Abstract
This study investigates the impact of Ti and V additions on the magnetic properties of nanostructured Fe–Sn alloys synthesized via a ball milling process. The structural properties, morphological features, and magnetic behavior of the resulting nanostructured materials were analyzed using various characterization techniques, including scanning electron microscopy, energy-dispersive spectroscopy, X-ray diffraction, and vibrating sample magnetometer. After subjecting the samples to a grinding time of 10 h, XRD analysis revealed the presence of characteristic peaks corresponding to FeSn phase. The average crystallite size ranged from 51 to 18 nm, while the lattice strain was measured between 0.184% and 0.259%. Interestingly, the grinding process led to an increase in coercivity, remanence magnetization, and squareness of the nanostructured FeSn samples, accompanied by a decrease in saturation magnetization. In the case of the nanostructured FeSnTiV samples, the addition of Ti or V to FeSn resulted in a reduction in saturation magnetization. Conversely, when both Ti and V were added, the saturation magnetization increased. However, the inclusion of Ti, V, or Ti-V compounds resulted in a decrease in coercivity, remanence magnetization, and squareness of the samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanostructured materials have garnered significant attention in materials science and nanotechnology due to their exceptional properties and characteristics. These materials possess structural features with dimensions typically ranging from 1 to 100 nm [1,2,3]. As a result of their high surface-to-volume ratio and the occurrence of quantum confinement effects, nanostructured materials exhibit unique properties and behaviors distinct from bulk materials [4]. Various fabrication techniques, including chemical synthesis, electrochemical deposition, and lithography, enable the production of nanostructured materials using a wide range of materials such as metals, semiconductors, polymers, and ceramics. Among the vast array of nanostructured materials available [5,6,7], FeSn nanostructures have emerged as a promising candidate for various applications such as magnetic storage, spintronics, and energy conversion. FeSn materials, comprising iron and tin with nanoscale structural features, can be synthesized using several methods such as chemical synthesis, thermal evaporation, and magnetron sputtering. Notably, FeSn nanostructures possess tunable magnetic and electrical properties, which can be controlled by adjusting their composition, size, and morphology. Consequently, the exploration and advancement of FeSn nanostructured materials hold immense potential for the future of advanced materials and nanotechnology [8,9,10,11]. The incorporation of Ti and V into FeSn nanostructured materials has demonstrated significant effects on their magnetic, morphological, and structural properties [12, 13]. These elemental additions offer the potential to enhance the magnetic characteristics of FeSn materials by increasing their magnetization and coercivity, rendering them highly suitable for applications in magnetic storage and spintronics. Specifically, Ti has been observed to facilitate the formation of FeSn nanoparticles with a more uniform size distribution, while also stabilizing the FeSn phase at lower temperatures [14]. Conversely, V has exhibited the ability to induce the formation of high aspect ratio nanowires and promote the emergence of the FeSn phase at elevated temperatures [15, 16]. Consequently, Ti- and V-doped FeSn nanostructured materials hold immense promise for a wide range of applications encompassing magnetic storage, spintronics, catalysis, and energy conversion. However, it is essential to conduct further research to gain a comprehensive understanding of the properties of these doped FeSn nanostructures and to optimize their performance for specific applications. By delving deeper into their unique characteristics, we can unlock their full potential and precisely tailor them to meet the diverse requirements of various technological fields. This knowledge will enable us to harness the advantages of Ti- and V-doped FeSn nanostructured materials and maximize their effectiveness in practical applications.
As part of the quest for a durable permanent magnetic material, extensive research has been carried out on the Fe–Sn binary systems using various non-equilibrium techniques, including mechanical alloying. The primary goal is to discover a new hard magnetic material by considering the magnitude and assessment of anisotropy energy. Thus, our focus in this study is on investigating the structural and intrinsic magnetic properties of the Fe–Sn binary system. This system consists of ferromagnetic compounds exhibiting considerable magnetic properties, all of which are metastable at room temperature. Notably, there is one highly metastable compound in the system that is often overlooked in reported Fe–Sn binary phase diagrams. Fayyazi et al. [17, 18] demonstrated that both Fe55Sn45 and Fe40Sn60 compositions confirm the formation of all three ferromagnetic compounds using the conventional method. This compelling evidence influenced our decision to choose these specific compositions for our study.
