Abstract
A facile synthesis of spirobiindane bis-coumarin hybrid molecules with C4-alkyl, C3-acyl, C3-ethoxycarbonyl or C3-nitrile groups has been achieved. Absorption, emission and solvatochromic characteristics of the bis-coumarins were evaluated in comparison with 3-acetylcoumarin. Studies show that the bis-coumarins with C3-ester and C3-cyano group show more than tenfold increase in fluorescence emission than others in hexane and ethyl acetate. Unlike in the cases of the bis-coumarins with an electron withdrawing acyl or ester group at C3 position where methanol quenches fluorescence emission there is no significant solvent effect in the case of an alkyl group at C4 position.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural and synthetic coumarins find many applications as drug candidates, optical brighteners, energy storage materials, triplet sensitizers etc. [1–4]. Among coumarins, bis-coumarins, where two coumarin units are present in a single molecule are a unique class of compounds with wide ranging biological activities [5]. Many bis-coumarins, e.g. desertonine 1 (Fig. 1) where two coumarin units are covalently linked directly or by a tether occur in plants, microorganisms and animals. Such natural and synthetic bis-coumarins are flexible and exhibit innumerable rotomeric populations. Therefore they may not efficiently exhibit synergic properties due to both the coumarin units. Bis-coumarins located on a rigid molecular frame work, on the other hand, are better suited for exhibiting inter and intramolecular synergic properties particularly those related to photochromic absorption and emission. In continuation of our interest in the synthesis of coumarins [6–10], we have conceived of novel bis-coumarins like 2 (Fig. 1) present on a rigid molecular motif. While there have been directed synthetic efforts towards linked bis-coumarins, to our knowledge there are no reports on the synthesis of bis-coumarins incorporated on a rigid molecular frame work like the one present on spirobiindane bisphenol 2. The spirobiindane bisphenol derivatives similar to 2 found use in the synthesis of foldamers [11–13], polymers [14], [15] and photo-electronic devices [16]. Such polymeric carbon networks were projected to have technological applications. Herein we describe synthesis, characterization and fluorochromic properties of bis-coumarins incorporated on the spirobiindane motif.
Experimental
The progression of all the reactions was monitored by TLC using hexanes (60–80 °C boiling mixture)/ethyl acetate mixture as eluent. Column chromatography was carried on silica gel (100–200 mesh SRL chemicals) using increasing percentage of ethyl acetate in hexanes. 1H NMR spectra (400 MHz), 13C NMR (100 MHz) and DEPT spectra were recorded for (CDCl3 + CCl4 1:1) solutions on a Bruker-400 spectrometer with tetramethylsilane (TMS) as internal standard and J-values are in Hz. IR spectra were recorded as KBr pellets on a Nicolet-6700 spectrometer. UV spectra were recorded using Hitachi ratio-beam spectrometer. Melting points were recorded using open-ended capillary tubes on VEEGO VMP-DS instrument. High resolution mass spectra were recorded on a Waters Micromass Q-TOF micro mass spectrometer using electron spray ionization mode. Organic solvents were distilled and dried before use.
Synthesis of 4-alkyl substituted spirocoumarin

4,4′,6,6,6′,6′-Hexamethyl-6,6′,7,7′-tetrahydro-2H,2′H-8,8′-spirobi[cyclopenta[g]chromene]-2,2′-dione 5a
A stirred solution of spirobiindane bisphenol 3 (220 mg, 0.65 mmol) in 75 % H2SO4 (2 ml) ethyl acetoacetate 4a (422 mg, 3.24 mmol) was added at 100 °C under the blanket of nitrogen atmosphere and refluxed for 2 h. After completion of reaction, mixture was diluted with ice cold water (10 ml) and extracted with DCM (3 × 10 ml). The combined organic layer was washed with saturated sodium bicarbonate solution (10 ml) for removal of excess H2SO4 and then washed with water (2 × 10 ml) and brine solution (10 ml) dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography on silicagel (100–200 mesh) using hexanes and EtOAC (8.2:1.8) as eluent to obtain bis-coumarin as reddish yellow colour solid, mp 101 °C, yield 68 %; IR (KBr) 2965, 2865, 1732, 1619, 1569, 1456, 1425, 1387, 1325, 1118, 886 cm−1; 1H NMR (400 MHz CDCl3 + CCl4) δ 7.34 (s, 1H), 6.70 (s, 1H), 6.23 (s, 1H), 2.49 (s, 3H), 2.32 (d, 1H, J = 13.2 Hz), 2.29 (d, 1H, J = 13.2 Hz), 1.49 (s, 3H), 1.41 (s, 3H) ppm; 13CNMR (100 MHz, CDCl3 + CCl4) δ 160.1 (C), 154.5 (C), 153.7 (C), 151.5 (C), 148.2 (C), 119.7 (C), 117.4 (CH), 114.5 (CH), 112.5 (CH), 59.4 (CH2), 57.6 (C), 43.5 (C), 32.0 (CH3), 30.4 (CH3), 18.8 (CH3) ppm; LCMS 440.90 (100 %), 415, 218, 134.

