Abstract
Kans grass (Saccharum spontaneum) is a weed species that is frequently found in many heavy metal-enriched waste dumps including fly ash pond sites. In this study, among a collection of phosphate-solubilizing bacterial strains isolated from the rhizosphere of Saccharum spontaneum present in the abandoned ash pond site of Mejia Thermal Power Station (MTPS-DVC), three strains were characterized for their plant growth-promoting abilities. The isolates identified as Bacillus anthracis strain MHR2, Staphylococcus sp. strain MHR3 and Bacillus sp. strain MHR4 had phosphate solubilization indices of 2.86, 2.31 and 2.40 and they produced soluble phosphates of 700, 600 and 640 mg l−1, respectively, in 4 days. In all the PSBs, pH significantly decreased, indicating the production of various organic acids. They showed other plant growth-promoting features like production of ammonia, siderophore, hydrocyanide and IAA. All of them were resistant to multiple heavy metals and antibiotics. Dry and fresh weight and shoot and root lengths of Brassica juncea L. increased in the presence of these isolates in pot cultures. The strains also increased phytoextraction ability of plants by enhancing the metal accumulation in plant tissues. Thus, the isolated indigenous and stress-adapted rhizobacteria may serve as potential biotechnological tool for the successful ecorestoration of various metal-contaminated sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saccharum spontaneum popularly known as ‘Wildcane’ or Kans grass is a native grass of the Indian subcontinent (Bhandari 1990; Pandey et al. 2015a). It is a tall perennial grass species with rhizomatous roots and grows up to 3 metres in height. Although it is a multifunctional species due to its ability to be used as biomass for ethanol and biogas production (Chandel et al. 2009), for a long time it has been neglected due to its bad reputation as weeds and its ability to quickly colonize croplands. But recently, S. spontaneum has attracted serious attention for its potential in ecological restoration and stabilization of various waste dumps like fly ash dumps, acid mine dumps and sewage sludge (Pandey et al. 2015b; Pandey and Singh 2014; Kumar et al. 2015). This research paper is centred about one such important dump site associated with all thermal power plants. Fly ash (FA) is a major coal combustion residue of thermal power stations and is composed of solid particles of ash, dust, soot and contains deadly heavy metals like lead, arsenic, cadmium, cobalt and mercury. Disposal of FA, as FA dump, is a serious environmental concern across the globe. Primarily, the FA is disposed of by either dry or wet disposal method. In the dry disposal, the FA is transported and disposed of by construction of a dry embankment (dyke). In the wet disposal method, the FA is mixed with water to form slurry. The slurry is disposed of in structures called ‘ash ponds’. Both of the ash dykes and ash ponds cause air, surface water and groundwater pollution (Mishra 2004). Air pollution is caused by emissions of hazardous gases from the power plants and windblown dust from the ash ponds. Rain or flooding can cause leaching of the toxic heavy metals from the ash dump sites and contaminate the underlying soil and groundwater reservoir. The ecological and human health risks associated with these dumps are indeed a large concern and demands a holistic approach for remediation (Borm 1997). Revegetation is one of the most widely sought after management strategies for the stabilization of waste dumps (Pandey and Singh 2014). As FA contains plant’s micro- and macro-nutrients, phytoremediation has been thought of as the easiest and cheapest way for the phytomanagement of FA basins (Haynes 2009). However, the revegetation process is a slow process because of the physical/chemical soil factors that restrict the establishment and growth of the plant communities. The limiting factors are an alkaline soil pH, high concentrations of soluble salts, a low content of humus, phytotoxic levels of some elements (e.g. B), deficiencies of others (e.g. N and P), multiple heavy metal stress and natural compaction and cemented layers of ash that restrict root growth (Haynes 2009). However, due to nature’s gift, some native tolerant plants are there that can overcome all of these barriers (Das et al. 2013). Inoculation of the plant roots with the rhizomicrobes may further facilitate the process of natural phytoattenuation of the degraded land (Kumari and Singh 2011). In nonagricultural conditions, the natural role of the plant growth-promoting rhizobacteria (PGPR), phosphate-solubilizing bacteria (PSB), mycorrhizal-helping bacteria (MHB) and arbuscular mycorrhizal fungi (AMF) are very important in restoring soil fertility than in conventional agriculture where the higher use of agrochemicals masks the contribution of the PSBs. Phosphate-solubilizing bacteria possess the ability to solubilize insoluble phosphates of soil into soluble forms by secreting various organic acids. By converting the plant unavailable phosphates into plant available forms, the PSBs stimulate plant nutrition and growth. In metalliferous soil, these PSBs play pivotal roles in reducing the metal stress on plants (Ahemad 2015; Kumar et al. 2009). Keeping the above environmental concerns in mind, this study was carried out to examine the phosphate solubilization and various plant growth-promoting activities of the rhizomicrobes present in the rhizosphere of S. spontaneum growing abundantly in the heavy metal-contaminated fly ash pond. Three strains were finally selected due to their good plant growth-promoting features. Acquisition of resistance to multiple heavy metals and antibiotics has made them suitable to survive in this heavy metal-contaminated environment. Moreover, their assistance to plants to uptake more heavy metal can make them ideal agents for application as bioinoculants in ecological restoration of vegetation in fly ash dump sites and minimize leaching of heavy metals to surroundings. This study also examined the ability of the isolated strains in enhancing the growth and lead accumulation ability of the Indian mustard plant Brassica juncea L. in pot culture. In spite of the all good PGPR traits in all the three strains, environmental application of one strain identified as Bacillus anthracis remains debatable. Although the strain was detected as nonpathogenic, the fear of relapse of virulence after environmental exposure would restrict its commercialization as a bioinoculant.
