Abstract
Assessment of effect of nanoparticles on plant growth is essential before adopting nanotechnology in agricultural sector. Four types of metal/metal oxide nanoparticles (NPs) viz. Zinc oxide (ZnO), Titanium oxide (TiO2), Copper oxide (CuO) and Silver (Ag) were studied for their effect on seed germination, vigour and yield in fodder crops, oat and berseem. Nanoparticles were synthesized and characterized before seed treatment. Seeds were treated with NPs at 750 mg (D1), 1000 mg (D2) and 1250 mg/kg of seed (D3). The effect of nanoparticles on seed germination and vigour was studied in the laboratory and seedling emergence rate, tiller number and seed yield were studied in the field. Nanoparticles (except TiO2 and CuO in berseem) enhanced germination at lower dose (D1), but reduction in root and shoot length was noticed at higher doses (D2 and D3). All four types of nanoparticles (NPs) in oat and only Ag in case of berseem enhanced germination to 100 % at lowest dose (D1). Substantial changes were noticed in field observations due to nanoparticle treatments regarding seedling emergence rate, tiller number and seed yield. Among the different NPs, TiO2 produced maximum seed yield at highest dose. No significant effect of nanoparticles on soil microbial populations was noticed during the field study. The experiment confirmed the dose-specific effect of nanoparticles on seed germination, crop growth and seed yield in oat and berseem crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology has opened up several applications in medicine, pharmacology and allied industries [1]. Nanomaterials are being widely used in several commercial applications such as fillers, semiconductors, drug carriers, electronics, cosmetics and catalysts [2]. Of late, applied studies in agriculture field particularly in crop protection, crop production and seed treatment have started emerging [3]. Nanoparticle literally ranges from 1 to 100 nm and due to this small size it acquires a wide array of advanced physical, chemical and electrical properties as compared to their bulk materials leading to some unprecedented advantages. Different studies showed both positive and negative effects of nanoparticles on plants, animals and microbes [4, 5].
Several promising results have been reported in the enhancement of seed germination, vigour and yield in wide array of crops due to nanoparticle application [6–8]. The nanoparticles of key elements/metals viz., SiO2, TiO2, ZnO were studied extensively for seed treatment purpose. Multi-cut forage crops like berseem, lucerne, sorghum, oat etc. need initial vigour and good germination to have more biomass during first cut. The restricted usage of herbicides in forages due to their utilization as animal feed has led to severe weed competition. The high initial vigour, foliage growth and good germination in thickly sown crop help in suppressing the weeds and enhancing the biomass.
Several reports showcasing the positive results in germination and plant growth [6–8] due to nanoparticle treatment and the inhibitory role of them have been reported [4, 5]. The base element of nanoparticle and choice of crop play an important role in deciding their effects. Similarly the dosage is also a crucial factor in deciding the toxicity of nanopartilces [4]. Nanosized TiO2 showed positive results in germination of spinach seeds [9] whereas, treatment with aluminium nanoparticles led to root inhibition in corn, soybean, cabbage and carrot. Lee et al. [5] found the negative relationship between concentration of nanoparticles and seedling length in Phaseolus radiatus and Triticum aestivum. The basic mode of impacting plant growth by NPs is obviously a matter of question and many researchers are trying to see it in the light of water penetration into seed, activation of antioxidant system, photochemical reaction of chloroplasts, chloroplast aging, nitrogen photoreduction and photosynthetic carbon reaction [10–13]. The contrasting results along with specificity of crop, nanoparticle and its dosage necessitates further studies of nanoparticles on different crops at varied doses to understand the interrelation of kind of nanoparticle, its dosage and its crop specificity.
The usage of nanoparticles in seed before sowing is comparatively safe as several metabolic, physiological and morphological changes occur before the plant biomass or seed production takes place. But certainly there is also need to study the effect of nanoparticles on soil microbes. Because soil microbes come into direct contact with nanoparticles during sowing of treated seeds. Hence, in the present experiment the authors tried to inquire the effect of seed treatment with nanoparticles on seed germination, vigour and yield attributing traits of fodder oat and berseem as well as the effect of treated seeds on soil microbes.
