Abstract
The present study has identified a free living bacterial strain from dump garbage soil, capable of forming large halo zone in National Botanical Research Institute’s Phosphate medium and Pikovskaya agar medium with phosphate solubilization index of 4.0. The phosphate solubilization ranged from 304.08 to 2073.01 ± 0.33 µg ml−1 in 3–12 days in NBRIP broth containing tricalcium phosphate and pH decreased from 7.0 to 3.8. The strain showed growth and color change in different nitrogen free media and nitrogen fixation in the supernatant was estimated to be 3.057 ± 0.34 and 7.87 ± 0.34 mg l−1 on 30th and 45th days of incubation. Indole acetic acid, ammonia, siderophore, and hydrogen cyanide production by the strain were quantified. 16s rDNA analysis confirmed the strain as Pseudomonas aeruginosa. Pot based assay with Indian mustard showed that seed coating of the strain significantly enhanced plant wet weight (up to 140%), plant dry weight (up to 88%), root length (up to 25%) and shoot length (up to 14%) over un-inoculated controls. It also showed that seed bacterization resulted in greater enhancement of plant growth than direct inoculation in the plant rhizoplane. Nitrogen and phosphorous content of the seed treated plants were 40.61 and 100% higher than the untreated controls further confirming the nitrogen fixation and phosphate solubilization efficiency of the strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen and phosphorous are regarded as the 1st and 2nd most important macronutrients essential for important metabolic pathways of plants. Although total P content of soil is many times higher than plant needs (400–1200 mg kg−1); most of the P is immobilized in the forms of organic and inorganic forms making only a small fraction available to plant (1 mg kg−1 or less) [1]. Phosphate solubilizing bacteria are soil inhabiting heterotrophs that secrete low molecular weight organic acids that dissolve and release P into solution from phosphatic minerals, particularly within the rhizosphere microenvironment [1]. Like P, N is also abundant but is unavailable to plants until bacteria convert it into ammonia by the process termed as biological N fixation. N and P added to soil by biological processes are environmentally sound alternative to chemical fertilizers as repeated use of fertilizers deteriorates soil quality and the surface run offs deteriorates nearby water bodies [2]. Therefore, agriculture scientists are shifting attention from N and P based inorganic fertilizers towards biofertilizers containing PSBs and diazotrophs for a self sustaining agriculture system. The present research, reports isolation and characterization of a plant growth promoting fluorescent Pseudomonas aeruginosa strain PGP. The isolate was screened and quantified for ammonia production, phosphate solubilization, N fixation, IAA production, siderophore production, hydrocyanic acid (HCN) production, and preliminary bio safety assessment was done to safeguard its field based application potential. Effect of the strain on Brassica juncea growth and N and P content was confirmed by pot based assay.
Material and Methods
Garbage soil sample was collected from soil sample in the suburbs of Sonarpur area, West Bengal, India where agro industrial waste is dumped off. Serial dilution was conducted followed by streaking in nutrient agar media for isolation of pure single colony. Potential isolates were screened for phosphate solubilization and selected on the basis of halo zone produced in National Botanical Research Institute’s Phosphate (NBRIP) agar growth medium and modified Pikovskaya medium [3, 4]. Among the 8–10 morphologically distinct isolates, one predominant strain showing visible fluorescence was selected for further study based on the large size of the halo zone.
Biochemical tests were carried out as per Bergey’s manual of systematic bacteriology [5]. Morphological test was performed by visualizing the Gram stained cells under phase contrast microscope. For 16s RNA identification, PCR amplification was done using universal primers 1492r and 27f [6]. The sequence was submitted to GenBank. Phylogenetic tree was constructed by Maximum Likelihood using boot strap value of 500. Evolutionary analysis was conducted in MEGA6 [7].
Phosphate solubilization was determined in NBRIP medium and Pikovskaya medium. The zone of solubilization was measured and phosphate solubilization efficiency (PSE) was calculated. The kinetics of Ca3 (PO4)2 solubilization mediated by the isolate was done by phosphomolybdate blue color method [8] in Pikovskaya’s broth (100 ml) (pH 7) and NBRIP broth. At various time intervals of 12 days incubation, 5 ml of the culture broth was sucked up, centrifuged (10,000 rpm, for 15 min) and the supernatant was collected. The available phosphorous (P) was determined by modified molybdenum blue method [8] and the color were read with spectrophotometer at 882 nm. Solubilization of tricalcium phosphate (TCP) was calculated from standard curve of KH2PO4 (10–100 μg ml−1) in the form of μg ml−1 of Pi released.
