Abstract
Isoproturon (IPU) degrading bacterium was isolated from herbicide treated soils. Morphological, biochemical and 16S rRNA sequencing revealed that the strain belonged to the phylogeny of the Bacillus sp. (99 % sequence similarity with Bacillus pumilus FM 201790.1) hence designated as B. pumilus K1. Biodegradation study was carried out using 200 mg L−1 IPU as sole source of carbon at three pH levels i.e. 6.5, 7.0 and 7.5 and three temperatures i.e. 25, 30 and 35 °C. In the first 5–10 days IPU biodegradation was slow, which was later accelerated. The IPU degrading potential of B. pumilus K1 was strongly influenced by pH and temperature with maximum degradation at pH 7.0 and 30 °C followed by pH 7.5 and 35 °C at the end of 20 days. However, at pH 6.5 and 25 °C least IPU degradation was observed. The optimum conditions for isoproturon degradation by this bacterial isolate were pH 7.0 and 30 °C temperature. Addition of supplementary carbon source enhanced 4.07 % IPU degradation. 4-Isopropylaniline was detected as IPU degradation by-product in the medium. The study clearly exhibited that B. pumilus K1 was able to metabolize IPU effectively and thus could be employed for development of field scale bioremediation technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The phenylurea herbicide isoproturon, 3-(4-isopropylphenyl)-1, 1-dimethylurea (IPU) is used for pre- and post emergence control of annual grasses and broad-leaved weeds in wheat, rye, and barley crops. This is among the most extensively used pesticides in conventional agriculture [1]. According to the official survey conducted by Directorate of Plant Protection, Quarantine and Storage, Govt. of India from 2005–2006 to 2009–2010, IPU is among the most consumed pesticide in the country with 7163 metric tonnes consumption per annum (www.pesticideinfo.org, retrieved on 24.06.2015 at 9.55 am). Although IPU has been banned in several countries or restricted to use 1.2 kg ha−1 year−1 since 2003, but in several countries it is still extensively being used. Ecotoxicological data suggests that IPU and some of its metabolites are harmful for aquatic invertebrates, freshwater algae and microbial communities [2–4]. IPU and its metabolites have also been suspected to be carcinogenic to human beings and animals [5, 6]. As a result of widespread and repeated use, IPU is frequently recorded as contaminant of agricultural catchments and water resources in various parts of the world, exceeding the European Commission drinking water limit of 0.1 µg L−1 [7, 8].
Considering these harmful effects of IPU, elimination of its build up in the environment is imperative [9]. Microbial degradation plays an important role in pesticide dissipation from the soil, which could prove to be a reliable, cost-effective remediation technique for IPU abatement [9, 10]. Several studies have already reported IPU degradation by bacterial isolates vi-a-vis repeated exposure to this herbicide over a longer period of time [11, 12]. Bacterial degradation of IPU in the contaminated soils is influenced by various physico-chemical and environmental factors [13]. Variety of soil bacteria and fungi have been isolated from different regions in the world which can utilize IPU as a carbon source [9, 14–16]. However, only Sphingomonas sp., Methylopila sp. and Sphingobium sp. strains have shown the complete IPU mineralization potential [1, 16, 17].
Biostimulation is the addition of nutrients to stimulate naturally occurring microbial populations [18]. Comprehensively, biostimulation could be perceived as introduction of adequate nutrients and oxygen in the medium in order to enhance pesticide degradation potential of microbes [19] or to promote cometabolism [20]. Biostimulation is usually paired under the enhanced bioremediation techniques along with bioaugmentation which is merely the introduction of specific microorganisms aimed at enhancing the biodegradation of target compound or serving as donors of the catabolic genes. The purpose of biostimulation is to accelerate the intrinsic degradation potential of a polluted matrix through the accumulation of nutrients, or some other limiting factors and has been used for a wide variety of xenobiotics [21]. Even though the diversity of natural microbial populations apparently exhibit the potential for pesticide remediation in the contaminated sites, however, lack of electron acceptors or donors, low nitrogen or phosphorus availability, late stimulation of the metabolic pathways responsible for degradation can hamper or delay the degradation. Under such circumstances, accumulation of exogenous nutrients can enhance the degradation of the toxic materials [22, 23].