Material and Methods
Seed Material
Seeds of oat and berseem were collected from Division of Seed Technology, Indian Grassland and Fodder Research Institute, Jhansi, Uttar Pradesh. Seed surfaces were examined for the cracks generated by desiccation and deterioration processes during storage under SEM (Fig. 1a, b). The cracks were conceptualized to act as the entry point of nanoparticles (NPs) into the seeds.
Synthesis and Characterization of NPs
Synthesis and characterization of nanoparticles were done by available resources in Department of Nanoscience and Technology, Tamil Nadu Agricultural University. NPs were characterized by Particle Size Analyzer (PSA), Scanning Electron Microscope (SEM), Transmission Electron Microscope (TEM) and X-Ray Diffraction (XRD) following standard procedures.
Synthesis of ZnO Nanoparticles
ZnO NPs were synthesized by adding 0.45 M aqueous solution of zinc nitrate (Zn(NO3)2·4H2O) and 0.9 M aqueous solution of sodium hydroxide (NaOH) in distilled water taken in two separate 250 ml glass beakers. The Zn(NO3)2 solution (100 ml) transferred to a burette was added drop wise (slowly for 40 min.) to the 100 ml of NaOH contained in the beaker placed over a magnetic stirrer with hot plate set at 55 °C with high-speed stirring. The beaker after adding 100 ml Zn(NO3)2 was removed from the hot plate, sealed with aluminium foil and kept undisturbed for 2 h for precipitation and settlement. The precipitated ZnO NPs were washed with Millipore water followed by ethanol and then vacuum dried at 60 °C [14]. Nanoparticles such synthesized were transferred to air tight screw cap vial (10 ml) and stored at ambient temperature for further investigations.
Synthesis of Ag Nanoparticles
The Ag NPs were prepared by using chemical reduction method according to the description outlined by Lee and Meisel [15]. Fifty milliliter of AgNO3 0.005 M taken in a beaker was boiled on a magnetic stirrer with hot plate. To this solution, 5 ml of 1 % trisodium citrate was added drop by drop from 10 ml measuring cylinder with vigorous mixing on the stirrer until pale yellow colour appeared. Then the beaker was removed and kept at ambient temperature where the following chemical reaction would have occurred.
Synthesis of CuO Nanoparticles
CuO NPs were synthesised using copper nitrate trihydrate (CuN2O6·3H20, Sigma-Aldrich), and sodium hydroxide anhydrous pellets (NaOH, Carlo erba) in the presence of polyvinyl alcohol (PVA, Sigma Aldrich) as starting precursor [16]. Sodium hydroxide was dissolved in deionized water and thus obtained solution (0.5 M, 50 ml) was added drop wise to an aqueous CuN2O6·3H20 solution (0.1 M, 50 ml) for 30 min. Sonication of the solution was performed using Sonics Model VCX 1500 until complete precipitation. Finally, precipitated powder was calcined at 600 °C for 2 h to obtain the nanoparticles.
Synthesis of TiO2 Nanoparticles
TiO2 NPs were synthesized by dissolving 0.5 g TiO2 pellets in 30 ml of NaOH solution (10 M) under vigorous stirring at room temperature for 2 h. Thus obtained yellow solution was irradiated in an ultra sonicator (Soncis, VCX 1500, 20 kHz and 350 W) for 2 h in ambient temperature. The resultant precipitate was then centrifuged, washed and decanted with deionized water several times and dried at 60 °C for 24 h to obtain the nanoparticles [17].
Scanning Electron Microscope (SEM)
Size and morphology of the nanoparticles were characterized by FEI QUANTA 250. Sample of test nano-particles (0.5–1.0 mg) was dusted on one side of the double sided adhesive carbon conducting tape, and then mounted on the 8 mm diameter aluminum stub. Sample surface were observed at different magnification and the images were recorded.