The aim of this research is to synthesize nanostructured FeSn materials that possess exceptional properties not commonly observed in their bulk counterparts. Nanostructured FeSn materials exhibit a significantly high surface-to-volume ratio, resulting in enhanced magnetic, electrical, and catalytic characteristics. Moreover, their small size renders them easily manageable and processable, making them highly suitable for a diverse range of applications. By incorporating Ti and V dopants into FeSn nanostructured materials, we can further enhance their properties by capitalizing on the unique electronic and magnetic attributes of these transition metals. The investigation of FeSn-doped nanostructured materials has opened up new avenues for the advancement of cutting-edge materials with superior properties, offering transformative potential across various fields. This research not only expands our comprehension of nanostructured materials but also lays the groundwork for the development of innovative applications that harness the remarkable capabilities of FeSn-doped nanostructures.
2 Experimental procedure
Nanostructured FeSn, FeSnTi, FeSnV and FeSnTiV alloys were synthesized via a powder metallurgy process using a planetary ball mill PM400 under an Argon atmosphere. The milling process was conducted at a speed of 300 RPM with varying durations. The elemental powder mixture for each sample consisted of Fe, Sn, Ti, and V, with particle sizes of 60 µm (99.5%), 55 µm (99.1%), 70 µm (99.98%), and 75 µm (99.3%) respectively. Chromed steel jars with a capacity of 250 ml were used for each sample, along with 20 mm chromed steel balls, maintaining a ball-to-powder mass ratio of 10:1. The preparation of the samples involved the utilization of 20 mm chromed steel balls, each weighing 32.6304 g, with approximately 6 balls used for every 20 g of the mixture. A detailed table presenting the total mass of the initial powder mixture for each 20 g sample is provided in Table 1 for reference.
The morphology of the powder particles was analyzed using a JEOL SEM XL30 scanning electron microscope, with backscattered electron imaging employed for observation. The structural evolution during the mechanical milling process was analyzed using Cu Ka radiation and an XPERT PRO X-ray diffraction (XRD) system, covering the 2θ range of 10° to 90°. In addition, magnetic measurements during milling were conducted using a Micro-Sense EV9 vibrating sample magnetometer (VSM).
3 Results and discussion
3.1 Structural analysis
Figure 1 displays the X-ray diffraction (XRD) patterns of the Fe–Sn–A (A: Ti and V) mixture powder before grinding. The XRD analysis of the initial powder samples confirmed the presence of cubic (bcc) iron (Fe), tetragonal tin (Sn), hexagonal close-packed (hcp) titanium (Ti), and cubic (bcc) vanadium (V), as illustrated in the pattern.
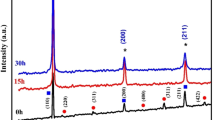
Figure 2 depicts the X-ray diffraction (XRD) patterns of the Fe–Sn mixture powder at various stages of grinding. With increasing grinding duration, the diffraction peaks exhibit broadening and reduced intensity. After 5 h of grinding, the appearance of a new FeSn phase becomes evident. At the 10-h mark, another phase of FeSn is observed [19]. Upon reaching 20 h of grinding, distinct peaks corresponding to FeSn emerge, with their intensities significantly increasing due to the prolonged milling time [20, 21]. Wang et al. [22] reported a decrease in FeSn peak intensity and an increase in peak width, suggesting a lower FeSn content and smaller crystalline grains within the samples. In addition, Yelsukov et al. [10, 23] conducted experiments demonstrating the typical occurrence of intermetallic formation preceding the formation of supersaturated solid solutions in alloy systems with limited solid solubility of one element in the other. Specifically, when milling the blended elemental powders of the Fe–Sn system, they observed the early-stage (1–2 h) formation of a mixture of α-Fe and FeSn intermetallic compounds. The proportion of intermetallic compounds reached approximately 40% with increasing milling time, especially when the initial powder mixture had a higher Sn content.