Materials and methods
Sample collection and isolation of phosphate-solubilizing bacteria (PSB)
The rhizosphere soil samples were collected around the roots of S. spontaneum present in the coal fly ash (FA) dump site of MTPS (Mejia Thermal Power Station) of DVC located in Bankura, Durlovpur, West Bengal, India. Pikovskaya’s (PKV) agar medium (glucose 10 g/l; tricalcium phosphate (TCP) 5 g/l; ammonium sulphate 0.5 g/l; sodium chloride 0.2 g/l; potassium chloride 0.2 g/l; magnesium sulphate 0.1 g/l; yeast extract 0.5 g/l; manganese sulphate trace; ferrous sulphate trace; agar 2%) was prepared, and pH was adjusted to 7.0 before sterilization (Pikovskaya 1948). 1gm of the rhizosphere sample was diluted in 10 ml of sterile distilled water and spread plated into PKV agar medium in various dilutions. The plates were incubated for 5–7 days in 30 °C. The bacterial colonies showing phosphate-solubilizing zones around them were considered as PSB. Pure cultures of the isolates were made by repeated subculturing for 2–3 times on fresh PKV plates and were maintained on PKV slants at 4 °C.
Identification of the bacterial strains
Identification of the strains was performed by morphological, biochemical, and molecular methods. Morphological identification was carried out by phase contrast microscopic view of the cells after Gram staining. Physiological and biochemical tests were carried out as per the methods outlined in Bergey’s Manual of Systemic Bacteriology (Krieg and Holt 1984). The 16S rDNA PCR was used to amplify 16S rRNA genes using primers f27 (5′-AGAGTTTGATCMTGGCTCAGTAC-3′) and r1492 (5′-GGYTACCTTGTTACGACTT-3′). The PCR mixture contained 1 ul template, 2.5 ul of 10× Taq DNA polymerase buffer, 5 mM MgCl2, 1 ul of dNTP at 2.5 mM, 0.5 ul of 2.5 unit Taq DNA polymerase, 3.75 pmol of each primer, and 0.5 ul of 2.5 unit Taq polymerase. The cycling conditions were, initial denaturation at 94 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 54 °C for 30 s and 72 °C for 1.5 min, followed by a final extension performed at 72 °C for 5 min. The PCR products were purified by the Quiagen gel extraction kit and sequenced by Sanger sequencing. The obtained gene sequences were compared with others in the GenBank databases using the NCBI BLAST at http://www.ncbi.n1m.nih.gov/blast/Blast.cgi. Sequences were submitted to NCBI GenBank database, and accession numbers were obtained. Phylogenetic tree was constructed by MEGA7 (Kumar et al. 2016). The evolutionary history was inferred using the Neighbor-Joining method of Saitou N and Nei M (1987). The bootstrap consensus tree inferred from 500 replicates is taken to represent the evolutionary history of the taxa analysed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) is shown next to the branches (Felsenstein 1985). The evolutionary distances were computed using the Kimura 2-parameter method (Kimura 1980 and are in the units of the number of base substitutions per site. The rate variation among sites was modelled with a gamma distribution (shape parameter = 1). The analysis involved 14 nucleotide sequences. All positions with less than 95% site coverage were eliminated. That is, fewer than 5% alignment gaps, missing data, and ambiguous bases were allowed at any position. There were a total of 407 positions in the final data set.
Measurements of phosphate solubilization
Bacterial isolates were screened for tricalcium phosphate (TCP)-solubilizing activity on PKV plates. The PSBs were spot inoculated on the agar plates aseptically and incubated at 30 °C for 7 days. A clear zone surrounding the colony indicated the phosphate solubilization and was measured as phosphate solubilization index (PSI). PSI was calculated as the ratio of the total diameter (colony + halo zone) to the colony diameter. All the observations were recorded in triplicates. For quantitative estimation of TCP solubilization, phospho-molybdate blue colour method (Murphy and Riley 1962) was used. Pikovskaya’s broth (100 ml) (adjusted to pH 7) prepared with sucrose and TCP (0.3 g/100 ml) was poured in 250-ml flasks. The flasks were sterilized, and exponentially growing cells were inoculated in them and placed on a rotary shaker for 5 days. At various time intervals, 5 ml of the culture broth was sucked up, centrifuged (10,000 rpm, for 15 min) and the supernatant was collected. The available phosphorous (P) was determined using spectrophotometer at 882 nm and calibrated with standard KH2PO4 curves. At each time, pH estimation of the culture supernatant was noted.
Screening for different plant growth-promoting activities
Detection and quantification of indole acetic acid (IAA) production in PSBs were done by the method of Loper and Scroth (1986) with modifications. The strains were inoculated in three test tubes containing 10 ml LB broth with l-Tryptophan (200 mg/L) and incubated at 30 °C. 5 ml of the bacterial culture from each tube was removed after 4 days of incubation and centrifuged at 10,000 rpm for 15 min at room temperature. For each PSB strain, 2 ml of supernatant was transferred to a fresh tube to which two drops of ortho-phosphoric acid and 4 ml of Salkowski’s reagent (l ml of 0.5 M FeC13 solution in 50 ml of 35% perchloric acid) was added. The mixture was incubated at room temperature for 30 and 120 min in the dark, and the intensity of the pink colour developed was recorded at 530 nm. A standard curve was prepared with pure IAA for quantification (Sarwart et al. 1992).
Ammonia production ability of the strains was tested by the method of Cappuccino and Sherman with modifications (1992). Overnight grown bacterial cultures were inoculated in 10 ml peptone broth and incubated at 30 °C for 48–72 h in a BOD shaker. After incubation, in each test tube 0.5 ml of Nessler’s reagent was added. Development of a yellow to dark brown colour indicated the production of ammonia. Quantification of ammonia production was done by Nesslerization spectrophotometric method. An overnight grown bacterial culture was inoculated in 10 ml peptone broth and incubated at 30 °C for 48–72 h with constant shaking. After that, 2 ml of the broth was taken in an eppendorf tube and centrifuged at 10,000 rpm for 5 min. Then, 40ul of Na–K-tartrate (25 g/50 ml) and 40ul of Nessler’s reagent were added sequentially to each tube containing the supernatant and the absorbance was measured at 450 nm. NH3–N standard curve was prepared for quantification. The concentration of the NH3 was calculated using the standard curve of ammonium sulphate solution in the range of 0.1–1 umol ml−1.
