelson SH, Wolinsky PR. When are CT angiograms indicated for patients with lower extremity fractures? A review of 275 extremities. J Trauma Acute Care Surg. 2017;82(1):133–7.
Darling RC III, Byrne J, Diagnosis of vascular trauma, Book chapter in A. Dua et al. (eds.), Clinical Review of Vascular Trauma, 33 https://doi.org/10.1007/978-3-642-39100-2_3, © Springer-Verlag Berlin Heidelberg 2014 review of sensitivity and specificity of CT Angiography in detecting vascular trauma.
Scaglione M, Laccetti E, Picascia R, Altiero M, Iacobellis F, Elameer M, Grassi R. Errors in imaging of abdominal and pelvic trauma. In: Patlas MN, Katz DS, Scaglione M, editors. Errors in emergency and trauma radiology. New York: Springer; 2019.
Iacobellis F, Laccetti E, Tamburrini S, et al. Role of multidetector computed tomography in the assessment of pancreatic injuries after blunt trauma: a multicenter experience. Gland Surg. 2019;8(2):184–96.
Iacobellis F, Iadevito I, Ierardi AM, et al. Imaging assessment of thoracic cage injuries. Semin Musculoskelet Radiol. 2017;21(3):303–14. https://doi.org/10.1055/s-0037-1602413.
The Royal College of Radiologists. BFCR(11)3 standards of practice and guidance for trauma radiology in severely injured patients. London: The Royal College of Radiologists; 2011.
Alonso RC, Nacenta SB, Martinez PD, Guerrero AS, Fuentes CG. Kidney in danger: CT findings of blunt and penetrating renal trauma. Radiographics. 2009;29(7):2033–53.
Katz DS, Lane MJ, Mindelzun RE. Unenhanced CT of abdominal and pelvic hemorrhage. Semin Ultrasound CT MR. 1999;20:94e107.
Guida F, Bocchini G, Sica G, Freeze A, Scaglione M. Errors in polytrauma. In: Romano L, Pinto A, editors. Errors in radiology. Milan: Springer; 2012. p. 27–37.
Yaniv G, Portnoy O, Simon D, Bader S, Konen E, Guranda L. Revised protocol for whole-body CT for multi-trauma patients applying triphasic injection followed by a single-pass scan on a 64-MDCT. Clin Radiol. 2013;68(7):668–75.
Wortman JR, Uyeda JW, Fulwadhva UP, Sospdickson AD. Dual-energy CT for abdominal and pelvic trauma. Radiographics. 2018;38(2):586–602. https://doi.org/10.1148/rg.2018170058.
Ptak T, Rhea J, Novelline R. Experience with a continuous, single-pass whole-body multi-detector CT protocol for trauma: the three-minute multiple trauma CT scan. Emerg Radiol. 2001;8:250–6.
Pinto A, Niola R, Tortora G, Ponticiello G, Russo G, Di Nuzzo L, Gagliardi N, Scaglione M, Merola S, Stavolo C, Maglione F, Romano L. Role of multidetector-row CT in assessing the source of arterial haemorrhage in patients with pelvic vascular trauma. Comparison with angiography. Radiol Med. 2010;115(4):648–67.
Yao DC, Jeffrey RB Jr, Mirvis SE, et al. Using contrast- enhanced helical CT to visualize arterial extravasation after blunt abdominal trauma: incidence and organ distribution. Am J Roentgenol. 2002;178:17–20.
Willmann JK, Roos JE, Platz A, Pfammatter T, Hilfiker PR, Marincek B, Weishaupt D. Multidetector CT: detection of active hemorrhage in patients with blunt abdominal trauma. AJR Am J Roentgenol. 2002;179(2):437–44.
Patterson BO, Holt PJ, Cleanthis M, Tai N, Carrell T, Loosemore TM, London Vascular Injuries Working Group. Imaging vascular trauma. Br J Surg.2012;99(4):494–505 Excellent systematic review of the literature analyzing all modalities in assessing vascular trauma.
Godt JC, Eken T, Schulz A, Johansen CK, Aarsnes A, Dormagen JB. Triple-split-bolus versus single-bolus CT in abdominal trauma patients: a comparative study. Acta Radiol. 2018;59(9):1038–44.
••Jeavons C, Hacking C, Beenen LF, Gunn ML. A review of split-bolus single-pass CT in the assessment of trauma patients. Emerg Radiol. 2018 Aug;25(4):367-374. The article review and compare the image quality and radiation dose of split-bolus single-pass computed tomography(CT) in the assessment of trauma patients in comparison to standard multi-phase CT techniques.