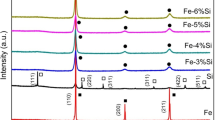
Figure 3 depicts the X-ray diffraction (XRD) patterns obtained from the Fe–Sn–A (A: Ti and V) mixture powder after 20 h of grinding. The XRD analysis revealed significant changes in the FeSnTi and FeSnV samples. Specifically, in the FeSnTi sample, the emergence of a distinct Fe0.975Ti0.025 phase [24, 25] is clearly observed, as illustrated in Fig. 3. Similarly, for the FeSnV sample, the XRD patterns indicate the formation of a new phase identified as Fe9V [26]. These findings demonstrate the successful synthesis of novel phases within the Fe–Sn–A (A: Ti and V) mixture powder through the grinding process.
The determination of crystallite size (D) was accomplished by evaluating the broadening (β) using the Scherrer equation [27]:
In turn, the lattice strain (ε) was calculated using the equation developed in Ref. [28] for the corresponding diffraction lines:
The above-mentioned equation was constructed with the variables λ = 1.7889 Å (Co radiation), β representing the full width at half-maximum, θ denoting the angle in radians, and ε representing the lattice strain.
Table 2 presents the variation of crystallite size and lattice strain of nanostructured FeSn/Ti/V at different milling times. During the milling process of nanostructured FeSn, the size of the crystallites decreases due to mechanical deformation induced by the milling balls. With increased milling time, the number of collisions between the milling balls and FeSn particles rises, resulting in a reduction in crystallite size. Simultaneously, the lattice strain increases during milling due to mechanical deformation causing a distortion in the FeSn lattice structure. The impact of the milling balls on FeSn particles compresses the lattice, reducing the average distance between Fe and Sn atoms. In the zoom section of Fig. 2, the X-ray diffraction peak shifts unequivocally validate the existence of substantial alterations in the lattice parameters when compared to those of the initial mixture [19, 29]. The compression leads also to an increase in lattice strain and higher lattice energy. Consequently, the reduction in crystallite size and the increase in lattice strain during milling are simultaneous effects arising from the mechanical deformation caused by the milling balls. The decrease in crystallite size enhances the surface area of FeSn particles, thereby potentially improving reactivity and catalytic properties. In addition, the increase in lattice strain can induce changes in the electronic and magnetic properties of the material, making it suitable for diverse applications.
The effect of adding Ti, V, or both elements to a material can influence its crystallite size in diverse ways, which depend on factors such as concentration and synthesis method. The presence of Ti, V, or both can serve as nucleation agents, promoting the formation of smaller crystallites. This occurs because the added elements introduce a higher degree of disorder in the lattice structure of the material, facilitating the generation of new nuclei with reduced sizes. Furthermore, the incorporation of these elements can induce lattice strain in the material. The introduction of Ti and V can cause lattice distortion due to their dissimilar atomic radii compared to the host material, resulting in an increase in lattice strain. However, the magnitude of this effect on lattice strain also relies on the concentration and synthesis method employed.
X-ray diffraction measurements are used to determine the bcc lattice parameter (a) of the FeSn phase in function of milling time, for the most intense peak using the following equation [30]:
Table 3 presents the evolution of the body-centered cubic (bcc) lattice parameter “a ” in nanostructured FeSn as a function of milling time. Prior to milling, the lattice parameter was measured at 0.2857 nm. After subjecting the sample to mechanical alloying for 20 h, the X-ray diffraction (XRD) analysis revealed the presence of broadened bcc lines, indicating a lattice parameter of 0.2934 nm. These results suggest that Sn effectively dissolves in iron, leading to the formation of a bcc α-Fe(Sn) solid solution. The observed increase in the lattice parameter, along with the absence of reflections from other phases in the diffractogram, further supports the formation of the solid solution.