Due to the persistence and toxicity of pesticides and their metabolites, biological means for detoxification have received serious attention as an alternative and eco-friendly technology over existing methods such as incineration and landfill [24]. To develop strong bioremediation technology for IPU remediation from the environment, it was accepted that screened indigenous microorganisms from polluted soil were often more effective to metabolize it than organisms from elsewhere. According to the National Bureau of Soil Survey and Land Use Planning (NBSS and LUP) Nagpur, Tarai region of Uttarakhand (Pantnagar Kashipur and Rudrapur) is the major food production zone of Indo Gangetic plains (IGP). Pantanagar Tarai agro-ecosystem has been categorized as 9.2a Agro-ecological Sub Region (AESR) of the country. This region is major food production zone of Uttarakhnad, therefore, pesticide application is also high in the region. Keeping in view the above facts, present investigation was carried out to isolate and characterize the native bacterial species able to degrade IPU by performing enrichment cultures from wheat field of Tarai agroecosystem, Pantnagar (Uttarakhand).
Material and Methods
Chemicals and Reagents
Analytical-grade (>99.5 % purity) 3-(4-isopropylphenyl)-1-methylurea (IPU) and its major metabolites [N-(4-isopropylphenyl)-N′-methylurea] (MDIPU), [N-(4-isopropylphenyl) urea] (DDIPU), and 4-isopropyl-aniline were procured from Sigma Aldrich, USA. All the stock solutions were prepared in methanol. Acetonitrile, dichloromethane and all other chemicals used during this investigation were of analytical grade and purchased from Himedia, India. The composition of mineral salt medium (MS) used for the enrichment, isolation and IPU degradation contained (g L−1) KH2PO4, 0.5; K2HPO4, 1.5; NH4NO3 1.0; MgSO4. 7H2O 0.2; NaCl 1.0; and FeSO4 0.025. Nutrient agar medium contained (g L−1): Peptone 10; Yeast extract 5.0 and NaCl 5.0. In experiments of pH effect on IPU degradation, the initial broth pH was adjusted using 0.01 N HCl or NaOH [16].
Collection of Soil Samples and Determination of Physico-Chemical Properties
Isoproturon treated composite surface soil samples (0–15 cm) were collected from foothill agro-ecosystem of north west Himalaya 29°3′0″N 79°34′04″E and 344 metre (msl) of Norman E. Borlaug Crop Research Centre, Pantnagar, India. The soil samples were sieved at 5 mm and subdivided into two aliquots. First aliquot was air dried for physico-chemical analysis, whereas, the second was preserved at 4 °C for isolation of IPU degrading bacteria. The physico-chemical properties of soil determined were sand, silt clay, soil pH, organic carbon (OC), organic matter (OM) content, available nitrogen (N), available phosphorous (P), and available Potassium (K) using standard laboratory methods. The mean soil particle size distribution of the collected samples was 47.3, 28.8 and 23.9 % sand, silt and clay respectively. The soil of the chosen agro-ecosystem was high in organic carbon and low in available nitrogen, phosphorus as well as potassium with neutral pH (Table 1). High organic carbon in the soil could be attributed to the decomposition of previously grown crop residues in the field, while low quantities of NPK in the tested soil samples might be due to the nutrient removal by previously grown crop because soil physico-chemical properties were determined at the beginning of Ravi season, after harvesting rice.
