Abstract
Three strains of bacteria (designated as YBL1, YBL2, YBL3 respectively) capable of degrading isoproturon, 3-(4-isopropylphenyl)-1, 1-dimethylurea, were isolated from the soils of two herbicide plants. Based on the comparative analysis of the 16S rRNA gene, and phenotypic and biochemical characterization, these strains were identified as Sphingobium sp. The optimum conditions for isoproturon degradation by these strains were pH 7.0, and temperature 30°C. Mg2+ (1 mM) enhanced the isoproturon degradation rate, while Ni2+ and Cu2+ (1 mmol l−1) inhibited isoproturon degradation significantly. These three strains also showed the ability to remove the residues of other phenylurea herbicides such as chlorotoluron, diuron and fluometuron in mineral salt culture medium. The N-demethylation was the first step of degradation of dimethylurea-substituted herbicides. Strain YBL1 was found capable of degrading both dimethylurea-substituted herbicides and methoxymethylphenyl-urea herbicides i.e. linuron (3-(3,4-dichlorophenyl)-1-methoxy-1-methylurea). Using the PCR method, partial sequences of the catechol 1,2-dioxygenase gene were obtained from these strains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Isoproturon is among the most extensively used herbicides in conventional agriculture, mainly in the control of the pre- and post-emergence of annual grasses in winter cereals. As a result of its widespread and repeated use, isoproturon is frequently detected in groundwater and surface waters in Europe (Johnson et al. 2001; Morvan et al. 2006; Sorensen et al. 2003), and the residue of isoproturon has affected aquatic invertebrates (Mansour et al. 1999), freshwater algae (Peres et al. 1996) and microbial communities (Widenfalk et al. 2004, 2008). So there is a serious need to develop remediation processes to eliminate or minimize the contamination of isoproturon in surface water and groundwater as well. Recently, much more attention has been paid to the fate of isoproturon in environmental conditions (Bending et al. 2006; Bending and Rodriguez-Cruz 2007; Celis et al. 2008; Walker et al. 2001, 2008), Biodegradation may be used as a reliable and cost-effective technique for pesticide abatement. A lot of strains (Kristensen et al. 2006; Radosevich et al. 1995; Topp et al. 2000) are known for their interesting catabolic capabilities to degrade a wide variety of environmentally hazardous compounds.
In previous studies, several bacteria (El-Sebai et al. 2004; Sorensen et al. 2001, 2008; Widehem et al. 2002) and fungi (Ronhede et al. 2005), able to catalyze phenylurea herbicides degradation have been isolated from the environment. The objective of our research was to isolate the isoproturon-degrading bacteria from the herbicide-contaminated soils and to characterize these bacteria with respect to their diversity, identity, and mechanism of isoproturon degradation. In the present study we describe the isolation of three isoproturon-degrading strains, all belong to the genus Sphingobium, from the soils of herbicide plants that had been treated with isoproturon for several years, and characterize the isoproturon degradation in aliquots and soils by these strains. This report also includes the removal of the methoxymethylphenylurea herbicide linuron at low degradation rate by the isolate YBL1 and the cloning of catechol 1,2-dioxygenase gene from all these three strains.
Materials and methods
Chemicals
Isoproturon (97% purity, 55 mg l−1 water solubility at 20°C), diuron (97.5% purity, 42 mg l−1 water solubility at 25°C), chlorotoluron (97% purity, 70 mg l−1 water solubility at 20°C), fluometuron (95% purity, 110 mg l−1 water solubility at 20°C), and linuron (97% purity) were donated by Jiangsu Kuaida Agrochemical Co., Ltd. 4-Isopropylaniline (4IA) (98% purity), 4-methylcatechol, catechol, 3-methylcatechol and cis,cis-muconic acid were purchased from Sigma (Munich, Germany). Aniline was purchased from Shanghai Chemical Co., Ltd. (Shanghai, China). Methanol used for liquid chromatography was HPLC-grade (Sigma-Aldrich, Germany).
Medium
The mineral salt medium (MSM) had the following composition (per liter): NaCl, 1.0 g; NH4NO3, 1.0 g; K2HPO4, 1.5 g; KH2PO4, 0.5 g; MgSO4 · 7H2O, 0.2 g; FeSO4, 0.025 g; in experiments of pH effect on degradation, the initial pH was adjusted by using HCl or NaOH. The Luria–Bertani (LB) medium was composed of tryptone (Oxoid) 10.0 g l−1, yeast extract (Oxoid) 5.0 g l−1 and NaCl 10.0 g l−1. All the media were sterilized at 121°C for 25 min.