In a comprehensive study conducted by E. P. Elsukov et al. [21], the impact of mechanical alloying for 32 h on the lattice parameters of FeSn was thoroughly investigated, with the lattice parameter “a” measured at 0.2953 nm. Building upon this investigation, E. P. Yelsukov et al. [23] further explored the relationship between the body-centered cubic (bcc) lattice parameter “a” and varying Sn concentrations. Remarkably, when the Sn concentration reached 40%, the value of “a” was observed to be approximately 0.2995 nm. This finding emphasizes the lattice parameter’s sensitivity to Sn content in the FeSn alloy, highlighting its pivotal role in influencing the material’s structural properties and overall performance.
3.2 Morphological examination
Table 4 and Fig. 4 presents the composition (in Wt. % or At. %) and the morphology of the initial mixture, respectively.
Figure 5 shows the SEM micrographs of FeSn nanostructured material milled for varying durations. FeSn is a material known for its diverse properties and morphology, which depend on the specific preparation and processing conditions employed. During the milling process, the nanocrystalline FeSn undergoes deformation and fragmentation, resulting in alterations to its morphology, crystallinity, and phase composition. Initially, at the beginning of milling, the nanostructured FeSn particles exhibit a relatively uniform shape and size, typically ranging in the tens of micrometers.
However, as the milling time reaches 5 h, the material undergoes notable plastic deformation and fragmentation, leading to a decrease in the average particle size. Consequently, particle agglomeration occurs, resulting from the increased surface energy and the formation of fresh interfaces between fragmented particles. Subsequently, at the 10-h mark, the nanostructured FeSn particles demonstrate a more intricate morphology, showcasing a blend of fragmented and partially welded particles. This process introduces a higher concentration of lattice defects and strain into the material due to deformation and fragmentation. Moreover, following 20 h of milling, the material’s morphology exhibits a highly fragmented structure, accompanied by a significant reduction in the average particle size. In addition, the material showcases a more disordered structure, owing to the abundance of lattice defects and the emergence of new interfaces between fragmented particles [31, 32]. Furthermore, as a consequence of the increased number of interfaces between fragmented particles, the material exhibits a higher surface area and a greater number of reactive surfaces. It is crucial to emphasize that the morphology of nanostructured FeSn can undergo substantial variations throughout the milling process, influencing particle size, shape, and the extent of fragmentation. These alterations hold significant implications for the material’s physical, chemical, and mechanical properties. Consequently, the investigation of these changes becomes a vital area of exploration within the realms of materials science and nanotechnology.
Figure 6 depicts the SEM micrograph of nanostructured FeSn/Ti/V after undergoing 20 h of milling. The inclusion of Ti, V, or both Ti and V within the nanostructured FeSn has a profound impact on its morphology during the milling process. These additional elements exert influence on the behavior of nanocrystalline FeSn, resulting in modifications to its morphology, crystallinity, and phase composition. Extensive research has demonstrated that the introduction of Ti enhances the deformation and fragmentation induced by milling, leading to a reduction in particle size and an increase in specific surface area. Ti atoms tend to diffuse into the FeSn lattice, creating fresh interfaces and promoting plastic deformation throughout milling. Moreover, the introduction of V, either alone or in combination with Ti, produces a more intricate morphology characterized by a mixture of fragmented and partially welded particles. Incorporating Ti and V into FeSn can generate ternary intermetallic compounds with larger particle sizes, inducing alterations in the material’s phase composition and lattice structure.
These compounds also serve as nucleation sites for the formation of new particles and interfaces during milling, resulting in a more heterogeneous morphology. However, the inclusion of vanadium can also lead to an increase in particle size, influenced by several factors.
Vanadium atoms can act as nucleation sites for the growth of new FeSn particles, effectively reducing the activation energy required for the formation of these particles and ultimately resulting in the growth of larger ones. In addition, the presence of vanadium can modify the reaction kinetics during the synthesis of FeSn, thereby contributing to the formation of larger particles.