Transmission Electron Microscope (TEM)
Sample was analyzed in FEI TECHNAI SPRIT make. Sample (NPs 0.50 mg) was diluted in suspensions by pure ethanol (15 ml) through ultrasonication. A drop of the suspension placed on 300-mesh lacy carbon coated copper grid upon drying, was examined and the images were recorded at different magnification.
X-ray Diffraction
The phase formation of powder samples was confirmed by X-ray diffraction (XRD) technique using an X-ray powder diffractometer (Rigaku Corporation Japan, Smart Lab 3 kW) with CuKα radiation (λ = 1.5405 Å) in slow scan in the 2θ range of 20°–80°.
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Test for Radical Scavenging Activity
Free radical scavenging activity of the nanoparticles, based on the scavenging activity of the stable 1,1-diphenyl-2- picrylhydrazyl (DPPH) free radical was determined by the method described by Braca et al. [18]. Sonicated nanoparticle solution (0.1 ml) was added to 3 ml of 0.004 % methanol solution of DPPH. Absorbance at 517 nm was determined after 30 min and the percentage inhibition activity was calculated as follows.
where, Ac = Control absorbance, As = Sample absorbance.
Seed Treatment and Germination Test at Initial and After Storage
Seeds were treated with four types of metal/metal oxide nanoparticles (NPs) viz. Zinc oxide (ZnO), Titanium oxide (TiO2), Copper oxide (CuO) and Silver (Ag) NPs at 0 mg (control), 750 mg (D1), 1000 mg (D2) and 1250 mg/kg of seed (D3) by means of an electric shaker for 10 min at 500 rpm. Standard germination test was followed [ISTA, 2008]. Total 400 seeds in 4 replicates were put in germinator as per between paper method. Seedling shoot and root lengths and dry-weight were measured at the end of the germination test.
Field Experiment
Treated seeds of oat and berseem were rinsed properly under running water, air dried and sown by wearing gloves and mask at an isolated and controlled field. Four border rows were used all around the field and entry was restricted in the field. Standard agronomic package of practice was followed to raise the crop. Seedling emergence rate was observed in 2 m row. Yield attributes viz. number of tiller/meter running row and seed yield (q/ha) were also recorded.
Soil Microbial Study
The observation on the various functional groups of microorganisms was carried out under laboratory condition by using standard microbiological enumeration method.
The total bacterial count was assayed by serial dilution and pour plate method using nutrient agar, total fungal count by using potato dextrose agar, total phosphate solubilising bacterial and fungal population using Pikovskaya’s agar medium [19], cellulolytic microorganisms by using Omeliansky’s medium [20], nitrogen fixing microorganisms by using N-free yeast extract mannitol agar medium [21].
Results and Discussion
Lab Experiment
Seed Surface
After harvesting, seeds were kept for a few days, it generates natural openings/cracks on its surface possibly due to desiccation and lipid peroxidation in seed coat. Openings on seed surfaces under SEM ranged from 2 to 40 µm in length and 2–6 µm in width (Fig. 1a).
Nanoparticles
Nanoparticles tested by the particle size analyser (Fig. 4d), SEM (Fig. 2), TEM (Fig. 3) and XRD (Fig. 4a–c) were pure and under nano size as well. Figure 4a–c show the room temperature X-ray diffraction (XRD) pattern of CuO, ZnO and TiO2, respectively. The diffraction peaks indicate good homogeneity with high purity. The X-ray diffraction (XRD) patterns were analyzed to confirm the phase formation and lattice parameters of all the three samples were obtained using PDXL2 (ICDD (PDF-2/Release 2013)) software. Formation of almost pure phase is confirmed in all three compounds. The result of DPPH radical scavenging activity showed that NPs had high antioxidation efficiency. Among the nanoparticles, ZnO had the highest scavenging activity of 78 % while the least was observed with CuO NPs (39 %) (Fig. 4d).