N fixation ability was evaluated by growing the strain on different N free solid agar media like Jensen media, Norris Glucose media, LGI media with mannitol and sucrose as carbon sources [9, 10]. The solid agar plates and culture tubes containing broths were incubated with the overnight grown strain at 30 °C and monitored for growth and color change for 7–30 days [11]. The spent culture was examined for presence of ammonia as indirect method for detection of the N. N fixation was estimated from cell free culture broth by adopting Micro kjeldahl method [12]. LGI medium with mannitol was inoculated with bacterial isolate and incubated at 30 °C for 30–45 days. The supernatant was used to determine the amount of fixed N through micro Kjeldahl method. Parallel control was run.
Ammonia production was detected using Nessler’s reagent. Overnight grown bacterial culture was inoculated in 10 ml peptone broth and incubated at 28 ± 1 °C for 48–72 h in incubator shaker. Later, 0.5 ml of Nessler’s reagent was added. The development of yellow to dark brown color indicated the production of ammonia. Quantitative estimation of ammonia production was done using Nesselerization spectrophotometric method [13]. Cell free supernatant was treated with Rochelle salt solution using K–Na Tartarate (25 g/50 ml) and Nessler’s reagent and optical density was read at 425 nm against a standard.
For indole acetic acid (IAA) determination, strain was inoculated in LB broth with and without tryptophan (control) and incubated at 28 ± 1 °C. An aliquot of 2 ml supernatant from different time points was added with two drops of orthophosphoric acid and 4 ml of Salkowski reagent [14]. The mixture was incubated for 25 min and intensity of pink color developed was recorded at 528 ± 1 nm with UV–VIS spectrophotometer against a standard graph of IAA (10–100 µg ml−1).
Siderophore production was determined on Chrome-azurol S Agar (CAS) medium. The bacterial strain was spot inoculated on CAS medium and incubated at 28 ± 1 °C for 48–72 h and observed for the formation of orange to yellow halo zones. For quantitative estimation of siderophore formation, 0.5 ml culture filtrate was mixed with 0.5 ml of CAS reagent. It was read at 630 nm against un-inoculated succinate as a reference. Percent Siderophore Units was calculated as per the method of Payne [15].
HCN production was determined on solid agar plates amended with or without 4.4 g glycine. Filter paper was soaked in a solution containing 0.5% picric acid and 1% Na2CO3 and was placed on the upper lids of plates. Control (uninoculated) plate was also kept. Development of color from yellow to light brown, moderate brown or strong brown was examined for apparent HCN production [16]. Quantitative estimation of HCN production was done by collecting supernatants at different time intervals (24–72 h) and were assessed spectrophotometrically at 427 nm with addition of above reagents and read against blank containing uninoculated media. Cyanide content was derived in μg ml−1 from a standard curve using KCN.
Pathogenic Pseudomonas possesses a class of compounds called phenazines that are responsible for their lethal effect [17]. Primers PhzF (5′-TAAGGATCCGGTAGTTCCAAGCCCCAGAAAC-3′) and PhzR (5′-CACATTTGATCTAGATGGGTCACGGCTATTCAG-3′) were used to amplify Phz (Phenazine Pigment) gene from the isolate [18]. As positive control, one multidrug resistant Pseudomonas strain from ATCC (ATCC® 27853™) was used.
Antibiotic susceptibility test was performed on Mueller–Hinton agar plate by disc diffusion [19]. The plates containing different antibiotics like ampicillin, tetracycline, chloramphenicol, streptomycin, rifampicin, vancomycin, penicillin, amikacin were examined for zone of inhibitions [19].