3.3 Magnetic measurements
Figure 7 illustrates the hysteresis curves of nanostructured FeSn ground for varying time intervals, providing valuable insights into its magnetic properties. Notably, an observable trend emerges as the grinding time increases, with the saturation magnetization exhibiting a decline while the coercivity demonstrates a corresponding increase. This trend highlights the susceptibility of FeSn’s magnetic properties to controlled manipulation of the grinding duration. The milled powders exhibit a significant level of magnetization. Trumpy et al. [33], in their study, observed that the magnetic properties of FeSn were influenced by both the low homogeneity range of milled FeSn and the presence of phases in the Fe–Sn system where iron content exceeded that of FeSn, resulting in a ferromagnetic behavior.
In a study conducted by E.P. Yelsukov et al. [23], an exploration was undertaken to investigate the magnetic properties displayed by Fe–Sn alloys in relation to their concentration levels. The findings of their research uncovered significant variations in the magnetic properties as a result of changes in concentration. Specifically, a discernible yet subtle dependence on the Sn concentration was observed within the range of 0–25 at%. These findings emphasize the intricate interplay between concentration levels and magnetic properties in Fe–Sn alloys, emphasizing the importance of meticulous consideration and analysis of these factors in future studies concerning this material.
Table 5 presents a comprehensive summary of the magnetic parameters that define the nanostructured FeSn samples subjected to different milling durations. Notably, a clear trend emerges as the milling time increases, with the coercivity, remanence magnetization, and squareness exhibiting a noticeable rise. Conversely, the saturation magnetization experiences a substantial decrease as the milling duration extends. These findings indicate that the magnetic properties of the FeSn material are inherently responsive to controlled adjustments in the milling time, thereby offering new possibilities for customized magnetic manipulation of this material.
During the milling process, the FeSn powder undergoes profound plastic deformation caused by the repetitive impacts between the milling balls and the powder particles. Consequently, this leads to the formation of small crystallites characterized by a high density of defects, including dislocations, grain boundaries, and other types of lattice imperfections. The presence of these defects within the nanocrystalline FeSn structure can significantly elevate the coercivity by serving as pinning sites for domain walls. These pinning sites impede the movement of domain walls, rendering demagnetization of the material more challenging. Furthermore, the reduced grain size of the nanocrystalline FeSn structure contributes to an increase in magnetic anisotropy energy, further reinforcing the coercivity [34, 35].
The augmentation of magnetic anisotropy energy contributes to an amplified remanence magnetization as the alignment of magnetic domains intensifies in the presence of an external magnetic field. This outcome arises from the enhanced stability of the magnetic domain structure facilitated by the heightened magnetic anisotropy energy, rendering the rotation or demagnetization of magnetic domains more challenging. Furthermore, the reduced grain size within the nanocrystalline FeSn structure also contributes to an increased squareness of the material. This arises from a reduction in domain wall pinning and an improvement in the homogeneity of the magnetic domain structure resulting from the smaller grain size. Consequently, the hysteresis loop of the material approaches a more rectangular shape, signifying a higher degree of magnetic ordering and a more ideal magnetic behavior [36, 37].
The decline in magnetization saturation of milled FeSn powder over time can be attributed to several influential factors. Primarily, the structural transformations taking place during the milling process play a crucial role. The severe plastic deformation endured by the FeSn powder gives rise to a nanocrystalline FeSn structure, characterized by a substantial density of defects including dislocations, grain boundaries, and various lattice imperfections. These defects contribute to structural disorder, ultimately leading to a reduction in the material’s magnetization saturation. Another significant factor is the progressive reduction in particle size with prolonged milling time. These factors contribute to an enhanced understanding of the decrease in magnetization saturation observed in the FeSn milled powder. First, the decrease can be attributed to the increased surface area and reduced volume of the material. This change in geometry reduces the number of magnetic moments that contribute to the overall magnetization, ultimately resulting in a decline in magnetization saturation. In addition, exchange coupling between magnetic moments in adjacent particles plays a significant role. As the particle size diminishes during the milling process, the number of neighboring particles increases, intensifying the exchange coupling effect. This coupling can lead to a reduction in magnetization saturation due to the presence of opposing magnetic moments within the material [38].