Enrichment cultures, isolation and characterization of the isoproturon-degrading bacteria
Soil samples were collected from the surface layer (0–10 cm) of two herbicide plants fields located in the cities of Nanjing and Suzhou, Jiangsu Province, China. The soil samples had been exposed to phenylurea herbicides for 10 years and contained isoproturon contamination usually above 0.5 mg kg−1. Briefly, about 5.0 g of the soil sample was added to an Erlenmeyer flask (250-ml) containing 100 ml MSM with the addition of isoproturon (30 mg l−1) as the sole carbon source and incubated at 30°C on a rotary shaker at 150 rev min−1 for a week. Every 5 days, about 5 ml of enrichment culture was subcultured for three times into fresh MSM containing 30 mg isoproturon l−1. Isoproturon removal was determined by HPLC in the culture from the second transfer. The enrichment culture capable of degrading isoproturon was serially diluted in MSM, 100 μl aliquots of fresh enrichment culture dilutions (10−4 to 10−6) plated on MSM agar containing 500 mg isoproturon l−1. The isoproturon agar was prepared by transferring isoproturon into the autoclaved and cooled medium (<50°C). After incubation at 30°C for 3 days, colonies were picked up and screened for their pure culture ability to degrade isoproturon. Three strains of bacteria, designated as YBL1, YBL2 and YBL3 respectively, were isolated from plates. Colonies were selected and purified by successive isolations. Isolated strains were preserved as stock in 15% glycerol and kept frozen at −70°C for further experiments.
Phenotypic and biochemical characterization of selected strains was performed according to Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994).
DNA extraction, PCR amplification, cloning and sequencing of partial 16S rRNA gene for identification
The extraction of total DNA and the PCR of the 16S rRNA gene were done following the protocol described by Huang et al. (2007). Sequencing reactions were performed by Invitrogen Biotechnology Co., Ltd. (Shanghai, China). The nucleotide sequences for the strains YBL3, YBL1, YBL2 have been deposited in the GenBank database under the accession nos. EU159273, EU159274 and EU159275 respectively.
Alignment of the partial 16S rRNA gene sequence was performed with sequences present in the GenBank database using BLAST (http://www.ncbi.nlm.nih.gov/blast/). Phylogenetic analysis was performed using MEGA version 4.0 (Tamura et al. 2007) software packages. The neighbor-joining (NJ) (Saitou and Nei 1987) method was used for phylogenetic analysis with the model of Kimura-2-Parameter. The robustness of the tree topology was assessed by bootstrap analysis, with 1,000 resembling replicates.
Degradation test
To study pure culture degradation ability of the isolated strains, the strains were grown in 250-ml Erlenmeyer flasks containing 100 ml of LB incubated at 150 rev min−1, 30°C. Cell cultures of all the strains were harvested in the late-exponential-growth phase by centrifugation at 4,000g for 5 min and washed twice with MSM. The cell pellets were suspended in MSM and adjusted to an optical density of 5.0 at 600 nm. About 1 ml of bacterial inoculum was inoculated into 100 ml MSM with the selected herbicide or other chemical (30 mg l−1, if undefined) as the sole source of carbon. All cultures were incubated at 150 rev min−1, 30°C. Samples were collected from the cultures at intervals and the concentration of the selected herbicide was determined by HPLC following the protocol described below. All degradation tests were performed in triplicate and repeated at least three times. The control experiment, without microorganism, was carried out under the same conditions. The results shown and described here are averages of one such experiment performed in triplicate.
Enzyme assays
Catechol 1,2-dioxygenase activity was assayed following the method of Ngai et al. (1990) by using cell extracts of strains YBL1, YBL2 and YBL3 grown on LB medium with supplementing catechol 6 h before harvesting. Cell suspensions were harvested by centrifugation, washed twice in distilled water, and resuspended in phosphate buffer (2 g of KH2PO4 per liter; pH 7.0) to which acetone (20%, v:v) was added. Cells were broken by ultrasonication for 5 min; each 0.5-min burst was followed by a 0.5 min cooling period. Unbroken cells and cell fragments were removed by centrifugation (15 min at 12,000g, 4°C); the supernatant fluid was kept on ice until it was assayed. The protein contents of cell extracts were determined by the Bradford method (Bradford 1976).