Hydrogen cyanide production was detected and measured by the method of Bakker and Schippers (1987). King’s B medium amended with 0.44% of l-Glycine was prepared. Whatman No. 1 filter paper strips were soaked in 0.5% picric acid and 2% Na2CO3 solution. Test tubes with the media were inoculated with 100 μL of the inoculum and incubated at 30 °C for 48 h. A change in colour of the filter paper from yellow to light brown or reddish brown colour indicated production of HCN. Amount of the total cyanide present in the filter paper was calculated using Eq. (1).
Siderophore production was assayed on the Chrome Azurol S agar (CAS) media as described by Clark and Bavoil (1994). CAS agar plates were prepared, spot inoculated with test organisms and incubated at 30 °C for 3 days. Developments of yellow–orange halo zones around the colonies were considered as positive for siderophore production. For quantification of siderophores, cells were grown in liquid CAS medium for 24 h. The supernatant was collected and 0.5 mL of cell-free culture was added to 0.5 mL of CAS reagent and the absorbance was measured at 630 nm against blank. Total siderophore amount was calculated using Eq. (2) and expressed as percent siderophore units:
where Ar = Absorbance of reference at 630 nm (CAS reagent); As = Absorbance of the sample at 630 nm.
Multiple heavy metals and antibiotic resistance ability of the isolates
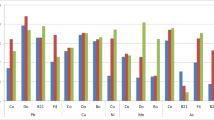
For determination of heavy metal tolerance assay of the strains towards arsenite (AsIII), cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), mercury (Hg), nickel (Ni), zinc (Zn), stock solutions of sodium arsenite, cadmium chloride, cupric sulphate, potassium chromate, lead acetate, mercuric chloride, nickel nitrate, zinc sulphate were prepared in distilled water. Sterilization was done by passing the solutions through 0.22-micron membrane filters. The MIC of the heavy metals for the three strains was determined by the plate dilution method of Aleem et al. (2003). The lowest concentration of the heavy metal that inhibited bacterial growth was considered as the MIC. Tubes containing 20 ml of melted LB agar and different concentrations of the metal were poured onto plates. Each plate was streaked with the three strains and incubated for 24–48 h. The experiment was conducted in triplicates.
Antibiotic resistance was tested using disc diffusion method. LB agar plate was spread plated with an aliquot of overnight grown bacterial culture. Filter paper discs containing different concentration of antibiotics, ampicillin 50 µg mL−1, chloramphenicol 30 µg mL−1, kanamycin 30 µg mL −1, rifampicin 5 µg mL−1, penicillin 10 µg mL−1, streptomycin 10 µg mL−1 and tetracycline 30 µg mL−1 were then placed on the plates. Plates were incubated at 28 °C. Growth was monitored, and the presence of inhibition zones around the discs was noted. Each test was replicated three times. Spots without antibiotics were kept as negative controls. The experiments were performed in triplicates.
Effect of the PGPR strains on growth and bioaccumulation of heavy metals in mustard seeds (Brassica juncea L.)
Indian mustard seeds of Brassica juncea (L.) Czern variety were used for seed coating with PSBs in pot assays. The pots (18 × 19 cm2) were filled up (2/3rd) with garden soil (autoclaved). The mustard seeds were first surface sterilized by immersing in 95% ethanol (30 s) and 0.2% mercury chloride (3 min). Traces of mercury chloride from the seeds were removed from the disinfected seeds by washing them 5 times with sterile distilled water. Surface-sterilized mustard seeds were soaked in 48-h old cultures of PSB isolates for two hours. One control pot was kept where uncoated seeds were sowed. Pots were kept in an area which gets ample sunlight and the temperature in the daytime varied from 25 to 30 °C and humidity varied from 70 to 50%. Hoagland nutrient solution where TCP is used as P source (Hoagland and Arnon 1950) and urea (0.32 g/200 ml) were applied to each pot after 3–4 days of sowing. Autoclaved water was used for watering daily. Three replicates were used for each treatment. Harvesting was made 45 days after inoculation and roots and shoot length and dry weight was measured. Root and shoot portions of the plants were first washed and then air-dried, and then, measurements were taken. For dry weights measurements, the shoot and root samples were oven-dried at 70 °C and the mean weight of the plants was expressed as g plant−1. In another set of experiment, lead was added in a concentration of 200 mg/kg with the soil in the pots for understanding the role of the heavy metal-resistant PSBs in the phytoextraction of metals by the plants. TCP was not added to avoid precipitation of lead in the presence of phosphate.
For determination of Pb accumulation in the shoots or roots, plants were harvested after a period of 45 days. They were washed in cold 0.2 mM CaSO4 twice and then rinsed with cold distilled water. Samples were oven-dried at 72 °C for 48 h. For Pb analysis, 200 mg of dried tissue was digested with HNO3 + HClO4 by the method of Temminghoff and Houba (2004). Inductively Coupled Plasma Atomic Emission Spectrophotometer (ICP-AES) was used for lead determination. Three independent replicates were taken per sample.
Statistical analysis
For statistical analysis, analysis of variance (ANOVA) and post hoc Fisher’s LSD test (p < 0.05) were used to compare treatment means.