Figure 8 displays the hysteresis curves of the nanostructured FeSn/V/Ti material, subjected to a 20-h milling process. Remarkably, the FeSnTiV material showcases exceptional magnetic properties. Moreover, the incorporation of Ti, V, or TiV contributes to a reduction in the material’s coercivity. This decrease enhances the material’s suitability for a wide range of magnetic applications, making it highly promising in terms of its magnetic performance.
Table 6 presents the magnetic parameters of various nanostructured FeSnTiV materials that underwent milling for up to 20 h. Notably, the nanostructured FeSnTiV material demonstrates the highest magnetic saturation value and the lowest coercivity value among the samples. In addition, the introduction of Ti, V, or TiV to the nanostructured FeSn leads to a notable decrease in coercivity. It is essential to acknowledge that the magnetic properties of the material are strongly influenced by the presence of these different elements within the FeSn matrix. The incorporation of Ti, V, or TiV induces modifications in the material’s microstructure and crystallographic characteristics, consequently resulting in changes in its magnetic properties. This highlights the significant role played by element doping in tailoring the magnetic behavior of nanostructured FeSnTiV materials.
The exceptional magnetic saturation observed in the FeSnTiV material can be attributed to the synergistic effect of Ti and V, which significantly enhances the magnetic properties of the material. In addition, the decrease in coercivity can be attributed to the reduction in the anisotropy constant within the nanostructured FeSn matrix. The incorporation of Ti, V, or TiV contributes to a reduction in the anisotropy constant, thereby lowering the coercivity of the material. This reduction can be attributed to several factors, including the introduction of lattice strain, grain refinement, and alterations in the crystal structure induced by the added elements. These changes collectively reduce the energy required to reverse the magnetization of the material, resulting in a lower coercive field. The studies conducted by Brzakalik and Hartmann [39, 40] further support these observations and provide valuable insights into the role of element doping on the magnetic behavior of nanostructured FeSnTiV materials.
4 Conclusion
In conclusion, this study successfully synthesized a magnetic Fe–Sn/A nanostructured alloy doped with Ti, V, and TiV through the ball milling process. The comprehensive characterization techniques employed in this study provided clear insights into the magnetic behavior of the FeSn nanostructures upon dopant addition. The incorporation of Ti and V resulted in significant modifications to the magnetic properties of the FeSn nanostructures, leading to improvements or reductions depending on the specific dopant used. The high surface-to-volume ratio exhibited by FeSn nanostructures imparted enhanced structural and magnetic properties compared to their bulk counterparts. Furthermore, their small size renders them versatile for a wide range of applications. The investigation of FeSn-doped nanostructured materials holds promising prospects for the development of advanced materials with superior properties and transformative applications in diverse fields such as energy storage, biomedicine, and environmental remediation.
References
A.A. Rempel, Nanotechnologies. Properties and applications of nanostructured materials. Russian Chem. Rev. 76(5), 435 (2007)