Enrichment Culture and Isolation of IPU Degrading Bacteria
Surface soil (0–15 cm) from wheat fields treated with IPU was used for the isolation of bacteria. Composite soil samples (5 g) in triplicate were taken in 250 mL Erlenmeyer flasks containing 100 ml of the minimal broth and 50 mg L−1 IPU. These flasks were incubated at 30 °C with continuous shaking at 150 rpm. After 10 days, 5 ml of broth culture from each flask was re-inoculated to 50 mL of fresh media with 100 mg L−1 IPU and cultured under the same conditions. This process was repeated five times and IPU concentration was increased up to 200 mg L−1. After 5 subcultures had been performed, 0.5 mL of culture broth was applied to mineral salt agar (MSA) plates supplemented with 200 mg L−1 IPU and 0.1 g L−1 dextrose as a source of carbon. The plates were incubated at 30 °C. After 48 h incubation a few colonies appeared on the plates. These bacterial colonies were streaked till the pure colonies obtained. After the successive enrichment culture, bacterial isolate showing prolific growth in IPU supplemented mineral salt agar medium was isolated from the plates and designated as K1.
Determination of IPU Minimum Inhibitory Concentration (MIC)
In order to determine the IPU MIC for bacterial isolate K1, it was streaked on mineral salt agar plates supplemented with IPU as sole source of carbon and energy at the concentrations of 50, 100, 150, 200, 300, 350 and 400 mg L−1. These agar plates were incubated at 30 °C for 72 h. After 72 h incubation appearance of bacterial colonies was recorded. On the basis of growth of bacterial isolate on mineral salt agar plates, isoproturon minimum inhibitory concentration was determined.
Identification of IPU Degrading Bacterial Isolates K1
The IPU degrading bacterial isolate K1 was identified on the basis of morphological and biochemical tests and 16S rRNA partial gene sequence analysis. Bacterial isolate K1 was tested for 24 different substrates (24 carbon sources and 2 enzyme activities viz. Catalase and Oxidase) (Table 2) to study their metabolizing abilities using KB009 part A and B HiCarbohydrate™ Kit. These tests were based on the principle of pH change and substrate utilization.
Effect of pH and Temperature on IPU Degradation
To optimize the best growth pH for bacterial isolate, 50 mL mineral salt broth (autoclaved at 121 °C for 20 min) in 100 mL Erlenmeyer flasks adjusted to pH 4.5, 5.5, 6.5, 7.0, 7.5, 8.5 and 9.5 were used. These flasks were amended with IPU to a concentration of 200 mg L−1 and inoculated with bacterial isolate K1. These flasks were incubated at 30 °C on an orbital shaker at 150 rpm. Samples were withdrawn regularly at 12, 24, 36, 48, 60 and 72 h incubation and optical density was recorded at 600 nm using UV/Vis Spectrophotometer (Varian). Similarly to optimize growth temperature, 50 mL autoclaved mineral salt broth amended with IPU to a concentration of 200 mg L−1 were inoculated with bacterial Isolate. These flasks were incubated at 20, 25, 30, 35, 40 and 45 °C on an orbital shaker at 150 rpm. Samples were withdrawn regularly at 12, 24, 36, 48, 60 and 72 h incubation and optical density was recorded at 600 nm. On the basis of growth curves plotted between OD600 nm and pH as well as OD600 and temperature, three pH values (6.5, 7.0 and 7.5) and three temperatures (25, 30 and 35 °C) were chosen for further IPU degradation study under laboratory conditions.
To assess the effect of pH on IPU biodegradation by bacterial isolate, 50 mL mineral salt broth (autoclaved at 121 °C for 20 min) in 100 mL Erlenmeyer flasks adjusted to pH values of 6.5, 7.0 and 7.5 were used. 200 mg L−1 IPU was added in the flasks as a sole source of carbon. The broth in the flasks was inoculated with 1 mL of bacterial inoculum (OD600 = 1.0). These flasks were incubated at 30 °C on an orbital shaker at 150 rpm for 20 days.