Results and discussion
Isolation of phosphate-solubilizing bacteria
Many (15–20) bacterial isolates were found, showing phosphate-solubilizing zones on Pikovskaya’s agar after 7 days of incubation. The isolates showed development of distinct phosphate solubilization zones, ranging from 10 mm to 20 mm. Three isolates showing sharp phosphate solubilization zones of 20, 15 and 18 mm, respectively, and displaying other PGPR properties were further chosen for quantification of phosphate solubilization.
Identification of the PGPR strains
The strains were identified based on morphological, biochemical and molecular methods. The accession numbers obtained from gene bank against the three strains were KT238975.1, KT238976.1 and KT238977.1, and they correspond for Bacillus anthracis strain MHR2, Staphylococcus sp. strain MHR3 and Bacillus sp. strain MHR4, respectively. The phylogenetic tree is shown in Fig. 1.
Measurement of phosphate solubilization
The halo zone diameters of Bacillus anthracis MHR2, Staphylococcus sp. strain MHR3 and Bacillus sp. strain MHR4 were 1.3, 0.85 and 1.05, respectively, against colony diameter zones of 0.70, 0.65 and 0.75. Their phosphate solubilization index (PSI) values were calculated to be 2.86, 2.31 and 2.40, respectively.
The solubilization of TCP in the liquid medium by the 3 different strains was followed by a drop in pH from an initial neutral pH (7.0) to acidic pH (between 4.0 and 4.66) after 5 days of incubation. In the blank treatment (control), no soluble P was detected and no drop in pH was observed while in the test cultures, the pH dropped significantly. On the fourth day of incubation, the maximum P solubilization was recorded by Bacillus anthracis sp. strain MHR2 (700 mg l−1) with a maximum drop in the pH to 4.0 followed by Bacillus sp. strain MHR4 (640 mg l−1) with a pH drop of 4.5. Among the isolates, the minimum concentration of soluble P (600 mg l−1) was observed in the culture of Staphylococcus sp. strain MHR3 and the minimum drop of pH of the medium was 4.86 on the fourth day of incubation. Although its pH further dropped to 4.7, this was not followed by further P solubilization. Bacillus sp. strain MHR4 which showed a minimum drop of pH to 4.5 on the fourth day of incubation showed highest P solubilization on its third day (660 mg l−1) with a medium pH value of 5.6. Figure 2 shows their phosphate solubilization efficiency (shown as concentration of free phosphate), growth curves and the concomitant decrease of pH in the PKV medium. Figure 4a shows the P solubilization zones of the 3 strains.
Phosphate solubilization efficiency of the three PSBs: Increase in soluble phosphate is associated with decrease in medium pH. 1A shows the concomitant decrease of pH in the PKV medium and the growth curves of the isolates. 1B shows the production of soluble P. Solid lines in (1A) stand for pH reduction, and broken lines stand for their O.D 660 values. Values are the means (n = 3) ± standard deviation
Plant growth-promoting activities
During measurement of IAA production, colour development became visible within minutes and continued to increase in intensity for a period of 30 min in all the three strains tested. In Bacillus anthracis sp. strain MHR2, the increase continued up to 120 min. Hence, the optical density was measured after 30 and 120 min. For the other 2 strains, no further significant increase occurred after 45 min. Figure 3a shows the IAA production by all the three PSBs.
Quantitative analysis of IAA, ammonia and siderophore production by the three strains. a shows indole acetic acid production, b shows ammonia production in peptone water using Nesslerization reaction and c shows siderophore production (%) by the three strains. Values are the means (n = 3) ± standard deviation
Production of ammonia was found to be weak in MHR2, but the other two strains MHR3 and MHR4 were good producers of ammonia. Figure 3b depicts the ammonia production by the selected strains at 48 and 72 h of incubation.
All the strains were positive for hydrogen cyanide production. Highest production of HCN was achieved by Staphylococcus sp. strain MHR3 and was recorded as 35.2 ppm while MHR2 produced 19.5 ppm and lowest production of 10.6 ppm was recorded by MHR4. Figure 4c shows pictures of HCN production in the plates.
All the three tested strains were good siderophore producers and all showed large orange halos around the colonies on CAS agar. As depicted in Fig. 3c, siderophore formation increased with increase in the incubation period. Highest zone was achieved by Bacillus anthracis sp. strain MHR2 on 72 h of incubation. It produced an orange halo zone of 2 cm (excluding colony diameter of 0.9 cm) on 72 h of incubation. Figure 4b shows pictures of siderophore formation on the agar plates.
Minimum inhibitory concentration (MIC) of heavy metals and antibiotic resistance of the isolates
All the bacterial strains showed different degrees of resistance towards the different heavy metals and antibiotics. The MIC of the heavy metals as detected by the plate-based assays is presented in Fig. 5. Strain MHR2 exhibited antibiotic resistance characteristics to kanamycin, rifampicin and streptomycin while MHR3 was resistant to ampicillin, and MHR4 was resistant to tetracycline and rifampicin.
Effects of PSBs on mustard seeds germination and growth and lead accumulation
All the three isolates showed a significant increase in shoot and root dry and fresh weights of mustard as compared to uninoculated control (Table 1). In comparison with the control plants, plant shoot and root length, plant fresh and dry weight increased in the PSB-treated plants. In case of single inoculations, best growth performances were recorded from the plants inoculated with Bacillus anthracis sp. strain MHR2. In comparison with single inoculations, mixed inoculation of the plants with the three strains together (consortia) gave better results.
The three PSBs acting in a consortium or individually also enhanced accumulation of lead in the Indian mustard plants. The treated plants showed higher accumulation of lead in comparison with the untreated ones (Table 2). Lead accumulation was significantly higher in the roots compared to shoots in both treated and untreated plants. Pb translocation from shoot to root was calculated by Eq. (3):
Here, Cshoot and Croot stand for metal concentration in the shoot (mg kg−1) and root of plant (mg kg−1), respectively. TF < 1 represents that translocation of metals from shoot to root was not made effectively. Bioaccumulation factor of Pb was further calculated by Eq. (4) (Ma et al. 2001):
Cshoot and Csoil are lead concentration in the plant shoot (mg kg−1) and soil (mg kg−1), respectively.