Y.T. Zhu, T.C. Lowe, T.G. Langdon, Performance and applications of nanostructured materials produced by severe plastic deformation. Scripta Mater. 51(8), 825–830 (2004)
P. Matteazzi, G. Le Caër, A. Mocellin, Synthesis of nanostructured materials by mechanical alloying. Ceram. Int. 23(1), 39–44 (1997)
G. Guisbiers, S. Mejía-Rosales, F.L. Deepak, Nanomaterial properties: size and shape dependencies. J. Nanomater. 2012, 20–20 (2012)
Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, H. Yan, One-dimensional nanostructures: synthesis, characterization, and applications. Adv. Mater. 15(5), 353–389 (2003)
G.D. Moon, S. Ko, Y. Min, J. Zeng, Y. Xia, U. Jeong, Chemical transformations of nanostructured materials. Nano Today 6(2), 186–203 (2011)
J.W. Park, C.M. Park, Nanostructured FeSn2/SnO2-based composites as high-performance anodes for lithium-ion batteries. J. Alloy. Compd. 803, 80–87 (2019)
M. Han, H. Inoue, S. Fang, C. John, L. Ye, M.K. Chan, J.G. Checkelsky, Evidence of two-dimensional flat band at the surface of antiferromagnetic kagome metal FeSn. Nat. Commun. 12(1), 5345 (2021)
D. Multer, J.X. Yin, M.S. Hossain, X. Yang, B.C. Sales, H. Miao, M. Zahid Hasan, Imaging real-space flat band localization in kagome magnet FeSn. Commun. Mater. 4(1), 17 (2023)
G.A. Dorofeev, E.P. Elsukov, Thermodynamic modeling of mechanical alloying in the Fe–Sn system. Inorg. Mater. 36, 1228–1234 (2000)
H. Gong, M. Qing, H. Wan, X. Yuan, P. Qiao, X. Liu, Y.W. Li, Fe-Sn bimetallic catalysts for an enhanced Fischer-Tropsch synthesis stability via oxygen removal and coking resistance. Fuel 311, 122115 (2022)
M. Yin, P. Nash, J.A. Kaduk, J.C. Schuster, Experimental investigation of the Fe-Sn-Ti ternary isothermal section at 873 K. J. Alloy. Compd. 693, 76–86 (2017)
Y. Liang, C. Wang, N. Liu, J. Zhang, New Ti-Fe-Sn-Y alloys designed for laser direct energy deposition. Mater. Charact. 187, 111866 (2022)
A.K. Pandey, P. Alvaredo, S. Milenkovic, F. Sket, Development of powders of Ti-Fe-Sn ultrafine eutectics for laser additive manufacturing. Powder Technol. 404, 117416 (2022)
Y. Han, S. Zhang, R. Bai, H. Zhou, Z. Su, J. Wu, J. Wang, Effect of nano-vanadium nitride on microstructure and properties of sintered Fe-Cu-based diamond composites. Int. J. Refract Metal Hard Mater. 91, 105256 (2020)
Y. Zhang, W. Wang, Z. Li, G. Huang, H. Zhang, F. Liu, Study of the brittleness mechanism of aluminum/steel laser welded joints with copper and vanadium interlayers. Opt. Laser Technol. 163, 109319 (2023)
B. Fayyazi, K.P. Skokov, T. Faske, D.Y. Karpenkov, W. Donner, O. Gutfleisch, Bulk combinatorial analysis for searching new rare-earth free permanent magnets: Reactive crucible melting applied to the Fe-Sn binary system. Acta Mater. 141, 434–443 (2017)
B. Fayyazi, K.P. Skokov, T. Faske et al., Experimental and computational analysis of binary Fe-Sn ferromagnetic compounds. Acta Mater. 180, 126–140 (2019)
H. Giefers, M. Nicol, High pressure X-ray diffraction study of all Fe–Sn intermetallic compounds and one Fe–Sn solid solution. J. Alloy. Compd. 422(1–2), 132–144 (2006)
Y.S. Kwon, K.B. Gerasimov, S.S. Avramchucka, Decomposition of FeSn intermetallic induced by mechanical milling. J Alloys Compd 359, 79–83 (2003)
E.P. Elsukov, I.V. Povstugar, Deformation-Induced Dissolution of the Intermetallic Compound FeSn in Nanocrystalline a-Fe. Phys. Metals Metallography 107(1), 80–89 (2009)
J. Wang, W. Zou, Z. Lu, Z. Lu, X. Liu, J. Xu, Y. Du, Anomalous Hall effect and magnetoresistance of (FexSn1− x) 1− y (SiO2) y films. J. Phys. D Appl. Phys. 40(8), 2425 (2007)