To study the effect of temperature on IPU degradation, 50 mL mineral salt broth in 100 ml Erlenmeyer flask was taken. 200 mg L−1 IPU was added as a sole source of carbon in the flasks and inoculated with 1 mL overnight bacterial inoculum (OD600 = 1.0). These flasks were incubated at 25, 30, and 35 °C on an orbital shaker at 150 rpm for 20 days. Uninoculated flasks were also maintained to determine abiotic degradation if any. All the experiments were conducted in triplicates for each pH and incubation temperature. Samples were withdrawn aseptically at 5, 10, 15 and 20 days incubation for determination of residual IPU concentration in the medium.
IPU degradation potential of bacterial isolate was further assessed in the presence of supplementary carbon source. Dextrose was added at a concentration of 0.5 g L−1 in the broth medium containing IPU at the concentration of 200 mg L−1. The flasks were incubated under optimized pH and temperature for 20 days. Residual IPU was determined using HPLC at 5, 10, 15 and 20 days.
Analytical Method and Conditions
To extract residual IPU 5 mL aliquots were taken at 5, 10, 15 and 20 days incubation from broth culture and centrifuged to obtain cell free supernatant. To estimate the biodegradation, residual IPU was extracted by adding 5 mL of cell free supernatant to an equal volume of dichloromethane. The extraction process was repeated thrice. The extract was dried over anhydrous Na2SO4 and solvent was evaporated to dryness using rotatory evaporator at 40 °C. The residues were dissolved in 1.0 mL of acetonitrile and water (75/25, v/v). All the samples were filtered by passing through acrodisc syringe filter of 0.2 μ membrane. The IPU extracts were then analyzed using Dionex HPLC equipped with auto sampler and Acclaim 120, C18 5 µm 4.6 × 250 mm column. Samples were eluted from the column using acetonitrile and water (75:25 v/v) at a flow rate of 1 mL min−1. The solutes were detected using UV detector at 243 nm. The HPLC analysis was performed at room temperature under isocratic conditions [11, 16]. Residual IPU concentration was quantified using a standard curve plotted between mass absorbance unit (mAU) and known concentration of isoproturon. Technical grade IPU and its major metabolites were used as standard for computation of residual IPU and metabolite identification. For this purpose 5, 10, 50, 100, 150, 200 and 250 ppm concentration of IPU and its reported metabolites were used to identify the by products of IPU degradation. The corresponding detection limits for IPU, MDIPU, DDIPU and 4IA were 0.75, 0.69, 0.86 and 0.89 µg L−1 respectively. The average extraction efficiency of this method was 96.00 ± 1.52 %. Percent IUP degradation was calculated using following formula:
where, As is the peak area of sample and Ac is the peak area of the control.
Statistical Analysis
The experimental data were processed for calculating standard error of the means and two factorial completely randomised design (CRD) analysis as available in the SPSS16, and expressed at 0.05 probability level.
Results and Discussion
Isolation and Characterization of IPU Degrading Bacterial Strain
IPU degrading bacterial strain K1 was isolated from the enrichment culture. This isolate was gram-positive, aerobic, spore-forming, and rod-shaped (Fig. 1). The bacterial isolate K1 was tested for 24 different substrates (24 carbohydrates as carbon source and 2 enzymes oxidase and catalase) to study their metabolizing abilities using KB009 part A and B HiCarbohydrate™ Kit. The tests were based on the principle of pH change and substrate utilization. K1 was positive to oxidase, catalase and 21 carbohydrates (Table 2).
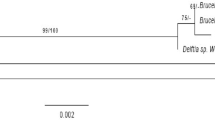
The 16S rRNA partial gene sequence analysis was carried out to identify the bacterial isolate K1 and deposited in the GenBank database under accession number KF279694. Homology search using BLAST revealed 99 % similarity of this sequence with 16S rRNA gene sequence of Bacillus pumilus (GenBank accession no. FM201790.1), giving the phylogenetic relationship of this bacterial isolate with several Bacillus species (Fig. 2), thus isolate was designated to be B. pumilus K1.