4,4′-Diethyl-6,6,6′,6′-tetramethyl-6,6′,7,7′-tetrahydro-2H,2′H-8,8′spirobi[cyclopenta[g]chromene]-2,2′-dione 5b
Yellow color solid, mp 101 °C, yield 74 %; IR (KBr) 2968, 2861, 1730, 1621, 1565, 1452, 1402, 1393, 1321, 1120, 889 cm−1; 1H NMR (400 MHz, CDCl3 + CCl4) δ 7.34 (s, 1H), 6.70 (s, 1H), 6.23 (s, 1H), 4.02 (q, J = 4.8 Hz, 2H), 2.49 (t, 3H, J = 4.8 Hz), 2.32 (d, 1H, J = 13.2 Hz), 2.29 (d, 1H, J = 13.2 Hz), 1.49 (s, 3H), 1.41 (s, 3H) ppm; 13CNMR (100 MHz, CDCl3 + CCl4) δ 160.1 (C), 154.5 (C), 153.7 (C), 151.5 (C), 148.2 (C), 119.7 (C), 117.4 (CH), 114.5 (CH), 112.5 (CH), 59.4 (CH2), 57.6 (C), 43.5 (C), 32.0 (CH3), 30.4 (CH3), 18.8 (CH3) ppm; LCMS 454.90 (100 %), 425, 223.

Synthesis of spirobiindane based bis-coumarins 7a–d
3,3′-Diacetyl-6,6,6′,6′-tetramethyl-6,6′,7,7′-tetrahydro-2H,2′H-8,8′-spirobi[cyclopenta[g] chromene]-2,2′-dione 7a
To a stirred solution of diformyl Spirobiindane bisphenol 6 (10 mg, 0.274 mmol) ethylacetoacetate 4a (96 mg, 0.60 mmol) in EtOH (2 ml), piperidine (2.6 mg, 0.032 mmol) in dry ethanol (2 ml) was added over a period of 5 min drop wise at rt under the blanket of dry nitrogen. This reaction was heated at reflux condition in a pre heated oil bath (80 °C) for 5 h for completion of reaction (TLC, 20 % EtOAc in hexanes; Rf = 0.3). Reaction mixture diluted with ethanol (1 ml). Evaporation of solvent under reduced pressure resulted in crude product as light yellow colour solid. Reaction mixture was extracted DCM (25 ml) and the organic solution was washed subsequently with water (3 × 25 ml) and brine (2 × 10 ml). This organic layer was dried over anhydrous Na2SO4. Crude product was subjected to column chromatography on SiO2 (25 g: 30 cm × 2 cm) using increasing amounts of EtOAc in hexanes as eluant. Colourless solid, Yield 75 %, mp above 250 °C; IR (KBr) υmax 3046, 2956, 2866, 1767, 1685, 1611, 1551, 1415, 1363, 1202, 1107, 974, 877, 769, 581, 456 cm−1; 1H NMR (400 MHz, CDCl3 + CCl4) δ 8.48 (s, 1H), 7.43 (s, 1H), 6.71 (s, 1H), 2.66 (s, 3H), 2.41 (d, 1H, J = 13.2 Hz), 2.37 (d, 1H, J = 13.2 Hz), 1.47 (s, 3H), 1.39 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3 + CCl4) δ195.0 (C), 158.9 (C), 157.6 (C), 155.6 (C), 149.5 (C), 147.3 (CH), 123.8 (C), 123.6 (CH), 118.3 (C), 112.3 (CH), 59.2 (CH2), 58.6 (C), 43.7 (C), 32.0 (CH3), 30.6 (CH3), 30.4 (CH3) ppm; HRMS (ESI, m/z) 519.1784 calcd for C31H28O6 (M+Na) found 519.1783.