Indian mustard (Brassica juncea) seeds were surface sterilized by immersion in 5% sodium hypochlorite for 15 min followed by repeated washing with sterile distilled water. Two grams of a sticker, gum arabic, was dissolved in 100 ml of the bacterial suspension (OD 1.5), stirred, and allowed to stand for 1 h. The bacterial sticker suspension was added at a rate of 0.1 ml g−1 of seeds in a plastic bag containing 100 seeds. The coated seeds were allowed to dry overnight. Seed germination was done on wet paper towels on plates. Control seeds were coated with water only. Germination index was calculated by the formula:
Control and treated seedlings (of 7 days old) were sowed in pots (5 seedlings per pot) containing soil compost mixture in triplicates. In another setup, untreated seeds were sowed but bacterial inoculum was injected (50 ml) in the rhizosphere soil. All the three experimental pots (in triplicates) were added with 150 ml of sterile nutrient solution (0.10 g l−1 MgSO4·7H2O; 0.14 g l−1 K2SO4; 0.74 g l−1 CaCl2·2H2O; 0.11 ml l−1 H3PO4 and 0.02 g l−1) and exposed in 14 h daylight condition. With regular intervals, plantlets were plucked and compared for growth parameters. Total N and P content in the 30 days old experimental plants/controls were determined. Plants (5 g) dried at 60 °C overnight and digested with a mixture of nitric acid and hydrogen peroxide followed by addition of hydrochloric acid. The digested samples were analysed for TKN (Total Kjeldahl Nitrogen) and phosphorous content (as PO4−) against standard concentration of each non metals as per standard protocol APHA 22nd edition 2012. For pot based assay, analysis of variance (ANOVA) with (p < 0.01) was used to compare means in all cases.
Results and Discussion
One Pseudomonas aeruginosa strain PGP was isolated from garbage soil of Sonarpur based on distinct zone formation in TCP containing NBRIP and Pikovskaya medium. The Gram negative strain was positive for citric acid production, catalase test, indole production test, methyl red, hydrogen sulphide production, starch hydrolysis, gelatin hydrolysis, triple sugar iron, urease test, H2S production and King’s base B test while it was negative for Voges–Proskauer and esterase activity. Figure 1a, b show the fluorescence of the strain in naked eye and under UV light, respectively. Figure 1c shows alpha haemolysis of the strain on human blood agar plate. This is a general feature of all Pseudomonas strains either pathogenic or non pathogenic. Ribotyping and BLAST search established the strain as Pseudomonas aeruginosa. The strain was assigned accession number KY317985.1 from GenBank. Figure 2 shows the phylogenetic tree of the isolate with nearest neighbors and other Pseudomonas strains.
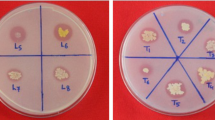
Figure 3a, b show the halo zones formed upon solubilization of TCP in Pikovskaya medium and NBRIP medium, respectfully.
Figure 4 shows the kinetics of phosphate solubilization and concomitant decrease of medium pH. Phosphate solubilizing efficiencies of the strain in NBRIP plate and PVK plates were 3.9 ± 0.9 and 4.1 ± 0.1 respectively. A close association was found between phosphate solubilization and growth rate which was an indicator of active metabolism of the strain. Maximum decrease of color intensity in NBRIP broth with bromophenol blue was 0.786 ± 0.2. This was calculated by subtracting OD660nm value of the control culture to the OD660nm value of the test culture. Production of various organic acids by the strain was further confirmed by cultivating the strain in MM9 agar media and observing the change of the color of methyl red from yellow to pink.
During the investigation on N fixation property, the strain showed visible growth and color change in all the N free solid agar media including Jensen medium, Norris media and LGI media. In the Jensen’s media and Norris media, a color change from pale green to blue qualitatively indicated the positive N2-fixing activity (Fig. 5a, b). Figure 5c, d show growth of the strain in LGI media with sucrose and mannitol. Figure 5e shows successive color change of LGI broth on different days on incubations. Figure 5f shows ammonia formed from the supernatant of culture in LGI media where strain was grown for 7 days. Amount of N fixed in the LGI medium by the strain PGP was found to be 3.057 ± 0.34 and 7.87 ± 0.34 mg l−1 after 30 and 45 days of incubations, respectively. Autolysis of cells during long incubation may have caused the increase in the amount of fixed N.