Neighbour-joining phylogenetic analysis resulting from the alignment of 16S rRNA partial gene sequences of B. pumilus K1 with those of other Bacillus sp. strains found in GenBank database. GenBank accession numbers are given in parentheses. Bootstrap value (in percent) is indicated at the respective nodes. The phylogenetic distance is shown on a scale bar
Determination of IPU MIC for Bacterial Growth
On the basis of bacterial growth on mineral salt agar plates supplemented with isoproturon as a sole source of carbon at different concentrations (50, 100, 150, 200, 300, 350 and 400 mg L−1), 350 mg L−1 IPU was found to show minimum inhibitory concentration for its growth.
Effect of pH and Temperature on IPU Degradation
Growth, pH and temperature for IPU degrading B. pumilus K1 were optimized under laboratory conditions. For this bacterial isolate was grown at pH-values from 4.5 to 9.5 and 20 to 45 °C temperature in MS medium supplemented with IPU as sole source of carbon at the concentration of 200 mg L−1. After 72 h observation bacterial strain exhibited maximum growth at pH 7.0 followed by 7.5 and 6.5. While 30 °C was the maximum growth temperature followed by 35 and 25 °C respectively. These three pH values and temperature regimes were chosen for further IPU degradation studies using B. pumilus K1.
Biodegradation of IPU by enriched bacterial isolate was studied for 20 days incubation at different pH and temperatures. Degradation of IPU in control and inoculated broth medium differed substantially at all the pH and temperatures. During first 5–10 days of incubation, IPU degradation was slow. However, it accelerated after 10 days and disappeared significantly (p < 0.05) from the broth medium at the end of 20 days (Tables 3, 4, 5). Biodegradation of IPU in control flasks was far less than the inoculated flasks and varied (p < 0.05) significantly.
Effect of Supplementary Carbon on the Degradation IPU
The bacterial isolate exhibited enhanced IPU degradation in the presence of dextrose in addition to IPU as a carbon source. IPU degradation at 5–10 days was slow but enhanced significantly at the end of experiment. Slight increase in IPU degradation was observed at 10 days incubation. As incubation proceeded IPU disappearance from the broth medium was accelerated and enhanced significantly. At the end of incubation (186.55 ± 0.49 mg L−1), total 93.23 % IPU was degraded by this bacterial isolate (Fig. 3). IPU degradation in control flasks and inoculated flasks was significantly (p < 0.05) different throughout the experiment. The increase in the IPU degradation efficiency due to supplementary carbon was 4.07 % at optimum pH and temperature. 4-Isopropylaniline (4IA) was accumulated as IPU degradation by-product in the medium (Table 6). However, no other reported metabolites were detected in the medium. This might be due to the potential of bacterial isolates to metabolize the IPU metabolites simultaneously in the medium.
Biodegradation of IPU has already been reported in several soils, repeatedly treated with this herbicide at various places such as UK, Denmark and France [1, 11–13]. Microbial degradation has been described as a primary mechanism responsible for IPU dissipation as well as other phenylurea herbicides from the soil [9, 10]. In the present investigation IPU degrading bacterial strain was isolated from Tarai agroecosystem, Pantnagar India using enrichment culture method. Phylogenetic analysis based on 16S rRNA revealed that the strain K1 showed 99 % similarity with the 16S rRNA sequence of B. pumilus (FM201790.1) and was clustered with several Bacillus strains. Since several bacteria have been reported to have the phenylurea herbicide degrading ability [1, 17, 29, 30], these earlier studies together with the work described here, indicate that different strains belonging to different genera are involved in the metabolism of phenylurea herbicides, but all these strains had different characteristics of extent and degree of degradation [16].