Germination Percentage
All the four NPs viz. ZnO, TiO2, CuO and Ag improved germination percentage significantly as compared to control in both crops (Fig. 5a). In oat, highest germination (100 %) was observed at D1 and it was reduced at subsequent higher doses (Fig. 1b) for all NPs. Lowest germination (89 %) was recorded at D3 of CuO. Except ZnO, in all treatments D3 was at par with control. In berseem, the same trend was observed, but, except ZnO (91 %), D3 showed higher germination percentage as compared to control. Ag at D1 showed maximum germination percentage (100 %) followed by CuO at D2 (99 %). Only TiO2 enhanced germination at higher doses.
Shoot Length
In oat, shoot length was increased significantly by NPs except CuO where it was reduced significantly at D3 as compared to control (Fig. 5b). TiO2 promoted maximum shoot length at D3 (5.90 cm). No variation was observed among TiO2 doses; otherwise the effect was higher at D1 as compared to D3. In berseem, CuO stimulated maximum shoot length at D1 (7.12 cm). ZnO and CuO showed decreasing but TiO2 and Ag showed increasing trend in shoot length. But in all cases, it was higher than the control.
Root Length
TiO2 promoted maximum root length at D3 (27.13 cm) in oat (Fig. 6a). In other cases NPs enhanced root length significantly at lower dose but, at higher doses it reduced significantly as compared to D1. In case of berseem, except ZnO, all the NPs stimulated root length significantly at higher doses. Maximum root length was found at Ag D3 (6.77 cm) and minimum at Ag D1 (5.05 cm).
Seedling Dry-Weight
Except TiO2, all NPs reduced seedling dry-weight as compared to control and the result showed significant variation even among doses (Fig. 6b). TiO2 disfavoured the seedling dry-weight at D1 (3.07 mg) and favoured it at higher doses, D3 was at par with control. Least dry-weight was found at D3 of Ag. Same trend was in case of berseem also where seedling dry-weight was subsequently increased at higher doses. Highest seedling dry-weight was observed at D3 of TiO2 (3.71 mg), but in case of other NPs D3 was lesser even than the control. Ag reduced seedling dry-weight as compared to control even at D2.
Field Experiment
Seedling Emergence Rate
NPs influenced the seedling emergence rate also. CuO NPs at D1 enhanced the emergence rate in both the cases whereas it reduced the rate at higher doses. In case of oat, Ag NPs at D1 could not enhance, but at D2 it enhanced the emergence rate at later stage of observation whereas D3 again reduced the trait. D2 of CuO NPs showed lowest emergence rate (Fig. 7) even lower than the control. In case of berseem, CuO NPs at D1 distinctly enhanced the trait at highest rate whereas TiO2 D3 showed lowest value.
Tiller Count
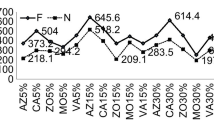
Number of effective tillers in oat was highest in case of D1 of CuO NPs whereas it was lowest in case of TiO2 D3 (2.47) (Fig. 8). Concentration of ZnO and Ag showed reciprocal relationship with magnitude of the trait whereas TiO2 triggered the trait at D2 as compared to D1 and D3. Tiller count was reduced in many cases as compared to control. In case of berseem, ZnO D1 favoured the trait by highest degree (429) followed by TiO2 D1 (395). Here all types of NPs had maximum value at D1 and it was reduced at subsequent doses.
Seed Yield
In case of oat (Fig. 9), CuO NPs enhanced the seed yield (q/ha) at D1 (19.50) and then reduced it by 4.3 % at D3. ZnO showed the same reducing trend with D1 as highest (19.00) and D3 as lowest (17.83). Ag NP showed maximum reduction from D1 (19.33) to D3 (18.67). But TiO2 enhanced the seed yield at higher doses with highest value at D3 (19.67). In control the seed yield was only 17.67 q/ha. In case of berseem, same trend was observed. CuO had highest value at D1 (2.31) followed by D2 (2.21) and D3 (2.07). ZnO also showed better yield at D1 (2.36) and 2.19 at D3. Ag provided same thrust to D2 and D3, but D1 was enhanced significantly by 16.90 % as compared to control. Exceptionally, like in oat, TiO2 enhanced seed yield incrementally.