Sartorelli KH, Frumiento C, Rogers FB, Osler TM. Nonoperative management of hepatic, splenic, and renal injuries in adults with multiple injuries. J Trauma. 2000;49(1):56–61 discussion 61-2.
Boscak AR, Shanmuganathan K, Mirvis SE, Fleiter TR, Miller LA, Sliker C, Steenburg SD, Alexander M. Optimizing trauma multidetector CT protocol for blunt splenic injury: need for arterial and portal venous phase scans. Radiology. 2013;268(1):79–88.
Melikian R, Goldberg S, Strife BJ, Halvorsen RA. Comparison of MDCT protocols in trauma patients with suspected splenic injury: superior results with protocol that includes arterial and portal venous phase imaging. Diagn Interv Radiol. 2016;22(5):355–9.
Uyeda JW, LeBedis CA, Penn DR, Soto JA, Anderson SW. Active hemorrhage and vascular injuries in splenic trauma: utility of the arterial phase in multidetector CT. Radiology. 2014;270(1):99–106.
Curry N, Hopewell S, Dorée C, Hyde C, Brohi K, Stanworth S. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Crit Care. 2011;15(2):R92. https://doi.org/10.1186/cc10096.
Anderson SW, Lucey BC, Rhea JT, Soto JA. 64 MDCT in multiple trauma patients: imaging manifestations and clinical implications of active extravasation. Emerg Radiol. 2007;14:151–9.
Kyongtae T. Bae Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches Radiology. 2010;256(1):32–61.
••Zarzaur BL, Dunn JA, Leininger B, Lauerman M, Shanmuganathan K, Kaups K, Zamary K, Hartwell JL, Bhakta A, Myers J, Gordy S, Todd SR, Claridge JA, Teicher E, Sperry J, Privette A, Allawi A, Burlew CC, Maung AA, Davis KA, Cogbill T, Bonne S, Livingston DH, Coimbra R, Kozar RA. Natural history of splenic vascular abnormalities after blunt injury: A Western Trauma Association multicenter trial. J Trauma Acute Care Surg. 2017 Dec;83(6):999-1005. The article describe the current management and outcomes of patients with splenic vascular injuries.
Leung V, Sastry A, Woo TD, Jones HR. Implementation of a split-bolus single-pass CT protocol at a UK major trauma centre to reduce excess radiation dose in trauma pan-CT. Clin Radiol. 2015;70(10):1110–5.
Dreizin D, Munera F. Blunt polytrauma: evaluation with 64-section whole-body CT angiography. Radiographics. 2012;32(3):609–31. https://doi.org/10.1148/rg.323115099.
Chidambaram S, Goh EL, Khan MA. A meta-analysis of the efficacy of whole-body computed tomography imaging in the management of trauma and injury. Injury. 2017;48(8):1784–93. https://doi.org/10.1016/j.injury.2017.06.003.
Leung V, Jones H, Sastry A. Can split bolus CT protocols prevent excess radiation dose in trauma CT? Clin Radiol. 2014;69:S23.
Hakim W, Kamanahalli R, Dick E, Bharwani N, Fetherston S, Kashef E. Trauma whole-body MDCT: an assessment of image quality in conventional dual-phase and modified biphasic injection. Br J Radiol. 2016;89(1063):20160160.
Marovic P, Beech PA, Koukounaras J, Kavnoudias H, Goh GS. Accuracy of dual bolus single acquisition computed tomography in the diagnosis and grading of adult traumatic splenic paren- chymal and vascular injury. J Med Imaging Radiat Oncol. 2017;61(6):725–31.
Stengel D, Ottersbach C, Matthes G, Weigeldt M, Grundei S, Rademacher G, Tittel A, Mutze S, Ekkernkamp A, Frank M, Schmucker U, Seifert J. Accuracy of single-pass whole-body computed tomography for detection of injuries in patients with major blunt trauma. CMAJ. 2012;184(8):869–76.
Stedman JM, Franklin JM, Nicholl H, Anderson EM, Moore NR. Splenic parenchymal heterogeneity at dual-bolus single-acquisition CT in polytrauma patients-6-months experience from Oxford. UK. Emerg Radiol. 2014;21(3):257–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest pertaining to this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Computed Tomography.
Rights and permissions
About this article
Cite this article
Iacobellis, F., Romano, L., Rengo, A. et al. CT Protocol Optimization in Trauma Imaging: A Review of Current Evidence. Curr Radiol Rep 8, 8 (2020). https://doi.org/10.1007/s40134-020-00351-5
Published:
DOI: https://doi.org/10.1007/s40134-020-00351-5