6,6,6′,6′-Tetramethyl-3,3′-dipropionyl-6,6′,7,7′-tetrahydro-2H,2′H-8,8′-spirobi[cyclopenta [g]chromene]-2,2′-dione 7b
Colourless solid, mp > 250 °C, yield 92 %; IR (KBr) υmax 3037, 2960, 2870, 1733, 1684, 1614, 1550, 1458, 1416, 1366, 1250, 1181, 949, 808 cm−1; 1H NMR (400 MHz, CDCl3 + CCl4) δ 8.50 (s, 1H), 7.44 (s, 1H), 6.72 (1H, s), 3.10 (q, 2H, J = 10.0 Hz), 2.41 (d, 1H, J = 13.2 Hz), 2.38 (d, 1H, J = 13.2 Hz), 1.47 (s, 3H), 1.39 (s, 1H), 1.15 (t, 3H, J = 8.0 Hz) ppm; 13C NMR (100 MHz, CDCl3 + CCl4) δ 198.2 (C), 158.9 (C), 157.5 (C), 155.5 (C), 149.5 (C), 147.3 (CH), 123.7 (C), 123.5 (CH), 118.4 (C), 112.2 (CH), 59.2 (CH2), 58.5 (C), 43.7 (C), 36.1 (CH2), 32.0 (CH3), 30.4 (CH3), 8.1 (CH3) ppm; HRMS (ESI, m/z) 547.2097 calcd for C33H32O6 (M+Na) found 547.2092.

3,3′-Dibutyryl-6,6,6′,6′-tetramethyl-6,6′,7,7′-tetrahydro-2H,2′H-8,8′-spirobi[cyclopenta [g]chromene]-2,2′-dione 7c
Colourless solid, mp > 250 °C, yield 94 %; IR (KBr) υmax 3037, 2960, 2870, 1733, 1684, 1614, 1550, 1458, 1416, 1366, 1250, 1181, 949, 808 cm−1; 1H NMR (400 MHz, CDCl3 + CCl4) δ 8.50 (s, 1H), 7.44 (s, 1H), 6.72 (1H, s), 3.10 (q, 2H, J = 10 Hz), 2.41 (d, 1H, J = 13 Hz), 2.38 (d, 1H, J = 13 Hz), 1.47 (s, 3H), 1.39 (s, 3H), 1.24-1.21 (m, 2H),1.15 (t, 3H, J = 8 Hz) ppm; 13C NMR (100 MHz, CDCl3 + CCl4) δ 198.2 (C), 158.9 (C), 157.5 (C), 155.5 (C), 149.5 (C), 147.3 (CH), 123.7 (C), 123.5 (CH), 118.4 (C), 112.2 (CH), 59.2 (CH2), 58.5 (C), 43.7 (C), 36.1 (CH2), 32.0 (CH3), 30.4 (CH3), 9.2 (CH2), 8.1 (CH3) ppm; HRMS (ESI, m/z) 575.2609 calcd for C33H32O6 (M+Na) found 575.2611.