Soil Microbial Study
Soil total bacterial colonies were higher with the application of CuO D1 (9.45 log CFU/g of soil) and ZnO D1 (9.46 log CFU/g of soil) at their lower dose followed by CuO D2 (9.43 log CFU/g of soil) and ZnO D2 (9.42 log CFU/g of soil), whereas, Ag D1 (9.42 log CFU/g of soil) recorded the lowest bacterial colony forming units at their lowest concentration (Table 1). The concentration of CuO NP and ZnO NP was inversely proportionate with the population of bacteria. Similarly, the population of total fungal colonies was found to be higher with CuO D1 (6.02 log CFU/g of soil) followed by CuO D2 and CuO D3 and other NP treatments had a negative effect on fungal colonies. Nitrogen fixing bacteria (5.46, 5.39 and 5.37 log CFU/g of soil in ZnO NP respectively) and cellulose decomposers (5.07, 5.06 and 5.00 log CFU/g of soil in TiO2 NP respectively) were significantly higher with the application of ZnO NP with irrespective of their dose followed by the application of TiO2 NP. Phosphate solubilising fungal (PSF) population was enhanced with the application of Ag NP (4.78, 4.77 and 4.74 log CFU/g of soil in incremental doses respectively).
In the above experiment, NPs enhanced germination significantly but at higher doses, in some cases, they reduced the germination. Zhang et al. [9] analysed the effects of nano-TiO2 and non nano-TiO2 on the germination and growth of naturally aged seeds of Spinacia oleracea by measuring the germination rate and the germination and vigor indexes. An increase of these indices was observed at 0.25–4 % of nano-TiO2 treatments. It was pointed out that a mixture of nanoscale TiO2 (nano-TiO2) and SiO2 (nano-SiO2) could increase nitrate reductase in soybean (Glycine max) and enhance its abilities of absorbing and utilizing water and fertilizer, stimulate its antioxidant system, and apparently hasten its germination and growth [8]. Navarro et al. [22] stated that engineered nanoparticles could sequester nutrients on their surfaces and thus serve as a nutrient stock to the organisms, particularly those engineered nanoparticles having high specific surface area. These positive effects could be probably due to the antimicrobial properties of engineered nanoparticles, which can enhance strength and resistance of plants to stress. Seed coat plays a very important role in protecting the embryo from harmful external factors. Seed coats can have selective permeability [23]. Pollutants, though having obvious inhibitory effect on root growth, may not affect germination if they cannot pass through seed coats. This may explain that seed germination in the present study was not affected by nanoparticles, instead germination was enhanced by NPs. In the present study, shoots could grow to a better degree even though root elongation was not up to the mark as compared to control. Radicles, after penetrating the seed coats, could contact the nanoparticles directly. Therefore, root elongation of sensitive plant species would have a dose-dependent response. Since roots are the first target tissue to confront with excess concentrations of pollutants, toxic symptoms seem to appear more in roots rather than in shoots [24]. In other study, nano-Al suspension had no obvious effect on cucumber, but, promoted the root growth of radish and rape, and significantly retarded root elongation of ryegrass and lettuce [25]. Zhang et al. [9] showed that during the growth stage the plant dry weight was increased as was the chlorophyll formation, the ribulose bisphosphate carboxylase/oxygenase activity and the photosynthetic rate. Gao et al. [13] and Xuming et al. [26] reported same kind of results. These results evidenced that the physiological effects were related to the nanometer-size particles. The authors also reported that the effects of non nano-TiO2 particles were not significant. Hong et al. [10, 11] analysed the effects of nano-TiO2 (rutile) on the photochemical reaction of chloroplasts of Spinacia oleracea as a theoretical basis and technical approach for the agricultural application of NPs. The obtained results evidenced that the nano TiO2 treatments induced an increase of the Hill reaction and of the activity of chloroplasts, which accelerated Fe–Cy reduction and oxygen evolution. Moreover non cyclic photophosphorylation activity was higher that cyclic photophosphorylation activity. The explanation of these effects, on the opinion of the authors, could be that the nano-TiO2 might enter the chloroplast and its oxidation–reduction reactions might accelerate electron transport and oxygen evolution. In other studies like in Riahi-Madvar et al. [27], the results show that while root growth is affected by the nanoparticles (NPs), other morphological properties including seed germination, shoot length, and dry biomass were the same as the control plants’ properties. This can be attributed to selective permeability of seed coats which confronts roots with excess in the NPs and low rate in transportation of this material to the shoot. Mahmoodzadeh et al. [28] showed that higher concentrations of nanoscale TiO2 (1200 and 1500 mg l−1) showed large radicle and plumule growth of seedling as compared to other concentrations and control. Shah and Belozerova [29] analysed the influence of metal nanoparticles on the growth of Lactuca seeds, this influence was tested by measuring the length of the root and shoot of the plant after 15 days of incubation. An increase in the shoot/root ratio compared to that of the control was evidenced. This also explains the increase in biomass accumulation by the studied plants. Each nutrient element under study (Zn, Ti, Ag and Cu) plays a vital role in photosynthesis and in many other indispensible physiological processes. For example, Zn plays an important role in many biochemical reactions within the plants like formation of chlorophyll and carbohydrates [30]. Similarly, trace amount of Cu is a component of regulatory proteins, participates in electron transport in photosynthesis and respiratory chains [31]. Sometimes plant development is negatively affected because NPs clog the root openings and both hydraulic and nutrient uptake in roots is inhibited. On the basis of several studies, the following principal factors that influenced toxicity in plants are, concentration of NPs, particle size and specific surface area, physiochemical properties of NPs, plant species, plant age/life cycle stage, growth media, NPs stability and diluting agents [32].
Regarding yield at field experiment, scanty literature has so far been published. Therefore, the authors cannot support the present work with much references. Prasad et al. [7] suggested that the micronutrients like Zn could be delivered into seeds through ZnO NPs. A higher amount of Zn was present in the seed when treated with nanoscale ZnO. This improved the germination, root growth, shoot growth, dry weight and pod yield of the treated seeds. In the present study, ZnO NPs enhanced seed yield at lower doses whereas decreased it at higher doses, but NPs surpassed the control at all doses. Roghayyeh et al. [33] have also reported an increase in pod weight, leaf, pod dry weight and yield of soybean when treated with nano-iron. However, the growth of Sesbania seedlings in gold solution did not show any remarkable difference between control and treated seedlings, even up to 200 ppm of gold NPs. Musante and White [34] have reported a decline in growth of Cucurbita pepo, on treatment with Ag and Cu NPs. Miao et al. [35] have also reported that Ag nanoparticles exert a negative effect on the growth of phytoplankton. Further, limited information is available on the mode of action of these nanoparticles on crop plants. Metal nanoparticles provide more surface area for valance electron exchange with bio-molecules, due to their higher surface area to volume ratio [29]. Therefore, use of metal nanoparticles can alter the antioxidant status of the treated plants, by virtue of their ability to participate in cellular redox reactions.
Since engineered NPs are introduced into the soil as a result of human activities, among the many fields that nanotechnology takes into consideration, it is also important to recall the analyses of the connections among nanoparticles, plants and soil where plants live and grow up. In the present study, NPs did not influence the soil microbes. The NPs adhered on surface of treated seeds got diluted in the soil and it could exert much impact on the plant as well as on the soil organisms under study. Shah and Belozerova [29] analysed the influence of metal nanoparticles on the soil microbial community and germination of Lactuca seeds. The results showed an insignificant influence of the nanoparticles in the soil on the number of colony forming units confirming the results of Tong et al. [36] and of Nyberg et al. [37]. On the contrary metal nanoparticles influence the growth of Lactuca seeds, this influence was tested by measuring the length of the root and shoot of the plant after 15 days of incubation. An increase if the shoot/root ratio compared to that of the control was evidenced. It is ascertained that nanoparticles tagged to agrochemicals or to other substances could reduce the injury to plant tissues and the amount of chemicals released into the environment; a certain contact is however unavoidable, due to the strong interaction of plants with soil growth substrates.