E.P. Yelsukov, E.V. Voronina, G.N. Konygin, V.A. Barinov, S.K. Godovikov, G.A. Dorofeev, A.V. Zagainov, Structure and magnetic properties of Fe100-xSnx (3.2<x<62) alloys obtained by mechanical milling. J. Magnet. Magnet. Mater. 166(3), 334–348 (1997)
Y. Cai, Y. Wu, Z.Y. Xie, H.S. Liu, Z.P. Jin, Phase equilibria in Fe–Sn–Ti ternary system at 1073 K and 1273 K. Calphad 49, 110–119 (2015)
A. Novitskii, I. Serhiienko, A. Nepapushev, A. Ivanova, T. Sviridova, D. Moskovskikh, V. Khovaylo, Mechanochemical synthesis and thermoelectric properties of TiFe2Sn Heusler alloy. Intermetallics 133, 107195 (2021)
B.F.O. Costa, G. Le Caër, B. Malaman, Evolution of a FeV sigma phase ball-milled in a mixture of argon and air. Hyperfine Interact. 183, 67–73 (2008)
A. Abada, S. Bergheul, A. Younes, Mechanical and structural behaviour of TiAlV nanocrystalline elaborated by mechanical milling technique. Micro Nano Lett. 15(14), 1023–1027 (2020)
N. Metidji, N.E. Bacha, A. Younes, D. Saidi, The effect of Ti addition on microstructure and magnetic properties of nanocrystalline FeAl 40 alloy powders prepared by mechanical alloying. Powder Metall. Met. Ceram. 59, 160–170 (2020)
W.F. Ehret, A.F. Westgren, X-ray analysis of iron-tin alloys. J. Am. Chem. Soc. 55, 1339 (1933)
A. Younes, M. Khorchef, A. Bouamer, H. Amar, Magnetic and structural behavior of Fe-CoO nanocomposites mechanically milled. In IOP Conference Series: Materials Science and Engineering 557, No. 1, p. 012064). IOP publishing
X. Zhang, J. Liu, Y. Qiao, A. Kong, R. Li, Y. Shan, Fe-boosting Sn-based dual-shell nanostructures from new covalent porphyrin frameworks as efficient electrocatalysts for oxygen reduction and zinc-air batteries. Electrochim. Acta 320, 134593 (2019)
Y.E. Lim, W.S. Choi, J.H. Kim, Y.N. Ahn, I.T. Kim, The Sn–red P-Fe–based alloy materials for efficient Li–ion battery anodes. J. Indust. Eng. Chem. (2023). https://doi.org/10.1016/j.jiec.2023.01.033
G. Trumpy, E. Both, C. Djega-Mariadassou, P. Lecocq, Mossbauer-effect studies of iron-tin alloys. Phys. Rev. B 2(9), 3477 (1970)
G. Venturini, B. Malaman, G. Le Caër, D. Fruchart, Low-temperature magnetic structure of FeSn 2. Phys. Rev. B 35(13), 7038 (1987)
A.P. Vokhmyanin, Symmetry analysis of the magnetic structures of alloys of the quasi-binary system Fe x Mn 1–x Sn 2. Phys. Met. Metallogr. 107, 115–122 (2009)
E. P. Yelsukov, E. V. Voronina, G.N. Konygin, S. K Godovikov, V. M. Fomin, Disordered Nanocrystalline Fe-Sn Alloys: 57Fe and 119Sn Mössbauer Spectroscopy Study. In Mössbauer Spectroscopy in Materials Science (Springer Netherlands, Dordrecht, 1999), pp. 283–290
B.C. Sales, J. Yan, W.R. Meier, A.D. Christianson, S. Okamoto, M.A. McGuire, Electronic, magnetic, and thermodynamic properties of the kagome layer compound FeSn. Phys. Rev Mater. 3(11), 114203 (2019)
N. Nakayama, K. Kosuge, S. Kachi, T. Shinjo, T. Takada, Magnetic properties of FeSn (OH) 6 and its oxidation product, FeSnO (OH) 5. Mater. Res. Bull. 13(1), 17–22 (1978)
K. Brzakalik, Structural and Magnetic Properties οf Fe3-xTixSn Disordered Alloys. Acta Phys. Pol. A 114(6), 1529–1536 (2008)
O. Hartmann, R. Wäppling, Muon spin precession in the hexagonal antiferromagnet FeSn. Phys. Scr. 35(4), 499 (1987)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abada, A., Younes, A. & Manseri, A. Magnetic and structural properties of nanostructured FeSn, FeSnTi, FeSnV and FeSnTiV alloys elaborated via ball milling process. J. Korean Phys. Soc. 84, 33–43 (2024). https://doi.org/10.1007/s40042-023-00947-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40042-023-00947-y