3,3′-Dibenzoyl-6,6,6′,6′-tetramethyl-6,6′,7,7′-tetrahydro-2H,2′H-8,8′-spirobi[cyclopenta [g]chromene]-2,2′-dione 7d
Yeild 89 %; mp 201 °C; IR (KBr) υmax 3052, 2959, 286, 1731, 1660, 1612, 1557, 1268, 1215, 1163, 930, 860, 765, 685 cm−1; 1H NMR (400 MHz, CDCl3 + CCl4) δ 8.10 (s, 1H), 7.84 (d, 2H, J = 8.0 Hz), 7.58 (t, 1H, J = 8.0 Hz), 7.45 (t, 2H, J = 8.0 Hz), 6.78 (s, 1H), 2.46 (d, 1H, J = 13.2 Hz), 2.41 (d, 1H, J = 13.2 Hz), 1.48 (s, 3H), 1.41 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3 + CCl4) δ 191.7 (C), 158.1 (C), 157.0 (C), 155.1 (C), 149.5 (C), 145.4 (CH), 136.6 (C), 133.7 (CH), 129.7 (CH), 128.6 (CH), 126.5 (C), 122.4 (CH), 118.1 (C), 112.6 (CH), 59.2 (CH2), 58.4 (C), 43.8 (C), 32.1 (CH3), 30.2 (CH3) ppm; HRMS (ESI, m/z) 643.2097 calcd for C41H32O6 (M+Na) found 643.2098; Crystals obtained from CH2Cl2, molecular formula: C41H32O6, monoclinic, space group P-1(No. 14), a = 12.1947(14), b = 14.2673(17), c = 19.6415(15) Å, α = 73.579 (9), β = 82.205 (9), γ = 84.827 (10), V = 3244.1(10) Å3, T = 293(2) K, Z = 5, ρc = 1.427 g cm–3, F(000) = 1630, graphite-monochromated MoKα radiation (λ = 0.71073 Å), μ (MoKα) = 0.106 mm−1, colourless (0.5 × 0.4 × 0.2 mm3), empirical absorption correction with SADABS (transmission factors: 1–0.8086), 2400 frames, exposure time 10 s, 2.60 ≤ θ ≤ 26.00, −16 ≤ h ≤ 9, −19 ≤ k ≤ 17, −26 ≤ l ≤ 27, 27648 reflections collected, 14809 independent reflections (R int = 0.1164), solution by direct methods (SHELXS97a) and subsequent Fourier syntheses, full-matrix least-squares on F 2o (SHELX97), hydrogen atoms refined with a riding model, data/restraints/parameters = 14809/0/855, S(F 2) = 1.103, R(F) = 0.0495 and wR(F 2) = 0.1140 on all data, R(F) = 0.3548 and wR(F 2) = 0.2051 for 7514 reflections with I > 2σ(I), weighting scheme w = 1/[σ2(F 2o ) + (0.1167P)2 + 1.646P] where P = (F 2o + 2F 2c )/3, largest difference peak and hole 0.416 and −0.172 e Å−3. Crystallographic data (excluding structure factors) for the structure reported here have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-779835. Copies of the data can be obtained on application to CCDC, 12 Union Road, Cambridge CB21EZ, UK (fax: (+44) 1223-336-033; e-mail: deposit@ccdc.cam.ac.uk).

Diethyl 6,6,6′,6′-tetramethyl-2,2′-dioxo-6,6′,7,7′-tetrahydro-2H,2′H-8,8′-spirobi[cyclopenta [g]chromene]-3,3′-dicarboxylate 9a
Colourless solid, yield 75 %, mp 203 °C; IR (KBr) υmax 3050, 2959, 2867, 1780, 1768, 1616, 1558, 1466, 1373, 1277, 1209, 876, 795 cm−1; 1H NMR (400 MHz, CDCl3 + CCl4) δ 8.55 (s, 1H), 7.41 (s, 1H), 6.72 (s, 1H), 4.40 (q, 2H, J = 8 Hz, J = 3 Hz), 2.43 (d, 1H, J = 13.2 Hz), 2.39 (d, 1H, J = 13.2 Hz), 1.48 (s, 3H), 1.41 (s, 3H), 1.41 (t, 2H, J = 8.0 Hz); 13C NMR (100 MHz, CDCl3 + CCl4) δ 163.3 (C), 157.6 (C), 156.5 (C), 155.4 (C), 149.4 (C), 148.7 (CH), 127.9 (C), 123.0 (CH), 117.9 (C), 112.3 (CH), 61.9 (CH2), 59.2 (CH2), 58.5 (C), 43.6 (C), 32.0 (CH3), 30.4 (CH3), 14.4 (CH3) ppm; HRMS (ESI, m/z) calcd for C33H32O8 (M+Na) 579.1995 and found 579.1996; Crystals obtained from CH2Cl2, molecular formula: C33H32O8, monoclinic, space group P21/c, a = 28.212 (3), b = 19.308 (3), c = 16.7559 (18) Å, α = 90, β = 109.382 (11), γ = 90, V = 8610.0 (18) Å3, T = 293(2) K, Z = 13, ρc = 1.395 g cm–3, F (000) = 3822, graphite-monochromated MoKα radiation (λ = 0.71073 Å), μ (MoKα) = 0.100 mm−1, colourless (0.5 × 0.4 × 0.2 mm3), empirical absorption correction with SADABS (transmission factors: 1–0.8086), 2400 frames, exposure time 10 s, 2.53 ≤ θ ≤ 29.30, −22 ≤ h ≤ 37, −25 ≤ k ≤ 22, −21 ≤ l ≤ 20, 39145 reflections collected, 19689 independent reflections (R int = 0.1493), solution by direct methods (SHELXS97a) and subsequent Fourier syntheses, full-matrix least-squares on F 2o (SHELX97), hydrogen atoms refined with a riding model, data/restraints/parameters = 19689/0/1126, S (F 2) = 1.103, R (F) = 0.0737 and wR (F 2) = 0.0739 on all data, R(F) = 0.4973 and wR (F 2) = 0.1328 for 7514 reflections with I > 2σ(I), weighting scheme w = 1/[σ2(F 2o ) + (0.1167P)2 + 1.646P] where P = (F 2o + 2F 2c )/3, largest difference peak and hole 0.135 and –0.153 e Å−3. Crystallographic data (excluding structure factors) for the structure reported here have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-780576. Copies of the data can be obtained on application to CCDC, 12 Union Road, Cambridge CB21EZ, UK (fax: (+44) 1223-336-033; e-mail: deposit@ccdc.cam.ac.uk).