Qualitative assay of N fixation of Pseudomonas aeruginosa strain PGP showing growth in N free Norris Glucose media a after 7 days, b after 30 days, c growth in N free LGI media with sucrose, d growth in N free LGI media with mannitol, e growth in N free LGI broth on day 1, day 5, day 7, day 14 and day 20 showing successive changes of color from blue to pink, f ammonia detected after addition of Nessler’s reagent in the culture supernatant proving presence of N in the N free LGI media (color figure online)
The strain was tested positive for other PGP traits like IAA production, ammonia, siderophore and HCN production. Figure 3c, d show plate based images of HCN and siderophore productions respectively. Figure 6a, b relate cell growth with bacterial IAA production while Fig. 6c, d relate cell growth with HCN production. Figure 6d shows ammonia production. Production of maximum IAA (78 μg ml−1) and maximum HCN (256 mM) were detected on 3rd day of growth while maximum ammonia (0.380 μg ml−1) was detected on 7th day of incubation. Siderophore production was maximum (40% siderophore units) on day 5 after which it started to decline.
Quantification of bacterial metabolites that help in plant growth a bacterial growth kinetics during IAA production, b IAA production in the culture supernatant obtained from the culture attaining that OD, c bacterial growth kinetics during HCN production, d HCN production in the culture supernatant obtained from the culture attaining that OD, e shows the ammonia production
Indian mustard Brassica juncea was selected (easy to handle and grows rapidly) for pot based confirmative assay of plant growth promotion aspect of the bacterium. Bacteria coated seed treatment gave better PGP effect than bacteria injected directly into the rhizosphere. This may be due to better bacterial colonization. No difference was noted in the germination index of the seeds between treated and untreated ones and 100% germination was noticed on day 5. Figure 7 compares the lengths of the plants in the three types of treatments on 5th, 10th, 15th and 30th days of harvesting. Figure 8 shows growth parameters of the different treatments of the mustard plants on different days of sowing. At 30 days of sowing the seed treated plants showed increased wet weight (up to 140%), dry weight (up to 88%), root length (up to 25%) and stem length (up to 14%) over un-inoculated controls. The rhizosphere treated plants showed root length, shoot length and plant wet weight increase of up to 6.25, 4.9, and 28% respectively over uninoculated controls. N fixing ability of the strain was further proved as the N content of the bacteria coated and rhizosphere inoculated plants was 40.61 and 19.9% higher respectively than the controls. Similarly P content of the bacterized plants was 100% higher than the untreated ones.
Plant growth performance assay with and without microbial inoculation a differences in roots and shoots lengths of the mustards with the three different treatments and b differences of roots and shoots dry and wet weights. ANOVA revealed significant variations (p < 0.01) in root length and shoot length between groups, and fresh and dry weight between groups
Although few Pseudomonas are well recognized as human pathogens and cause variety of antibiotic resistant infections, most Pseudomonas isolated from close association of plants are beneficial to plants and exert antagonistic effect only against plant pathogens [20]. But still as antibiotic resistant Pseudomonas aeruginosa strains are well recognized as human pathogens, biosafety assessment of the strain was carried out by conducting antibiotic susceptibility test and detection of the gene phenazine. Normally, phenazine gene is present in human pathogenic forms [17, 18]. The positive control (human pathogen) showed positive PCR product but the test sample showed no amplicon. No amplification of the phenazine gene along with antibiotic susceptible nature of the isolate (large zone of inhibitions were found around all antibiotic discs tested) was reasonable enough to think non hazardous nature of this plant growth promoting Pseudomonas aerginosa strain PGP. Literature also supports the nonpathogenic nature of plant growth promoting fluorescent Pseudomonas species [20].
Recently, the ability to fix N has been recognized within the Pseudomonas genus sensu stricto [21, 22]. Specific strains of fluorescent Pseudomonas spp. have been isolated from different crop rhizosphere and have been shown to enhance different plants growth in pot and field studies [21, 22]. This study adds up one more strain in the ever expanding list of Pseudomonas with PGPR activities including N fixation. Among the PGP attributes studied, strain PGP produced 77 μg ml−1 of IAA, 257 mM HCN, 0.40 μg ml−1 of ammonia and 40% siderophore units. The mustard growth assay study has demonstrated that inoculation of mustard with Pseudomonas aeruginosa strain PGP significantly increased plant P and N uptake resulting in enhanced dry and wet biomass. However, further studies on pathogencity of this strain are required before recommending this isolate as bioinoculant in biofertilizer production.