Soil experiment
Fluvo-Aquic soil was collected from Sheyang, Jiangsu Province, China. Red soil was collected from Yingtan, Jiangxi Province, China. Magan Soil was collected from Nanjing, Jiangsu Province, China. All these soils were collected from the top layer of 0–10 cm and had never been treated with isoproturon. The chemical and physical properties of the soil are shown in Table 1. Soil samples were dried at room temperature, then sieved to 5 mm and stored at 4°C. Soil samples were sterilized as described by Zhang et al. (2006). Subsamples (50 g) of fresh soil and sterile soil were weighed, and the solution of isoproturon was added to obtain a final concentration of 30 mg kg−1 of soil, and mixed well. One set of each, fresh soil and sterile soil, was inoculated by diluted isolates. The inoculum was thoroughly mixed into the soils under sterile conditions, and the moisture content of soils was adjusted to 25% (w/w of dry weight of soil; 70% of field capacity of Magan soil). The final density of isoproturon-degrading strains for each experiment was approximately 108 cells per gram soil. Each soil microcosm was incubated at 30°C in the dark. About 1.0 g of the each soil sample was collected and the concentration of isoproturon was detected everyday. Experiments were conducted in triplicate.
Analytical methods and conditions
For isoproturon extraction from liquid culture, a 1 ml sample taken from 100 ml liquid culture was extracted with 4 ml dichloromethane. The extract was dried over anhydrous Na2SO4 and then evaporated at room temperature. The residues were dissolved in 200 μl of methanol. An aliquot of the solution (5 μl) was injected into a HPLC system for detection.
For isoproturon extraction from soil, 1.0 g of soil sample was taken out from 50.0 g soil and dried over anhydrous Na2SO4 and then was extracted with 5.0 ml of dichloromethane, the mixture was shaken for 1 h at 200 rev min−1 on a rotary shaker and then centrifuged. About 1.0 ml supernatant was decanted into a centrifuge tube, and the organic solvent allowed to evaporate at room temperature. The residues were dissolved in 1.0 ml of methanol and then the solution was passed through a filter with a pore size of 0.2 μm. The extraction of diuron and chlorotoluron from soil was performed using the same method.
Isoproturon concentration in the extracts was determined by reverse-phase HPLC (Waters 600 Controller, Rheodyne 7725i Manual injector and 2487 Dual k Absorbance Detector; Waters Co., Milford, MA). Detection of isoproturon was recorded at 241 nm. The separation column for the HPLC (internal diameter, 4.6 mm; length, 25 cm) was filled with Kromasil 100−5 C18. The mobile phase was methanol:water (70:30, V:V), and the flow rate was 1.0 ml min−1. The concentrations of the other phenylurea herbicides were determined using HPLC conditions identical to those described for isoproturon except the wave length 243 nm.
The identification of the metabolites during the degradation of isoproturon was determined by using HPLC-MS as described by Mascolo et al. (2001). The concentrations of isoproturon as well as the detection of isoproturon metabolites were monitored by HPLC-MS using an LCQ Deca XP (Thermo Fisher Scientific, USA). Samples, injected via a 100 ml Rheodyne loop, were eluted at a flow rate of 0.6 ml min−1 through a Kromasil 100−5 C18 equipped with a 20 mm pre-column by running a gradient from methanol/water (60/40, v/v). The flow from the HPLC was split to allow 50 ml to enter the ionspray interface. The mass spectrometric conditions (positive ions) were according to the protocol described by Mascolo et al. (2001).
Cloning of the dioxygenase gene from these strain
The extraction of total DNA was completed according to the protocol described as above. The dioxygenase gene were cloned from three isolates using a PCR-based technique. The primers were designed as follows: catAf 5′-CCATTGAAGGGCCGCTCTATGT-3′ and catAr 5′-ACCGAARTTGATCTGCGT(G,C)GTCA-3′. The PCR reaction was comprised of the same quantity as the reaction of 16S rRNA gene amplification mixture described by Huang et al. (2007) except for the primers. The mixture was subjected to 30 cycles of the following conditions: denaturation for 1 min at 95°C, annealing for 1 min at 55°C, and a final extension for 0.5 min at 72°C, with a final 5 min at 72°C extension step after cycling was complete. Sequencing reactions were performed by Invitrogen Biotechnology.
The phylogenetic tree based on partial sequences of catechol 1,2-dioxygenase gene was constructed following the same protocol as used for the construction of phylogenetic tree based on 16S rRNA gene.