It was noted that plant shoot and root lengths (and fresh and dry weights) decreased in the lead-treated control plants than the control plants from soil without lead (data not shown). However, in the PSB-treated plants from soil with lead, the decreases of plant root/shoot lengths or their dry/wet weights were not prominent. So, it can be assumed that the special plant growth beneficial traits of the metal-resistant PSB may have resisted the damaging effects of the toxic metal on the plants by producing iron chelators, siderophores, organic acids, etc.
Discussion
Ecological restoration of abandoned fly ash ponds and dykes has become important parts of the sustainable development strategy in many countries including India. Green capping is one of the most popular methods to re-vegetate abandoned ash ponds. It has many advantages such as prevention of soil erosion, reduction of dust emission by stabilization of the surface areas of ash dump sites and prevention of potential ground water contamination by minimizing leaching of heavy metals. Finally, addition of a native vegetation cover is aesthetic in nature and profitable in the long term. PGPRs have been proposed to play a great role in the formation of vegetation cover by colonizing roots of plants and promoting plant health through a variety of mechanisms like the production of phytohormones, suppression of pathogens, phosphate solubilization and enhancing nutrient uptake (Khan et al. 2007). Among the plant growth-promoting bacteria, PSB improves plant growth by introduction of various enzymes, organic acids, siderophores, plant hormones and other biotechnological products into soil–plant interface. PSB produces various organic acids like monocarboxylic, monocarboxylic hydroxy, monocarboxylic ketogluconic, dicarboxylic, dicarboxylic hydroxy and tricarboxylic hydroxy acids to solubilize inorganic phosphate compounds (Chen et al. 2006). The acids produced by them help in the mobilization of the insoluble and biounavailable heavy metals to plant available forms. Several PSB exhibiting both heavy metal detoxification characters and plant growth-promoting features have been explored and have been implicated in phytoremediation of metalliferous soils (He et al. 2013; Misra et al. 2012). In the present study, we screened for beneficial bacteria present in the rhizosphere of Saccharum spontaneum which is normally found in different types of serpentine soils. So far no studies have been explored in isolation of such heavy metal mobilizing PSBs from the FA-contaminated environment. Few reports are present on the ability of this underutilized weed for ecorestoration of bare FA dump sites and encouraging revegetation in those bare lands (Pandey et al. 2015b). Pandey et al. (2015a, b) first time reported the changes in physicochemical properties of the barren FA dump sites following revegetation by the studied plant. In the present study, we wanted to investigate whether the rhizobiome of the plant plays any supporting role in the ecorestoration process by the plant. We found that the rhizobacteria isolated from S. spontaneum caused a decrease in the pH of the culture medium indicating production of multiple organic acids. Many reports are present on the ability of bacteria from serpentine soil to be able to solubilize ‘unavailable’ forms of heavy metal-bearing minerals by excreting organic acids. The acids may have role in phosphate solubilization, heavy metal mobilization and plant growth promotion. Our isolated PSBs are ideal plant growth-promoting rhizomicrobes as all of them are positive for IAA, ammonia, HCN and siderophore production. IAA is an important phytohormone and acts as an important signal molecule in the control of overall plant growth and plant microbe interaction. Ammonia is an inorganic volatile component that indirectly influences plant development and has been implicated as a weapon of biocontrol (Howell et al. 1988). HCN production by the rhizobacteria has been postulated to play an important role in the biocontrol of pathogens. As we all know iron is a vital nutrient for plants. But most of the iron present in soil occurs as insoluble hydroxides and oxyhydroxides and is inaccessible to plants and microorganisms. Certain bacteria can acquire this iron by secretion of low molecular weight iron chelators called siderophores which can form strong association with the metals for complexing iron. While doing so, siderophore helps in the sequestration of different heavy metals also and thus protect the plants from the damaging effects of the heavy metals (Rajkumar et al. 2010). All our isolates are resistant to multiple heavy metals and antibiotics. Many PGPRs isolated from different plants growing in heavy metal-enriched environment have been shown to be resistant to heavy metal (Jiang et al. 2008; Rajkumar et al. 2005). To survive under metal stress conditions, PGPRs have evolved several mechanisms like precipitation of metal as insoluble salts, accumulation and sequestration of the metal ions inside cell, biotransformation of toxic metal to less toxic forms to tolerate the uptake of heavy metal ions. For observing the effects of our isolated PGPRs on growth promotion of other plants, we selected Brassica sp. as there are many reports of these plants to tolerate multiple heavy metals and even they have shown good growth in fly ash amended soil (Kumar et al. 2008). In our pot culture-based experiment, we observed improved growth performance of the Indian mustard seedlings in the presence of the isolates than the control untreated plants. Lead was added with normal soil to see the whether the PGPR could enhance phytoabsorbtion of lead by the plants. More heavy metal accumulation was observed in the roots and shoots of the plant in the presence of the isolates than the control group. In a similar type of experiment, Wu et al. (2006) also observed enhanced phytoextraction of heavy metals by B. juncea inoculated with rhizobacteria isolated from plants of a Pb–Zn mine tailing.