6,6,6′,6′-Tetramethyl-2,2′-dioxo-6,6′,7,7′-tetrahydro-2H,2′H-8,8′-spirobi[cyclopenta [g]chromene]-3,3′-dicarbonitrile 9b
Colourless solid, yield 85 %, mp above 250 °C; IR (KBr) υmax 3046, 2956, 2866, 2011, 1767, 1685, 1611, 1551, 1415, 1363, 1202, 1107, 974, 877. 769, 581, 456 cm−1; 1H NMR (400 MHz CDCl3 + CCl4) δ 8.55 (s, 1H), 7.41 (s, 1H), 6.72 (s, 1H), 2.47 (d, 1H, J = 13.2 Hz), 2.36 (d, 1H, J = 13.2 Hz), 1.48 (s, 3H), 1.41 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3 + CCl4) δ 157.6 (C), 156.5 (C), 155.4 (C), 149.4 (C), 148.7 (CH), 123.0 (CH), 117.9 (C), 117.5 (CH), 112.3 (C), 103.5 (C), 59.2 (CH2), 58.5 (C), 43.6 (C), 32.0 (CH3), 30.4 (CH3) ppm; HRMS (ESI, m/z) 485.l572 calcd for C29H22N2O4 (M+Na) found 485.l570.
Results and Discussion
Synthesis of spirobiindane based bis-coumarins
Our first target in the synthesis of spirobiindane based bis-coumarins was the C4-alkyl courmains 5, which can be generated by reacting alkyl acetoacetates with spirobiindane bisphenol 3 by double Von-Pechman condensation [17]. The bisphenol 3 was prepared by methanesulfonic acid catalyzed deep-seated rearrangement of bisphenol A [18]. The spirobiindane mounted bisphenol 3 underwent facile iterative Von-Pechmann condensation when treated with two equivalents of ethyl acetoacetate 4a under strongly acidic conditions to furnish the bis-coumarin 5a in good yield (Scheme 1). Reflecting its C2 symmetric nature, bis-coumarin 5a exhibited three singlets for the methyl groups and a double doublet for the methylene group in its 1H NMR spectrum. As anticipated the 13C NMR spectrum of 5a displayed 15 signals out of which six were in aliphatic region. The DEPT-135 spectrum, when analyzed along with the 13C NMR spectrum, confirmed the assigned structure. The Von-Pechmann condensation of spirobiindane bisphenol 3 with β-keto esters 4 to form C4 alkyl bis-coumarin 5 was generalized by reacting β-keto ester 4b with bisphenol 3 to provide 5b in good yield (Scheme 1).