Results
Isolation and characterization of the strains
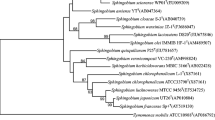
Three different isoproturon-degrading strains were isolated from the enrichment culture. Strain YBL1 was isolated from the soil collected from Nanjing and other two strains were isolated from the soil collected from Suzhou. These strains could grow on LB agar, forming slightly yellow, smooth and wet colonies within 2–3 days at 30°C. All strains were non-fluorescent yellow-pigmented, and found to be Gram-negative, strictly aerobic, non-spore-forming, and rod-shaped. Positive to oxidase, catalase and nitrate reductase tests, while negative to urease and amylase tests, sensitive to 3% NaCl, and resistant to 100 mg streptomycin l−1. Upon comparison of a partial 16S rRNA gene sequence (>1,200 bp) obtained from these strains with sequences from the GenBank database, the highest degree of similarity for these three strains (>99%) was obtained with the 16S rRNA gene sequence of Sphingobium fuliginis TKPT (Prakash and Lal 2006). A dendrogram illustrating the results of 16S rRNA gene analysis is presented in Fig. 1. These results indicated that strains YBL1, YBL2 and YBL3 belong to the genus Sphingobium. Although three strains were similar to each other on the basis of phenotypic and biochemical characteristics, the strain YBL2 could be distinguished from the other two on the basis of its colony size and the growth rate. The colony of YBL2 was much larger in size than the colonies of other two strains, and the growth rate of YBL2 in LB liquid medium was faster than YBL1 and YBL3 under the same conditions. Strain YBL3 was significantly different from the other two strains on the basis of comparative analysis of the 16S rRNA gene.
Phylogenetic tree based on partial 16S rRNA gene sequence was constructed by the neighbor-joining method and was rooted by using Rhodospirillum rubrum as the outgroup. Bootstrap values (%) are indicated at the nodes (only greater than 50% were shown). The scale bars represent 0.02 substitutions per site. The strains overstriking in figure could degrade isoproturon
Isoproturon degradation in MSM
In the degradation test, more than 99% of the initial isoproturon (50 mg l−1), added to the medium as the sole carbon source, was degraded by strain YBL2 at the inoculation rate of 107 cells ml−1 within 20 h, and the strain was also able to utilize 4IA as sole source of carbon for growth (Fig. 2). As shown in Fig. 3, strain YBL2 showed optimum degradation activity at pH 7.0, which dropped sharply above pH 9.0 or below pH 6.0, and the strain lost the ability to degrade isoproturon at pH 5.0. The optimum temperature for isoproturon degradation by YBL2 was 30°C, however it can degrade isoproturon between 20 and 35°C as well. Supplementing the medium with glucose considerably enhanced the isoproturon degradation rate; the addition of Cu2+, Ni2+ at the rate of 1 mM inhibited the capability of YBL2 to degrade isoproturon, while the Mg2+ at the same concentration enhanced the isoproturon degradation rate under the same conditions (Fig. 4). The concentration of isoproturon (within the chemical solubility in water) had no apparent effect on the degradation rate (data not shown). The degradation ability of diuron (10 mg l−1), chlorotoluron (10 mg l−1), fluometuron (10 mg l−1) by strain YBL2 is presented in Fig. 5, the degradation of 4IA (10 mg l−1) is shown in Fig. 2. Diuron and 4IA were completely degraded by YBL2 within 36 h. Isolates YBL1 and YBL3 were found to have similar characteristic to strain YBL2 for the degradation of isoproturon and 4IA (data not shown). Strain YBL1 can also degrade the methoxymethylphenylurea herbicide linuron at low degradation rate (Fig. 5b). The degradation of acetochlor and metsulfuron-methyl was not observed in the medium when inoculated by any of the strains YBL1, YBL2 or YBL3.
a Growth of strain YBL2 (▲) and degradation of isoproturon (■), the control (♦) was performed under the same conditions to the treatment except inoculating with strain. b Growth of strain YBL2 (▲) and degradation of 4IA (■), the control (♦) was performed under the same conditions to the treatment except inoculating with strain.. Error bars represent standard error in all of these figures
a Effect of muriate (1 mmol l−1) on the degradation of isoproturon by strain YBL2 (detected at 10 h after inoculation), the medium is MSM without MgSO4. b Effect of carbon and nitrogen source on the degradation of isoproturon (detected at 10 h after inoculation). I, MSM; II, MSM without NH4NO3; III, MSM added glucose. Error bars represent standard error in all these figures. The curve represent the number of YBL2 cells, the column represent the degradation of isoproturon in culture medium
Degradation of isoproturon in soils
Three different soils i.e. Magan soil, Flvo-Aquic soil and Red soil were used to check the degradation activity of strains. All of these three strains degraded isoproturon significantly (>80%) in Magan soil, and Flvo-Aquic soil within 2 days, while in Red soil the degradation activity was very poor (Fig. 6a). The degradation rate of other phenylurea herbicides, such as chlorotoluron and diuron was also observed to be higher in Magan soil (Fig. 6b). These strains also have the ability to degrade isoproturon in fresh soil (non-sterilized), but the degradation rate was slower in fresh soil than in sterilized soil (Fig. 6c).