Among the three strains studied, highest phosphate solubilization was found by MHR2. It also showed highest IAA production (27 mg l−1) where the other two strains produced almost similar amount of IAA (~20 mg l−1) (Fig. 3a). This result is in good correlation with the value shown in Table 2 concerning the efficiency of PGPR on plant growth promotion. It is also in good agreement with result from Table 2 showing lead accumulation by the strains. Higher IAA production by that strain MHR2 resulted in higher fresh and dry biomass of B. juncea suggesting that there was a significant relationship between the plant growth-promoting potential of PGPR strains and their level of IAA production. The results of this study match with the result of Gravel et al. (2007) who also observed a significant relation between plant growth-promoting activity of PGPR and level of IAA production. The strains showed a cumulative effect on plant growth as plant growth rate was highest when the strains were applied as consortia. In addition, this study also shows that increased phosphate solubilization by PGPR can play an important role in metal uptake by plant in metal-contaminated soils (Zaidi et al. 2006; Ma et al. 2009). Varying level of metal tolerance has been reported from other PGPRs also. Many of them also showed antibiotic resistance like our strains (Jiang et al. 2008; Rajkumar et al. 2005). Increased use of antibiotics in healthcare and agriculture has aggravated transfer of the antibiotic-resistant genes through horizontal gene transfer. It has been proposed that under environmental conditions of metal stress, metal and antibiotic-resistant bacteria adapt faster by the spread of plasmid-based R-factors than by mutation and natural selection (Silver and Misra 1988). The multiple antibiotic resistance property of the strains would make them stronger for establishing themselves in any introduced soil. In natural environments also, these isolates would gain better chance of survival having higher competence. A large body of evidence suggests that PGPR enhances the growth, seed emergence and crop yield, and contribute to the protection of plants against certain pathogens and pests (Beneduzi et al. 2012). Due to the possession of these multifarious properties, these strains have the potential to be used as bioinoculant to promote growth of a phytocover in the fly ash dumping grounds and other mine tailings and promote phytoextraction of heavy metals and minimize leaching of heavy metals in the surroundings and thus would be helpful in the phytorestoration of the degraded landscape. On the other hand, commercialization of the PGPR strains containing antibiotic-resistant genes needs detailed understanding on the mechanism of resistance of the strains towards the heavy metals and antibiotics. If the resistance genes for the antibiotics and biocides/metals are located physically on the same plasmid, metal/biocide exposure can also promote horizontal gene transfer of antibiotic resistance. The number of infections caused by antibiotic-resistant bacteria is rising worldwide. So precautions need to be taken to minimize mobilization of diverse range of antibiotic resistance genes present in the environmental microbial community into pathogenic bacteria via HGT (Pal et al. 2015). Although not much conclusive experiments are present on HGT between heavy metal-resistant PGPRs and pathogenic bacteria, more studies are essential before their environmental release. Pal et al. (2015) first carried out large-scale identification of bacterial taxa, metals and environments of particular concern for co-selection of resistance against antibiotics, biocides and metals. According to them, plasmids provide limited opportunities for biocides and metals to promote HGT of antibiotic resistance through co-selection, whereas ample possibilities exist for indirect selection via chromosomal biocide/metal resistance genes.
Among the three isolates in this study, B. anthracis strain MHR2 needs special discussion due to renowned popularity of Bacillus anthracis as the only causative agent of the deadly disease anthrax. Being serious and often fatal, this disease mostly affects wild and domestic herbivores (zoonotic disease) due to its highly resistant long lasting spores. Ecology-related studies reveal the existence of vegetative spores of this obligatory pathogen in soil having high pH > 6.1 and high calcium levels. Anthrax spores are best survived in black soil rich in nutrient organic matter, ambient moisture and temperatures above 15 °C (Hugh-Jones and Blackburn 2009). Recent researchers have, however, revealed that due to very specific nutrient and temperature requirements it is unlikely for anthrax bacilli spores to grow outside the host body. Experimental evidences have reported poor growth of vegetative spores outside host tissue, due to specific physiological and nutrient requirements. The vegetative spores face antagonism from other bacterial strains thereby reducing their efficiency to survive in environmental soil samples deficient of required nutrients. This leads to a decline in the viability of the spores, and it becomes difficult for B. anthracis to cause anthrax even when favourable conditions return (Turnbull 1991, 1992). Early bacteriological history dated back in 1940s to 1960 (Buchanan et al. 1966) evaluated seven specific strains having similarities with B. anthracis namely B. anthracis similis, B. anthracis simulans, B. anthracis symptomatici, B. anthracoides, B. anthraciformis and B. pseud(o)anthracis, which although resemble B. anthracis in vitro, but have failed to induce anthrax in laboratory animals. Bacillus anthracis contains 3 main virulence factors which are coded on 2 plasmids, pXO1 and pXO2. Absence of any one of these factors results in the formation of an avirulent type, unable to cause virulence. Sterne (1939, 1959) showed a specific environmental strain named as the Sterne strain which was not virulent and did not contained virulence plasmids and capsule. The Sterne strain had naturally lost its ability to produce a capsule, or a layer of polysaccharides, which protects it from being consumed and destroyed by our defensive immune system cells. This strain sometimes finds importance in microbiology laboratories for testing the ability and accurately identifying and diagnosing anthrax, and also a source for anthrax research. This being a predominant strain has been used for immunization of domesticated animals against anthrax worldwide since many decades. The Sterne strain lacks pXO2 plasmid and may regain it becoming capsulated from gene transfer of other strains, however, since its discovery in 1930 no case of virulence has been reported to cause anthrax neither in animals nor in humans (Sterne 1939, 1959).