Mechanism of nanotoxicity remains unknown; however, it would be closely related to the chemical composition, chemical structure, particle size and surface area of the nanoparticles. Toxicity of nanoparticles may be attributed to two different actions [38]: (1) chemical toxicity based on the chemical composition, e.g., release of (toxic) ions; and (2) stress or stimuli caused by the surface, size and/or shape of the particles. Attentions to designing appropriate experimental set up and interpretation are needed to provide a defensible scientific understanding of the biological effects of nanoparticles [39]. In order to understand the possible benefits of applying nanotechnology to agriculture, the first step should be to analyze penetration, transport and mode of action of NPs in plants.
Conclusion
Nanoparticles tested in the investigation were supportive in enhancing the germination and seedling vigour of the oat and berseem. In conclusion, the present experiment confirmed the dose-specific effect of NPs on seed germination, crop growth and seed yield of two important fodder crops. All the four NPs viz. ZnO, TiO2, CuO and Ag at lower dose significantly enhanced germination percentage, seedling vigour and yield traits as compared to control in both the crops. The crops responded to NPs differentially regarding all the traits under study. Apparently no toxicity was observed. The technology holds immense potential in various sectors of agriculture if all the issues with NPs are addressed properly. However, the findings are to be verified under large scale field condition before recommending to farmer for adoption.
References
Tarafdar JC (2012) Perspectives of nanotechnological applications for crop production. NAAS News 12:8–11
Pramanik P, Maity A (2013) Nanopollution—a growing issue. Natl Environ Sci Acad Newsl 16(7):2
Subramanian KS, Tarafdar JC (2011) Prospects of nanotechnology in Indian farming. Indian J Agric Sci 81:887–893
Yang L, Watts DJ (2005) Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett 158:122–132
Lee WM, An YJ, Yoon H, Kwbon HS (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ Toxic Chem 27:1915–1921
Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3(10):3221–3227
Prasad TN, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad TS, Sajanlal PR, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35(6):905–927
Lu CM, Zhang CY, Wen JQ, Wu GR, Tao MX (2002) Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci 21:168–172
Zhang L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 106:279–297
Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P (2005) Effects of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res 105:269–279
Hong F, Yang F, Liu C, Gao Q, Wan Z, Gu F, Wu C, Ma Z, Zhou J, Yang P (2005) Influence of nano-TiO2 on the chloroplast aging of spinach under light. Biol Trace Elem Res 104:249–260
Yang F, Liu C, Gao F, Su M, Wu X, Zheng L, Hong F, Yang P (2007) The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol Trace Elem Res 119:77–88
Gao F, Hong F, Liu C, Zheng L, Su M, Wu X, Yang F, Wu C, Yang P (2006) Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol Trace Elem Res 111:239–253
Moghaddam AB, Nazari T, Badraghi J, Kazemzad M (2009) Synthesis of ZnO nanoparticles and electrodeposition of polypyrrole/ZnO nanocomposite film. Int J Electrochem Sci 4:247–257
Lee PC, Meisel D (1982) Adsorption and surface—enhanced Raman of dyes on silver and gold sols. J Phys Chem 86:3391–3395
Wongpisutpaisan N, Charoonsuk P, Vittayakorn N, Pecharapa W (2011) Sonochemical synthesis and characterization of copper oxide nanoparticles. Energy Proced 9:404–409
Arami H, Mazloumi M, Khalifehzadeh R, Sadmezhaad SK (2007) Sonochemical preparation of TiO2 nanoparticles. Mater Lett 61:4559–4561
Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I (2001) Antioxidant principles from Bauhinia t arapotensis. J Nat Prod 64:892–895