a Degradation of isoproturon in Magan soil, Fluvo-Aquic soil and Red soil at 2 days after inoculation. b Degradation of isoproturon, chlorotoluron, and diuron in Magan soil at 24 h after inoculation. c Degradation of isoproturon in sterilized and non-sterilized Magan soil at 24 h after inoculation. Error bars represent standard error
Detection of the metabolites in the isoproturon degradation
A metabolite which has same mass as 3-(4-isopropylphenyl)-1-methylurea (MDIPU) was detected in the catabolic products of isoproturon, and another metabolite with mass 212, which is same as that of 3-(3-chloro-4-methylphenyl)-1-methylurea, was detected in the degradation of chlorotoluron by YBL2, analyzed by HPLC-MS. But none of the metabolites were detected in the degradation of isoproturon, chlorotoluron and diuron by YBL1 and YBL3 using HPLC. A mutant of YBL2 was obtained after several subcultures, and the mutant strain degraded isoproturon to MDIPU, but could not degrade MDIPU further.
Catechol, which was thought of as the key intermediate in the process of phenyl-ring cleavage of most aromatic hydrocarbons, was detected at the beginning of aniline degradation by strain YBL2 and also by the other two strains. Moreover cis,cis-muconic acid, the first metabolite after phenyl-ring cleavage, was also detected in the degradation products of aniline by HPLC. The activity of catechol 1,2-dioxygenase was 0.78, 0.67 and 0.79 μmol min−1 (mg protein)−1 in YBL1, YBL2 and YBL3 respectively.
Cloning of the dioxygenase gene from these isoproturon-degrading strains
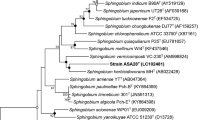
Using PCR method with the primers designed according to the dioxygenase gene sequences downloaded from the NCBI, a DNA fragment of about 420 bp was amplified (Fig. 7). The nucleotide sequences of the dioxygenase genes for strains YBL1, YBL2, YBL3 have been deposited in the GenBank database under the accession nos. EU825701, EU825702 and EU825703 respectively. A BLAST sequence search showed that these three genes had 83% sequence similarity to the dioxygenase gene catA of Sphingomonas sp. KA1 plasmid pCAR3 (GenBank accession No. AB270530), while the sequence similarity with other sequences was below 80%. The sequences from YBL2 and YBL3 have 99% sequence similarity with each other, but both of these two sequences showed only 79% sequence similarity with the sequence obtained from the strain YBL1 (Fig. 8).
Discussion
On the basis of phenotypic and physiological characterization and 16S rRNA gene sequence analysis, the three isolates capable of degrading isoproturon were identified as Sphingobium sp., and these strains are the first isoproturon-degrading strains discovered in this genus. The isolation of these strains confirmed that members of the genus Sphingobium characteristically play an important role in the biodegradation of organic waste (Dai and Copley 2004; Prakash and Lal 2006; Sharma et al. 2006). These three strains and another strain Sphingomonas sp. Y57 (Sun et al. 2006), which was isolated from the soil collected from Changzhou, were obtained from different soils collected from three different herbicide-manufacturing plants, however all of these three soils had been contaminated by isoproturon for a long time. The isolation of these strains suggested that exposure of soil to herbicide enhances the abundance and activity of herbicide-degrading bacteria (Ostrofsky et al. 1997; Pussemier et al. 1997).
Since several different bacteria that have the ability to mineralize phenylurea herbicides have been recently isolated (Dejonghe et al. 2003; El-Sebai et al. 2004; Sorensen et al. 2001; Widehem et al. 2002), these earlier studies together with the work described here, indicate that different strains belonging to different genera are involved in the metabolism of phenylurea herbicides, but all these strains had different characteristics of extent and degree of degradation. These phenylurea herbicide-degrading bacteria can be classified into three types. The first type is represented by the strain Sphingomonas sp. SRS2, the first isoproturon-degrading strain isolated by Sorensen (2001) which can also degrade the closely related dimethylurea herbicides, such as diuron and chlorotoluron, starting by N-demethylation followed by further degradation of the phenyl structure. Strains YBL2 and YBL3, which can degrade chlorotoluron, diuron, fluometuron and 4IA, also belonged to this group. The second type was the strain Methylopila sp. TES (El-Sebai et al. 2004), which only degraded isoproturon and slowly mineralized 4IA, but it was unable to degrade other phenylurea herbicides, and there was apparently no transient accumulation of any known isoproturon metabolites, such as MDIPU. In contrast to Sphingomonas sp. SRS2, strains Arthrobacter globiformis D47 (Turnbull et al. 2001) and Variovorax sp. WDL1 (Dejonghe et al. 2003) represent a third kind of phenylurea herbicide-degrading strains. This group is able to transform various phenylurea herbicides to their corresponding aniline derivatives, but then unable to degrade the corresponding aniline further. Strain YBL1 could metabolize most of the phenylurea herbicides including dimethylphenylurea and methoxymethylphenylurea i.e. linuron, though the degradation of linuron was at a low rate, meanwhile this strain was also able to degrade 4IA and aniline, so it may be assumed that the strain YBL1 was different from all three of types of phenylurea herbicide-degrading strains.