The research work conducted by Mukherjee et al. has found three potential strains including Staphylococcus, Bacillus anthracis and Bacillus species capable for use in phytoremediation. Although the three strains have shown equally commendable results in various PGPR tests conducted, MHR2 results surpass the other two MHR3 and MHR4 in terms of IAA production and phosphate solubilization. Commercialization of MHR2 and MHR3 would pose no problem as these strains do not find evidence of being reported as environmentally unsafe or have reported evidence of pathogenicity due to field applications. The use of Bacillus species (Bai et al. 2002) and Staphylococcus species (Ipek et al. 2014) has earlier been studied and reported for their phosphate-solubilizing ability (Wani and Khan 2010) and plant growth-promoting ability (Kumar et al. 2012). But commercialization of B. anthracis would not be possible due to the report of horizontal gene transfer among virulent B. anthracis strains in field (Saile and Koehler 2006). Unfortunately, though the studies conducted for the strain MHR2 did not show sign of pathogenicity under laboratory environment, the question of mutation in this particular species or reoccurrence of its virulence remains questionable. It has also been found that relapse of virulence in the Sterne strain has not occurred during its course of existence since 1930. Keim et al. (1997), have emphasized on the extremely conservative nature of B. anthracis as only 3% of its genome has any changes. Although at this point the authors cannot confirm that whether this Bacillus anthracis strain MHR2 is a type of Sterne strain or one of the seven nonpathogenic varieties of B. anthracis identified by Buchanan et al. (1966), further experiments would be conducted to identify the strain by DNA hybridization study (Turnbull 1999). Our finding of nonvirulent strain of B. anthracis supports the observation of Turnbull et al. (1992) that not all Bacillus anthracis can cause anthrax. According to him, the nonvirulent B. anthracis isolates lacking capsule or toxin producing genes exist in the environment. According to Turnbull et al. (1992, 1999), these types of strains may contain high spore counts initially but do not support the perception that when distributed environmentally they will be a source of anthrax, keeping in mind its enriched requirement to flourish as a pathogen. But still as scarce work has been done on acquisition of virulence by a nonvirulent anthrax strain, we would not recommend environmental exposure of the strain right now without undergoing further experiments. Horizontal gene transfer has been noticed in pathogenic B. anthracis strain in the rhizosphere of grass species (Saile and Koehler 2006). Although we have not found any previous report of nonvirulent strain of B. anthracis as PGPR, evidences are accumulating that like many other bacteria, B. anthracis also can interact with plants and even they promote plant growth to enhance its rate of transmission among grazing hervivors (Ganz et al. 2014). Ganz et al. (2014) have shown that B. anthracis increased the rate of establishment of Enneapogon desvauxii (native grass) by 50%. Moreover, the interaction of the strain with its host enhanced soil community composition also. However, this is the first report of occurrence of an avirulent strain of B. anthracis as PGPR in soil rhizosphere of a weed.
Conclusions
The present study concludes that S. spontaneum which is one of the most abundantly colonized grasses in the ash pond houses many phosphate-solubilizing bacteria in its rhizosphere. As we have identified three different bacteria capable of plant growth promotion as well as source of phytoremediation of contaminated soil, we go by the fact that all the three strains MHR2, MHR3 and MHR4 are potential candidate for phytoremediation. However, among the three strains only MHR3 and MHR4 would be recommended for field-based application by the authors. Considering the risks of environmental dissemination of antibiotic-resistant genes MHR3 and MHR4 would serve as better candidates than MHR2 as they carry less number of antibiotic-resistant genes. Although not a pathogen, the avirulent strain Bacillus anthracis MHR2 cannot be recommended for field-based application due to the fear of debateable relapse of virulence.
References
Ahemad M (2015) Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: a review. 3. Biotech 5:111–121
Aleem A, Isar J, Malik A (2003) Impact of long-term application of industrial wastewater on the emergence of resistance traits in Azotobacter chroococcum isolated from rhizosphere soil. Bioresour Technol 8:7–13
Bai Y, D’Aoust F, Smith DL, Driscoll BT (2002) Isolation of plant-growth-promoting Bacillus strains from soybean root nodules. Can J Microbiol 48(3):230–238
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas SPP-mediated plant growth-stimulation. Soil Biol Biochem 19:451–457
Beneduzi A, Ambrosini A, Passaglia Luciane MP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol. 35(4):1044–1051
Bhandari MM (1990) Flora of the Indian desert. Pbl. MPS Repros, Jodhpur, pp 390–391
Borm PJA (1997) Toxicity and occupational health hazards of Coal Fly Ash (CFA): a review of data and comparison to coal mine dust. Ann Occup Hyg 6:6590–6676
Buchanan RE, Holt JG, Lessel EF (1966) Index Bergeyana. E&S Livingstone Ltd, Edinburgh
Cappuccino JC, Sherman N (1992) In: Microbiology: A Laboratory Manual, 3rd edn. Benjamin/cummings Pub. Co., New York, pp 125–179
Chandel AK, Narasu ML, Chandrasekhar G, Manikyam A, Rao LV (2009) Use of Saccharum spontaneum (wild sugarcane) as biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae VS3. Bioresour Techno 100:2404–2410
Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34:33–41
Clark VL, Bavoil PM (1994) Methods in Enzymology 235(A). Academic Press, London, pp 315–372
Das M, Agarwal P, Singh R, Adholeya A (2013) A study of abandoned ash ponds reclaimed through green cover development. Int JPhytoremediation 15:320–329
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Ganz HH, Turner WC, Brodie EL, Kusters M, Shi Y, Sibanda H, Torok T, Getz WM (2014) Interactions between Bacillus anthracis and Plants May Promote Anthrax Transmission. PLoS Negl Trop Dis 8(6):e2903. doi:10.1371/journal.pntd.0002903
Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 39:1968–1977
Haynes RJ (2009) Reclamation and revegetation of fly ash disposal sites-challenges and research needs. J Environ Manage 90:43–53
He H, Ye Z, Yang D, Yan J, Xiao L, Zhong T, Yuan M, Cai X, Fang Z, Jing Y (2013) Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb. Zn uptake by Brassica juncea. Chemosphere 90:1960–1965
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Circular Calif Agricul Exper Stat Circ 347:1–32
Howell CR, Beier RC, Stipanovic RD (1988) Production of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium pre-emergence damping-off by the bacterium. Phytopathology 78:1075–1078
Hugh-Jones M, Blackburn J (2009) The ecology of Bacillus anthracis. Mol Aspects Med 30(6):356–367
Ipek M, Pirlak L, Esitken A, FigenDönmez M, Turan M, Sahin F (2014) Plant Growth-Promoting Rhizobacteria (PGPR) increase yield, growth and nutrition of strawberry under high-calcareous soil conditions. J Plant Nutr 37(7):990–1001
Jiang C, Sheng X, Qian M, Wang Q (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72(2):157–164
Keim P, Kalif A, Schupp J, Hill K, Travis SE, Richmond K, Adair DM, Hugh-Jones M, Kuske C, Jackson P (1997) Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. J Bacteriol 179(3):818–824
Khan MS, Zaidi A, Wani PA (2007) Role of phosphate solubilizing microorganisms in sustainable agriculture—a review. Agron Sustain Dev 27:29–43
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120
Krieg NR, Holt JG (1984) Bergy’s manual of determinative bacteriology, vol 1. Baltimore, The Williams and Wilkins Co
Kumar KV, Singh N, Behl HM, Srivastava S (2008) Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere 72:678–683
Kumar KV, Srivastava S, Singh N, Behl HM (2009) Role of metal resistant plant growth promoting bacteria in ameliorating fly ash to the growth of Brassica juncea. J Hazard Mater 170:51–57
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167(8):493–499
Kumar A, Ahirwal J, Maiti SK, Das R (2015) An Assessment of Metal in flyAsh and Their Translocation and Bioaccumulation in Perennial Grasses Growing at the Reclaimed Opencast Mines. Int J Environ Res 9:1089–1096
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kumari B, Singh S (2011) Phytoremediation of metals from fly ash through bacterial augmentation. Ecotoxicology 20:166–176
Loper JE, Scroth MN (1986) Influence of bacterial sources on indole-3 acetic acid on root elongation of sugarbeet. Phytopathology 76:386–389
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kenelly ED (2001) A fern that hyper accumulates arsenic. Nature 409:579–582
Ma Y, Rajkumar M, Freitas H (2009) Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. HAZMAT-9242
Mishra UC (2004) Environmental impact of coal industry and thermal power plants in India. J Environ Radioact 72:35–40
Misra N, Gupta G, Jha PN (2012) Assessment of mineral phosphate solubilizing properties and molecular characterization of zinc tolerant bacteria. J Basic Microbiol 52:549–558
Murphy J, Riley JR (1962) A modified solution method for determination of phosphate in natural water. Anal Chim Acta 27:31–36
Pal C, Bengtsson-Palme J, Kristiansson E, Larsson DGJ (2015) Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genom 16:964
Pandey VC, Singh N (2014) Fast green capping on coal fly ash basins through ecological engineering. Ecol Eng 73:671–675
Pandey VC, Prakash P, Bajpai O, Kumar A, Singh N (2015a) Phytodiversity on fly ash deposits: evaluation of naturally colonized species for sustainable phytorestoration. Environ Sci Pollut R 22:2776
Pandey VC, Bajpai O, Pandey DN, Singh N (2015b) Saccharum spontaneum: an underutilized tall grass for revegetation and restoration programs. Genet Resour Crop Ev. 62:443–450
Pikovskaya RI (1948) Mobilization of phosphorous in soil in the connection with vital activity of some microbial species. Mikorobiologiya 17:362–370
Rajkumar M, Nagendran R, Lee KJ, Lee WH (2005) Characterization of a novel Cr6 + reducing Pseudomonas sp. with plant growth-promoting potential. Curr Microbiol 50:266–271
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149
Saile E, Koehler TM (2006) Bacillus anthracis multiplication, persistence, and genetic exchange in the rhizosphere of grass plants. Appl Environ Microbiol 72(5):3168–3174
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sarwart M, Arshad M, Matens DA, Frankenberger WT (1992) Tryptophan-dependent biosynthesis of auxins in soil. Plant Soil 147:207–215
Silver S, Misra TK (1988) Plasmid-mediated heavy metal resistance. Annu Rev Microbiol 42:717–743
Sterne M (1939) The use of anthrax vaccines prepared from avirulent (uncapsulated) variants of Bacillus anthracis. Onderstepoort J. Vet. Sci. Anim. Industry 13:307–312
Sterne M (1959) “Anthrax,” in Infectious Diseases of Animals Vol. 1 eds Stableforth A W, Galloway IA, editors. (London: Butterworths;): 16–52
Temminghoff EJM, Houba VJG (2004) Digestion with HNO3-H2O2-HF. In: Temminghoff EJM, Houba VJG (eds) Plant Analysis Procedures, 2nd edn. Kluwer, Dordrecht, pp 16–19
Turnbull PC (1991) Anthrax vaccines: past, present and future. Vaccine 9(8):533–539
Turnbull PCB (1999) Definitive identification of Bacillus anthracis—a review. J Appl Microbiol 87:237–240
Turnbull PCB, Hutson RA, Ward MJ, Jones MN, Quinn CP et al (1992) Bacillus anthracis but not always anthrax. J Appl Bacteriol 72(1):21–28
Wani PA, Khan MS (2010) Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol 48(11):3262–3267
Wu SC, Cheung KC, Luo YM, Wong MH (2006) Effects of inoculation of plant growth-promoting rhizobacteria on metal uptake by Brassica juncea. Environ Pollut 140(1):124–135
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64:991–997
Acknowledgements
We acknowledge our host institute Techno India University for funding this research work. We are also grateful to DVC-MTPS authority and particularly to Mrs Kalyani Pyne for providing us the fly ash sample.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, P., Roychowdhury, R. & Roy, M. Phytoremediation potential of rhizobacterial isolates from Kans grass (Saccharum spontaneum) of fly ash ponds. Clean Techn Environ Policy 19, 1373–1385 (2017). https://doi.org/10.1007/s10098-017-1336-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-017-1336-y