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Omeliansky WL (1902) Ueber die Garung der Cellulose. Proc Indiana Acad Sci Cent F Bakt II 8:225–231
Subba Rao NS (1999) In soil microbiology, 4th edn. Oxford and IBH Pub. Co Pvt. Ltd., New Delhi
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao A, Quigg A, Santschi PH, Sigg L (2008) Environmental behaviour and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386
Wierzbicka M, Obidzin´SKA J (1998) The effect of lead on seed imbibition and germination in different plant species. Plant Sci 137:155–171
Sresty TVS, Rao KVM (1999) Ultrastructural alterations in response to zinc and nickel stress in the root cells of pigeonpea. Environ Exp Bot 41:3–13
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Xuming W, Fengqing G, Linglan M, Jie L, Sitao Y, Ping Y, Fashui H (2008) Effects of nano-anatase on Ribulose-1, 5-bisphophate carboxylase/oxygenase mRNA expression in spinach. Biol Trace Elem Res 126:280–289
Riahi-Madvar A, Rezaee F, Jalali V (2012) Effects of alumina nanoparticles on morphological properties and antioxidant system of Triticum aestivum. Iran J Plant Physiol 3(1):595–603
Mahmoodzadeh H, Nabavi M, Kashefi H (2013) Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J Ornam Plants 3(1):25–32
Shah V, Belozerova I (2009) Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut 197:143–148
Corredor E, Testillano PS, Coronado MJ, González-Melendi P, Fernández-Pacheco R, Marquina C, Ibarra MR, de la Fuente JM, Rubiales D, Pérez-De-luque A, Risueño MC (2009) Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol 9:45–54
Rico C, Majumdar S, Duarte-Gardea M, Peralta JR, Gardea-Torresdey JL (2011) Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem 59:485–498
Adhikari T, Kundu S, Biswas AK, Tarafdar J, Subba Rao A (2012) Effect of copper oxide nano particle on seed germination of selected crops. J Agric Sci Technol A 2:815–823
Roghayyeh SMS, Mehdi TS, Rauf SS (2010) Effects of nano-iron oxide particles on agronomic traits of soybean. Not Sci Biol 2:112–113
Musante C, White JC (2010) Toxicity of silver and copper to Cucurbita pepo: differential effects of nano and bulk-size particles. Environ Toxic. doi:10.1002/tox.20667
Miao AJ, Quigg A, Schwehr K, Xu C, Santschi P (2007) Engineered silver nanoparticles (ESNs) in coastal marine environments: bioavailability and toxic effects to the phytoplankton Thalassiosira weissflogii. In: Second international conference on the environmental effects of nanoparticles and nanomaterials, 24–25th sep, London UK
Tong Z, Bischoffff M, Nies L, Apppplegate B, Turco RF (2007) Impact of fullerene (C60) on a soil microbial community. Environ Sci Technol 51:2985–2991
Nyberg L, Turco RF, Nies L (2008) Assensing the impact of nanomaterial on anaerobic microbial communities. Environ Sci Technol 42:1938–1943
Brunnnner TI, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ (2006) In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and effect of particle solubility. Environ Sci Technol 40:4374–4381
Murashov V (2006) Comments on ‘‘particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles’’ by Yang L, Watts DJ, Toxicology Letters, 2005, 158, 122–132. Toxicol Lett 164:185–187
Acknowledgments
The authors duly acknowledge the facilities provided by Department of Nanoscience and Technology, Tamil Nadu Agriculture University, Coimbatore and Innovation Centre, Bundelkhand University, Jhansi to conduct the laboratory experiment and Indian Grassland and Fodder Research Institute, Jhansi to conduct rest of the study. To the best of the knowledge there is no conflict of interest involved in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maity, A., Natarajan, N., Vijay, D. et al. Influence of Metal Nanoparticles (NPs) on Germination and Yield of Oat (Avena sativa) and Berseem (Trifolium alexandrinum). Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 595–607 (2018). https://doi.org/10.1007/s40011-016-0796-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0796-x