Sorensen et al. (2001) have previously pointed out that the initial N-demethylation of isoproturon to MDIPU was the first and a limiting step in the degradation of isoproturon. The transient accumulation of MDIPU in the catabolism of isoproturon by YBL2 indicates that the degradation of isoproturon was also initiated by N-demethylation of the dimethylurea side chain. The degradation was inhibited at MDIPU during the mineralization of isoproturon by mutant strains. This result indicated that there are two different enzymes in the cell to catalyze the two N-demethylations of the dimethylurea side chain or there was no other N-demethylation at all after the first step, or the two N-demethylations were brought about under different conditions or in different locations in the cell. The reason due to which the strain YBL2 lost the ability to degrade MDIPU remains to be clarified.
The pathway of degradation of aromatic compounds usually involves the incorporation of two atoms of oxygen into the aromatic ring and subsequent cleavage of the dihydroxylated compound. Such cleavage could either be ortho (between the two hydroxylated carbons) or meta (between a hydroxylated and non-hydroxylated carbon). There is one phenol ring in the molecule of isoproturon. Catechol and cis,cis-muconic acid were detected in the degradation of aniline by all of our isolates. The catechol 1,2-dioxygenase gene responsible for catechol cleavage was also cloned from all of these three strains. In the experiment of catechol degradation by the enzyme, the three isolates failed to show the appearance of a yellow coloration. All these results indicated that our strains can cleave the phenyl structure in the degradation of isoproturon via ortho-cleavage rather than meta-cleavage of catechol. More interestingly, although strain YBL2 has closer relation to strain YBL1 than to strain YBL3 on the basis of 16S rRNA gene analysis, inverse results were obtained on the catechol 1,2-dioxygenase gene analysis.
In the degradation test, the degradation rate of isoproturon by YBL2 in the culture medium supplemented with glucose was better than that in the culture medium with isoproturon only, and the counting results by MPN indicated that the enhancement of the degradation ability was a consequence of the augmentation of YBL2 degradation efficiency rather than co-metabolic activity. All of these three stains were unable to degrade isoproturon in the MSM containing Cu2+ or Ni2+ (1 mmol l−1), and there was no cell survival at all in these conditions (Fig. 4). These results indicated that all of three strains were sensitive to these two heavy metal ions.
Bioremediation is a cost-effective method to degrade pollutant chemicals into innocuous products. Successful removal of pesticides from soil by implanted bacteria has been reported for many compounds (Barles et al. 1979; Karpouzas and Walker 2000; Kearney et al. 1986). Previous studies (Bending et al. 2003; El-Sebai et al. 2007) have pointed out that soil pH was the most important factor to influence isoproturon degradation in soil. In this study, the pH range for YBL2 degrading isoproturon in MSM was pH 5.0–9.0, but the pH of the Red soil (pH 4.6) was out of this range, while the pH of the Magan soil (pH 6.7) and Fluvo-Aquic soil (pH 8.3) were in the range of pH, this may be the major reason that these strains degrade isoproturon much more slowly in red soil than in Magan soil and Fluvo-Aquic soil. The degradation rate of isoproturon by these strains in sterilized soil was faster than in fresh (non-sterilized) Magan soil. This may be attributed to the presence of other soil microflora in fresh soil, which affects the degradation efficiency of isolates; however it was not very significant. Although there were some limiting factors affecting the degradation of isoproturon by these strains, the inoculation of strain YBL2 was able to significantly enhance the removal rate of isoproturon in Magan soil and in Fluvo-Aquic soil. These results highlight the potential of the bacterium for use in the clean-up of isoproturon- and 4IA-contaminated environments, especially in soils with neutral or slightly alkaline pH i.e. Magan soil and Fluvo-Aquic soil.