It is well known that pesticide biodegrading activity of the soil micro biota is deeply influenced by pedoclimatic conditions [31, 32]. Among the physico-chemical properties, pH and temperature are considered to play a key role being responsible for the regulation of pesticide degrading capabilities of microorganisms [11, 33–35]. Although the effect of pH on IPU degradation by soil microflora is well described by many previous studies [9, 11, 15, 16], the mechanisms responsible for this regulation have not been explained [16]. However, the pH is known to affect the growth and survival of microbial populations [36–38] which ultimately affects their activity. This suggests that pH might have an effect on the growth or survival of the bacterial population which might partly explain the variation in IPU degradation rate observed at different pH values. However, a more specific effect of pH on IPU degradation (abiotic and biotic IPU transformation, etc.) cannot be excluded. Further research should be carried out to study the transcriptional and enzymatic regulation occurring in B. pumilus on exposure to IPU at different pH values. The experimental results indicate that this bacterial isolate has the ability to degrade significant quantities of IPU at the pH range of 7.0–7.5 with an optimum activity at pH 7.0 and reduced activity at pH 6.5. The data recorded over the incubation time indicated that during the first 5–10 days, there was slow biodegradation of IPU, which might represent a lag phase while it got accelerated as the incubation proceeded, most likely due to induction/activation of enzymes in the inoculated cultures. Temperature plays a key role in bacterial metabolism. Slight variation in temperature may increase or decrease the metabolic rates of the microorganisms. In the present investigation along with pH, temperature has also been considered as a key factor for IPU degradation by B. pumilus K1. Experimental data revealed maximum IPU degradation at 30 °C incubation. It is likely that 30 °C might be more conducive to bacterial growth than other incubation temperatures [39].
Addition of supplementary carbon to the system having xenobiotic compounds increased the IPU biodegradation potential of bacterial isolate which is often because of the increase in metabolic activity of the microbes involved [40]. However, some studies have reported that the addition of auxiliary carbon to the system declined the degradation of pesticides [41, 42]. This might be because of the fact that dextrose being the easily available carbon source as compared to xenobiotics and therefore, the bacterium preferred dextrose over pesticide as an energy source. IPU degradation in control flasks was far less than in inoculated one. Abiotic IPU loss in control flasks might be due to chemical reactions at the set conditions [43].
Conclusion
In order to facilitate the process of biodegradation and bioremediation actively in the environment, there is a need to understand the fate of pesticides after their application and the metabolic potential of the soil microbial community in relation to the physico-chemical and biological properties of the environment. These conditions at each contaminated site vary, so all physico-chemical factors must be taken into account while studying the biodegradation of pesticides. The results of this study may imply that the enriched indigenous soil bacterial isolate B. pumilus K1 possesses efficient catalytic enzyme system to degrade IPU. So such bacterial strains might be useful for bioremediation of pesticide polluted soil and water environments. However, further research will be needed to apply this bacterial isolate at microcosm. Commercial scale bioremediation studies are needed because this isolate is being reported for the first time as IPU degrader in laboratory conditions.
References
Sorensen SR, Ronen Z, Aamand J (2001) Isolation from agricultural soil and characterization of a Sphingomonas sp. able to mineralize the phenylurea herbicide isoproturon. Appl Environ Microbiol 67(12):5403–5409