Conclusion
Phosphate solubilization, N fixation and other plant growth promoting properties can unravel the opportunity of this non pathogenic plant growth promoting fluorescent Pseudomonas isolate for its use as bio-inoculant in biofertilizer formulations.
References
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorous and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Ladha JK, de Bruijn FJ, Malik KA (1997) Introduction: assessing opportunities for nitrogen fixation in rice-a frontier project. Plant Soil 124:1–10
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganism. FEMS Microbiol Let 170:265–270
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate solubilizing bacteria. Curr Microbiol 43:51–56
Garrity G (2005) The proteobacteria, part B the gammaproteobacteria. Bergey’s Manual of Systematic Bacteriology. Springer, New York, pp 323–379
Roychowdhury R, Mukherjee P, Roy M (2016) Identification of chromium resistant bacteria from dry fly ash sample of Mejia MTPS Thermal Power Plant, West Bengal, India. Bull Environ Contam Toxicol 96:210–216
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 28:2725–2729
Drummond L, Maher W (1995) Determination of phosphorous in aqueous solution via formation of the phosphoantimonylmolybdenum blue complex. Re-examination of optimum conditions for the analysis of phosphate. Anal Chim Acta 302:69–74
Döbereiner J (1988) Isolation and identification of root associated diazotrophs. Plant Soil 110:207
Baldani JI, Baldani VLD (2005) History on the biological nitrogen fixation research in graminaceous plants: special emphasis on the Brazilian experience. An Acad Bras Ci 77:549–579
Boddey RM, De Oliveira OC, Urquiaga S, Reis VM, De Olivares FL, Baldani VLD, Döbereiner J (1995) Biological nitrogen fixation associated with sugar cane and rice: contributions and prospects for improvement. Plant Soil 174:195–209
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and waste water. American Public Health Association, Washington, DC
Mukherjee P, Roychowdhury R, Roy M (2017) Phytoremediation potential of rhizobacterial isolates from Kans grass (Saccharum spontaneum) of fly ash ponds. Clean Technol Environ Policy 19:1373–1385
Brick JM, Bostock RM, Silverstone SE (1991) Rapid insituassay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol 57(535–538):20
Payne SM (1994) Detection, isolation, and characterization of siderophores. Meth Enzymol 235:329–344
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas sp. mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Cezairliyan B, Vinayavekhin N, Grenfell-Lee D, Yuen GJ, Saghatelian A, Ausubel FM (2013) Identification of Pseudomonas aeruginosa Phenazines that Kill Caenorhabditis elegans. PLoS Pathog 9:e1003101
Jamunadevi S, Balashanmugam P, Muralitharan G, Kalaichelvan PT (2012) Molecular characterization of pathogenic and non-pathogenic Pseudomonas aeruginosa with special reference to phenazine gene. J Mod Biotechnol 1:70–74
Zaidan MRS, Noor Rain A, Badrul AR, Adlin A, Norazah A, Zakiah I (2005) In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop Biomed 22:165–170
Jan AT, Azam M, Ali A, Haq Q (2011) Novel approaches of beneficial Pseudomonas in mitigation of plant diseases: an appraisal. J Plant Interact. 6:195205
Gopalakrishnan S, Srinivas V, Prakash B, Sathya A, Vijayabharathi R (2015) Plant growth-promoting traits of Pseudomonas geniculata isolated from chickpea nodules. Biotech 5:653–661
Yan Y, Yang J, Dou Y, Chen M, Ping S et al (2008) Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. PNAS 105:7564–7569
Acknowledgements
The authors acknowledge Techno India University for providing all the necessary facilities for carrying out the work. The authors are also grateful to Good Earth Enviro Care, Kolkata, West Bengal for carrying out N and P analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest among the authors.
Additional information
Significance Statement
The present study isolated one plant growth promoting rhizobacterium that in addition to general PGP properties showed dual possession of phosphate solubilisation and nitrogen fixation. This strain would be perfect for bioinoculum preparation due to its efficient plant growth promotion by increasing nutrient uptake.
Rights and permissions
About this article
Cite this article
Roychowdhury, R., Qaiser, T.F., Mukherjee, P. et al. Isolation and Characterization of a Pseudomonas aeruginosa Strain PGP for Plant Growth Promotion. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 353–360 (2019). https://doi.org/10.1007/s40011-017-0946-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-017-0946-9