References
Barles RM, Topp EE, Blackwell BA (1979) Accelerated parathion degradation in soil inoculated with acclimated bacteria under field conditions. Arch Environ Contam Toxicol 8:647–660. doi:10.1007/BF01054867
Bending GD, Lincoln SD, Sorensen SR (2003) In-field spatial variability in the degradation of the phenyl-urea herbicide isoproturon is the result of interactions between degradative Sphingomonas spp. and soil pH. Appl Environ Microbiol 69:827–834. doi:10.1128/AEM.69.2.827-834.2003
Bending GD, Lincoln SD, Edmondson RN (2006) Spatial variation in the degradation rate of the pesticides isoproturon, azoxystrobin and diflufenican in soil and its relationship with chemical and microbial properties. Environ Pollut 139:279–287. doi:10.1016/j.envpol.2005.05.011
Bending GD, Rodriguez-Cruz MS (2007) Microbial aspects of the interaction between soil depth and biodegradation of the herbicide isoproturon. Chemosphere 66:664–671. doi:10.1016/j.chemosphere.2006.07.099
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Celis E, Elefsiniotis P, Singhal N (2008) Biodegradation of agricultural herbicides in sequencing batch reactors under aerobic or anaerobic conditions. Water Res 42:3218–3224. doi:10.1016/j.watres.2008.04.008
Dai M, Copley SD (2004) Genome shuffling improves degradation of the anthropogenic pesticide pentachlorophenol by Sphingobium chlorophenolicum ATCC 39723. Appl Environ Microbiol 70:2391–2397. doi:10.1128/AEM.70.4.2391-2397.2004
Dejonghe W, Berteloot E, Goris J (2003) Synergistic degradation of linuron by a bacterial consortium and isolation of a single linuron-degrading variovorax strain. Appl Environ Microbiol 69:1532–1541. doi:10.1128/AEM.69.3.1532-1541.2003
El-Sebai TE, Lagacherie B, Soulas G (2004) Isolation and characterization of an isoproturon-mineralising Methylopela sp. TES from French agricultural soil. FEMS Microbiol Lett 239:103–110. doi:10.1016/j.femsle.2004.08.017
El-Sebai TE, Lagacherie B, Soulas G (2007) Spatial variability of isoproturon mineralizing activity within an agricultural field: geostatistical analysis of simple physicochemical and microbiological soil parameters. Environ Pollut 145:680–690. doi:10.1016/j.envpol.2006.05.034
Holt J, Krieg N, Sneath P (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams and Wilkins, Baltimore
Huang X, He J, Sun J (2007) Isolation and characterization of a metsulfuron-methyl degrading bacterium Methylopila sp. S113. Int Biodeter Biodegr 60:152–158. doi:10.1016/j.ibiod.2007.02.005
Johnson AC, Besien T, Bhardwaj C (2001) Penetration of herbicides to groundwater in an unconfined chalk aquifer following normal soil applications. J Contam Hydrol 53:101–117. doi:10.1016/S0169-7722(01)00139-5
Karpouzas DG, Walker A (2000) Factors influencing the ability of Pseudomonas putida epI to degrade ethoprophos in soil. Soil Biol Biochem 32:1753–1762. doi:10.1016/S0038-0717(00)00093-6
Kristensen KE, Jacobsen CS, Hansen LH (2006) Genetic labelling and application of the isoproturon mineralizing Sphingomonas sp. strain SRS2 in soil and rhizosphere. Lett Appl Microbiol 43:280–286. doi:10.1111/j.1472-765X.2006.01956.x
Kearney PC, Karns JS, Muldoon MT (1986) Coumaphos disposal by combined microbial and UV-ozonation reactions. J Agric Food Chem 34:702–706. doi:10.1021/jf00070a028
Mansour M, Feicht EA, Behechti A (1999) Determination photostability of selected agrochemicals in water and soil. Chemosphere 39:575–585. doi:10.1016/S0045-6535(99)00123-X
Mascolo G, Lopez A, James H (2001) By-products formation during degradation of IPU in aqueous solution II: chlorination. Water Res 35:1705–1713. doi:10.1016/S0043-1354(00)00428-0
Morvan X, Mouvet C, Baran N (2006) Pesticides in the groundwater of a spring draining a sandy aquifer: temporal variability of concentrations and fluxes. J Contam Hydrol 87:176–190. doi:10.1016/j.jconhyd.2006.05.003
Ngai K-L, Neidle EL, Ornston CN (1990) Catechol and chlorocatechol 1,2-dioxygenase. Methods Enzymol 188:122–126. doi:10.1016/0076-6879(90)88022-3
Ostrofsky E, Traina S, Tuovinen O (1997) Variation in atrazine mineralization rates in relation to agricultural management practice. J Environ Qual 26:647–657
Peres F, Florin D, Grollier T (1996) Effect of the phenylurea herbicide isoproturon on periphytic diatom communities in freshwater indoor microcosms. Environ Pollut 94:141–152. doi:10.1016/S0269-7491(96)00080-2