Mansour M, Feicht EA, Behechti A, Schramm KW, Kettrup A (1999) Determination photostability of selected agrochemicals in water and soil. Chemosphere 39(4):575–585
Widenfalk A, Bertilsson S, Sundh I, Goedkoop W (2008) Effects of pesticides on community composition and activity of sediment microbes—responses at various levels of microbial community organization. Environ Pollut 152(3):576–584
Vallotton N, Eggen RIL, Chevre N (2009) Effect of sequential isoproturon pulse exposure on Scenedesmus vacuolatus. Arch Environ Contam Toxicol 56(3):442–449
Behera BC, Bhunya SP (1990) Genotoxic effect of isoproturon (herbicide) as revealed by three mammalian in vivo mutagenic bioassays. Indian J Exp Biol 28(9):862–867
Hoshiya T, Hasegawa R, Hakoi K, Cui L, Ogiso T, Cabral R, Ito N (1993) Enhancement by non-mutagenic pesticides of GST-P positive hepatic foci development initiated with diethylnitrosamine in the rat. Cancer Lett 72(1–2):59–64
Spliid NH, Koppen B (1998) Occurrence of pesticides in Danish shallow ground water. Chemosphere 37(7):1307–1316
Muller K, Bach M, Hartmann H, Spiteller M, Frede HG (2002) Point- and nonpoint-source pesticide contamination in the Zwester Ohm catchment, Germany. J Environ Qual 31(1):309–318
Hussain S, Devers-Lamrani M, El Azhari N, Martin-Laurent F (2011) Isolation and characterization of an isoproturon mineralizing Sphingomonas sp. strain SH from a French agricultural soil. Biodegradation 22:1637–1650
Pieuchot M, PerrinGanier C, Portal JM, Schiavon M (1996) Study on the mineralization and degradation of isoproturon in three soils. Chemosphere 33(3):467–478
Bending GD, Lincoln SD, Sorensen SR, Morgan JAW, Aamand J, Walker A (2003) In-field spatial variability in the degradation of the phenyl-urea herbicide isoproturon is the result of interactions between degradative Sphingomonas spp. and soil pH. Appl Environ Microbiol 69(2):827–834
El-Sebai T, Lagacherie B, Cooper JF, Soulas G, Martin-Laurent F (2005) Enhanced isoproturon mineralisation in a clay silt loam agricultural soil. Agron Sustain Dev 25(2):271–277
El-Sebai T, Lagacherie B, Soulas G, Martin-Laurent F (2007) Spatial variability of isoproturon mineralizing activity within an agricultural field: geostatistical analysis of simple physicochemical and microbiological soil parameters. Environ Pollut 145(3):680–690
Badawi N, Ronhede S, Olsson S, Kragelund BB, Johnsen AH, Jacobsen OS, Aamand J (2009) Metabolites of the phenylurea herbicides chlorotoluron, diuron, isoproturon and linuron produced by the soil fungus Mortierella sp. Environ Pollut 157(10):2806–2812
Hussain S, Sorensen SR, Devers-Lamrani M, El-Sebai T, Martin-Laurent F (2009) Characterization of an isoproturon mineralizing bacterial culture enriched from a French agricultural soil. Chemosphere 77(8):1052–1059
Sun JQ, Huang X, Chen QL, Liang B, Qiu JG, Ali SW, Li SP (2009) Isolation and characterization of three Sphingobium sp. strains capable of degrading isoproturon and cloning of the catechol 1, 2-dioxygenase gene from these strains. World J Microbiol Biotechnol 25(2):259–268
El-Sebai T, Lagacherie B, Soulas G, Martin-Laurent F (2004) Isolation and characterisation of an isoproturon-mineralising Methylopila sp. TES from French agricultural soil. FEMS Microbiol Lett 239(1):103–110
Scow KM, Hicks KA (2005) Natural attenuation and enhanced bioremediation of organic contaminants in groundwater. Curr Opin Biotechnol 16(3):246–253
Couto MNPFS, Monteiro E, Vasconcelos MTSD (2010) Mesocosm trials of bioremediation of contaminated soil of a petroleum refinery: comparison of natural attenuation, biostimulation and bioaugmentation. Environ Sci Pollut Res 17(7):1339–1346
De Lorenzo V (2008) Systems biology approaches to bioremediation. Curr Opin Biotechnol 19(6):579–589
Kadian N, Gupta A, Satya S, Mehta RK, Malik A (2008) Biodegradation of herbicide (atrazine) in contaminated soil using various bioprocessed materials. Bioresour Technol 99(11):4642–4647
Cosgrove L, McGeechan PL, Handley PS, Robson GD (2010) Effect of biostimulation and bioaugmentation on degradation of polyurethane buried in soil. Appl Environ Microbiol 76(3):810–819