Next we targeted synthesis and characterization of bis-3-acylcoumarins 7, as such coumarins are expected to exhibit useful flourochromic properties [19]. Initially, the spirobiindane bisphenol 3 was converted into bis-2-hydroxybenzaldehyde 6 by iterative Duff reaction (Scheme 2) [20]. Among the three options for conversion of bisphenol 3 to bis-2-hydroxybenzaldehyde 6, namely Reimer Teimann reaction [21] and Vilsmeier reaction [22] and Duff reaction, last one worked well to provide 6 in good yield. The Knoevenagel condensation [23] of bis-2-hydroxybenzaldehyde 6 with 2 equivalents of ethyl acetoacetate 4a in presence of a catalytic amount of piperidine provided spirobiindane based bis-coumarin 7a (Scheme 2). The 1H NMR spectrum of 7a exhibited three singlets as anticipated from its C2 symmetric nature. A singlet at δ 8.5 ppm was the diagnostic peak assignable to olefinic C4-H. In addition to other anticipated signals the 13C NMR spectrum of 7a exhibited two carbonyl carbon signals at δ 195 and 159 ppm. The conversion of bis-2-hydroxybenzaldehyde 6 into bis-coumarin 7a proved to be general as we synthesized 7b–d by employing three more β-keto esters 4b–d (Scheme 2). In each case spectral data compared well with the parent bis-coumarin 7a. Structure of the spirobiindane based 3-acylcoumarin 7d was unequivocally established on the basis of single crystal X-ray diffraction data analysis (Fig. 2).
Next, we conducted the Knoevenagel condensation of spirobiindane bis-2-hydroxy benzaldehyde 6 with two more active methylene compounds namely diethyl melonate 8a and ethyl cyanoacetate 8b which provided spirobiindane based coumarins 9a and 9b respectively without any difficulty (Scheme 3). The 1H, 13C and DEPT-135 NMR Spectra of 9a–b matched well with the bis-3-acetylcoumarin 7a. In addition, single crystal X-ray diffraction data analysis of 9a unambiguously confirmed its assigned structure (Fig. 3).
Photophysical Properties
3-Acylcoumarins, in general, exhibit rich photochemical and photophysical properties useful for many technological applications [24]. Upon excitation 3-acylcoumarins increase their dipole moment through contribution of resonance forms B and C as shown in Fig. 4. Owing to stabilizing dipolar nature of the resonance forms B and C (Fig. 4) coumarins with electron donating groups at C7 position show good solvatochromic effects, which can be exploited for the characterization of micelles [25]. We have recorded absorption and emission spectra of spirobiindane bis-coumarins 5a, 7a, 7b, 7d, 9a, 9b and compared the data with that of the parent 3-acetylcoumarin.
UV–Vis spectral analysis
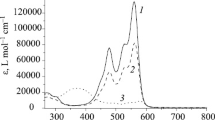
3-Acetylcoumarins exhibit two intense UV absorption bands with λmax located around 300 and 350 nm attributable to π–π* excitation of two major resonance forms B and C (Fig. 4) [26, 27]. The absorption spectral data of spirobindane bis-coumarins 5a, 7a, 7b, 7d, 9a, 9b and 3-acetylcoumrin in three solvents namely hexane (non-polar), ethyl acetate (EtOAc, moderately polar, aprotic) and methanol (MeOH, polar protic) are gathered in Table 1. Similar to the parent 3-acetylcoumrin, all the bis-coumarins prepared in this study show two almost equally intense bands located around 300 and 350 nm (Fig. 5). While the parent 3-acetylcoumrin exhibits bathochromic shift and hypochromic effect on change of solvents from hexane to methanol there is no such trend for bis-coumarins which indicate that solvent does not interfere for the UV absorption of the molecule. However, the hyperchromic effect seen in bis-coumarins (Fig. 5; Table 1) may be attributable to the presence of two chromophores in close proximity.