Prakash O, Lal R (2006) Description of Sphingobium fuliginis sp. nov., a phenanthrene-degrading bacterium from a fly ash dumping site, and reclassification of Sphingomonas cloacae as Sphingobium cloacae comb.nov. Int J Syst Evol Microbiol 56:2147–2152. doi:10.1099/ijs.0.64080-0
Pussemier L, Goux S, Vanderheyden V (1997) Rapid dissipation of atrazine in soils taken from various maize fields. Weed Res 37:171–179. doi:10.1046/j.1365-3180.1997.d01-18.x
Radosevich M, Traina SJ, Hao YL (1995) Degradation and mineralization of atrazine by a soil bacterial isolate. Appl Environ Microbiol 61:297–302
Ronhede S, Jensen B, Rosendahl S (2005) Hydroxylation of the herbicide isoproturon by fungi isolated from agricultural soil. Appl Environ Microbiol 71:7927–7932. doi:10.1128/AEM.71.12.7927-7932.2005
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sharma P, Raina V, Kumari R (2006) Haloalkane dehalogenase linB is responsible for beta- and delta-hexachlorocyclohexane transformation in Sphingobium indicum B90A. Appl Environ Microbiol 72:5720–5727. doi:10.1128/AEM.00192-06
Sorensen SR, Ronen Z, Aamand J (2001) Isolation from agricultural soil and characterization of a Sphingomonas sp. able to mineralize the phenylurea herbicide isoproturon. Appl Environ Microbiol 67:5403–5409. doi:10.1128/AEM.67.12.5403-5409.2001
Sorensen SR, Albers CN, Aamand J (2008) Rapid mineralization of the phenylurea herbicide diuron by Variovorax sp. strain SRS16 in pure culture and within a two-member consortium. Appl Environ Microbiol 74:2332–2340. doi:10.1128/AEM.02687-07
Sorensen SR, Bending GD, Jacobsen CS (2003) Microbial degradation of isoproturon and related phenylurea herbicides in and below agricultural fields. FEMS Microbiol Ecol 45:1–11. doi:10.1016/S0168-6496(03)00127-2
Sun J, Huang X, He J (2006) Isolation identification of isoproturon degradation bacterium Y57 and its degradation characteristic. China Environ Sci 26:315–319 in Chinese
Tamura K, Dudley J, Nei M (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi:10.1093/molbev/msm092
Topp E, Zhu H, Nour SM (2000) Characterization of an atrazine-degrading Pseudaminobacter sp. isolated from Canadian and French agricultural soils. Appl Environ Microbiol 66:2773–2782. doi:10.1128/AEM.66.7.2773-2782.2000
Turnbull GA, Ousley M, Walker A (2001) Degradation of substituted phenylurea herbicides by Arthrobacter globiformis strain D47 and characterization of a plasmid-associated hydrolase gene, puhA. Appl Environ Microbiol 67:2270–2275. doi:10.1128/AEM.67.5.2270-2275.2001
Walker A, Jurado-Exposito M, Bending G (2001) Spatial variability in the degradation rate of isoproturon in soil. Environ Pollut 111:407–427. doi:10.1016/S0269-7491(00)00092-0
Walker A, Bromilow RH, Nicholls PH (2008) Spatial variability in the degradation rates of isoproturon and chlorotoluron in a clay soil. Weed Res 42:39–44. doi:10.1046/j.1365-3180.2002.00260.x
Widehem P, Ait-Aissa S, Tixier C (2002) Isolation, characterization and diuron transformation capacities of a bacterial strain Arthrobacter sp. N2. Chemosphere 46:527–534. doi:10.1016/S0045-6535(01)00192-8
Widenfalk A, Bertilsson S, Sundh I (2008) Effects of pesticides on community composition and activity of sediment microbes—responses at various levels of microbial community organization. Environ Pollut 152:576–584. doi:10.1016/j.envpol.2007.07.003
Widenfalk A, Goedkoop W, Svensson JM (2004) Effects of the pesticides captan, deltamethrin, isoproturon, and pirimicarb on the microbial community of a freshwater sediment. Environ Toxicol Chem 23:1920–1927. doi:10.1897/03-345
Zhang XH, Zhang GH, Li SP (2006) Isolation and characterization of a dichlorvos-degrading strain DDV-1 of Ochrobactrum sp. Pedosphere 16:64–71. doi:10.1016/S1002-0160(06)60027-1
Acknowledgments
This work was supported by Agricultural Technology Transfer Program (grant 2007.100) and National Foundation Program for Nature Resources of Science and Technology (2005DKA21201-11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, JQ., Huang, X., Chen, QL. et al. Isolation and characterization of three Sphingobium sp. strains capable of degrading isoproturon and cloning of the catechol 1,2-dioxygenase gene from these strains. World J Microbiol Biotechnol 25, 259–268 (2009). https://doi.org/10.1007/s11274-008-9888-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9888-y