Kanissery RG, Sims GK (2011) Biostimulation for the enhanced degradation of herbicides in soil. Appl Environ Soil Sci 2011:843450. doi:10.1155/2011/843450
Siddique T, Benedict CO, Muhammad A, William TF (2003) Enrichment and isolation of endosulfan-degrading microorganisms. J Environ Qual 32:47–54
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analysis of soils. Agron J 54:464–465
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India Pvt. Ltd, New Delhi
Walkley A, Black IA (1934) an examination of the Degtjareff method for determining organic carbon in soils: effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–263
Olsen SR, Cole CV, Watanabe FS, Dean, LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Department of Agriculture Circular No. 939
Dejonghe W, Berteloot E, Goris J (2003) Synergistic degradation of linuron by a bacterial consortium and isolation of a single linuron-degrading variovorax strain. Appl Environ Microbiol 69:1532–1541
Widehem P, Ait-Aissa S, Tixier C (2002) Isolation, characterization and diuron transformation capacities of a bacterial strain Arthrobacter sp. N2. Chemosphere 46:527–534
Smith EA, Prues SL, Oehme FW (1997) Environmental degradation of polyacrylamides. 2 Effects of environmental (outdoor) exposure. Ecotoxicol Environ Saf 37:76–91
Andrea MM, Peres TB, Luchini LC, Pettinelli A (2000) Impact of long-term pesticide applications on some soil biological parameters. J Environ Sci Health B 35:297–307
Bending GD, Shaw E, Walker A (2001) Spatial heterogeneity in the metabolism and dynamics of isoproturon degrading microbial communities in soil. Biol Fertil Soils 33:484–489
Walker A, Jurado-Exposito M, Bending GD, Smith VJR (2001) Spatial variability in the degradation rate of isoproturon in soil. Environ Pollut 111:407–415
Rasmussen J, Aamand J, Rosenberg P, Jacobsen OS, Sorensen SR (2005) Spatial variability in the mineralisation of the phenylurea herbicide linuron within a Danish agricultural field: multivariate correlation to simple soil parameters. Pest Manag Sci 61:829–837
Russell JB, Dombrowski DB (1980) Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol 39(3):604–610
Sun CQ, O’Connor CJ, Turner SJ, Lewis GD, Stanley RA, Roberton AM (1998) The effect of pH on the inhibition of bacterial growth by physiological concentrations of butyric acid: implications for neonates fed on suckled milk. Chem Biol Interact 113(2):117–131
Rousk J, Brookes PC, Baath E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75(6):1589–1596
Hussain S, Arshad M, Saleem M, Khalid A (2007) Biodegradation of α and β-endosulfan by soil bacteria. Biodegradation 18:731–740
Kumar M, Philip L (2006) Enrichment and isolation of a mixed bacterial culture for complete mineralization of endosulfan. J Environ Sci Health B 41:81–96
Awasthi N, Manickam N, Kumar A (1997) Biodegradation of endosulfan by a bacterial coculture. Bull Environ Contam Toxicol 59:928–934
Goswami S, Singh DK (2009) Biodegradation of α and β endosulfan in broth medium and soil microcosm by bacterial strain Bordetella sp. B9. Biodegradation 20:199–207
Giri K, Rai JPN (2012) Biodegradation of endosulfan isomers in broth culture and soil microcosm by Pseudomonas fluorescens isolated from soil. Int J Environ Stud 69(5):729–774
Acknowledgments
Laboratory facilities provided by G.B. Pant University of Agriculture and Technology, Pantnagar and technical assistance for HPLC analysis from Dr. R.N. Ram, Professor and Head, Department of Fishery Biology, College of Fisheries are gratefully acknowledged. Authors are also grateful to two anonymous referees for critical review, providing valuable suggestions and strengthening the manuscript. Thanks are due to Genetech Labs Pvt. Ltd., Biotech Park, Kursi Road Jankipuram, Lucknow for 16S rRNA gene sequencing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Giri, K., Suyal, D.C., Mishra, G. et al. Biodegradation of Isoproturon by Bacillus pumilus K1 Isolated from Foothill Agroecosystem of North West Himalaya. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 87, 839–848 (2017). https://doi.org/10.1007/s40011-015-0667-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-015-0667-x