Fluorescence Emission Spectral Analysis
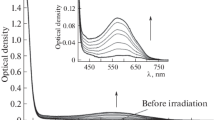
The fluorescence emission spectral data of spirobiindane bis-coumarins 5a, 7a, 7b, 7d, 9a, 9b and 3-acetylcoumrin in three solvents namely hexane (non-polar), EtOAc (moderately polar, aprotic) and MeOH (polar protic) are gathered in Table 2 (see also Fig. 6). Fluorescence quantum yields for the newly made bis-coumarins 5a, 7a, 7b, 7d, 9a, 9b were measured with respect to 3-acetylcoumarin (0.011 in hexane) and were found to be in the range of 0.02–0.09 in hexane which indicates that spirobiindane based bis-coumarins exhibit better fluorescence emission (Fig. 6). Compared to 3-acetylcoumarin the bis-coumarins 9a and 9b which have ester and cyano substitutions at C3 position respectively exhibit ten-fold increase in fluorescence emission in hexane and about 15-fold increase in EtOAc. Excepting the bis-coumarin 5a others 7a, 7b, 7d, 9a, 9b exhibit about ten-fold quenching by the polar protic solvent (MeOH) in moving from the non-polar (hexane) solvent, which shows that the excited states are Inoue hard-anionic [28]. Among the bis-coumarins studied, one with C3 ester group 9a exhibits maximum Stokes shift of 84 nm in hexane. The solvatochromic effect on 5a includes a red shift of about 59 nm on emission maximum when the medium is changed from non polar (hexane) to polar protic solvent (MeOH) and the near absence of quenching on changing the solvent from hexane to methanol. These observations indicate Inoue soft-anionic character of the excited state of 5a. Excepting the coumarin 7d all other bis-coumarins exhibit large Stokes shift of 59–84 nm similar to that of the parent 3-acetylcoumarin. Since excitation increases the molecular dipole moment in the coumarin motif both reorganization of the solvent and conformational changes are expected to be involved in stabilization of the excited state, thus making Stokes shift larger [29].
Conclusion
Some perpendicularly disposed rigid bis-coumarins embedded on spirobiindane frame work were synthesized from spirobiindane bisphenol in two high yielding steps. Newly synthesized bis-coumarins possess electron-donating C4-alkyl group or electron-withdrawing C3-acyl/ester/cyano groups. Both absorption and emission characteristics of the bis-coumarins show that they have better photochromic properties compared to 3-acetylcoumarin which arise out of synergy between two perpendicularly disposed coumarin units.
References
Hepworth J (1984) Comprehensive heterocyclic chemistry I. In: Katritzky AR, Rees CW (eds). Pergamon, Oxford, vol 3, pp 737–883
Geen GR, Evans JM, Vong AK (2005) Comprehensive heterocyclic chemistry II. In: Katritzky AR, Rees CW, Scriven EF (eds). Pergamon, Oxford, vol 5, pp 469–500
Padwa A (2008) Comprehensive heterocyclic chemistry III. In: Katritzky AR, Ramsden C, Scriven E, Taylor R (eds) Rhbne-Poulenc Rore, Dagenham, vol 5, pp 554–671
Murry RDH, Mendez J, Brown SA (1982) The natural coumarins: occurrence, chemistry and biochemistry. Wiley, New York
Hidayat H, Javid H, Ahmed AH, Karsten K (2012) The chemistry and biology of bicoumarins. Tetrahedron 68:2553–2578
Rao HSP, Sivakumar S (2006) Condensation of α-aroylketene dithioacetals and 2-hydroxyarylaldehydes results in facile synthesis of a combinatorial library of 3-aroylcoumarins. J Org Chem 71:8715–8723
Rao HSP, Vasantham K (2011) Nitroketene dithioacetal chemistry: synthesis of coumarins incorporating nitrothiophene moiety. J Chem Sci 123:411–420
Rao HSP, Swamy TV (2012) Synthesis of coumarin flavone hybrids. Lett Org Chem 9:218–220
Rao HSP, Swamy TV (2013) Synthesis and transformations of 4H-chromene 4-hydroxy-2H-pyran-2-one and 4H-chromene 4-hydroxycoumarin conjugates. J Chem Sci 125:777–790
Rao HSP, Swamy TV (2013) Synthesis of spirobiindane based bis-flavanones and bis-chroman-4-ones by Kabbe Reaction. Lett Org Chem 10:307–310
Ramesh V, Veera E (2011) Conformationally rigid aromatic amino acids as potential building blocks for abiotic foldamers. Org Biomol Chem 9:367–369
Kohler B, Enkelmann V, Oda M, Pieraccini S, Spada GP, Scherf U (2001) Novel chiral macrocycles containing two electronically interacting arylenechromophores. Chem Eur J 7:3000–3004
Kendhale AM, Rajesh G, Pattuparambil R, Rajamohanan K, Hoffmann HJ, Gangadhar J, Sanjayan R (2008) Unusual structural architecture from spirobi(indane) building blocks. Chem Commun 22:2541–2543
Fritsch D (2011) Synthesis and gas permeation properties of spirobischromane-based polymers of intrinsic microporosity. Mol Phys 212:1137–1146
Du N (2010) System and method of sensing and removing residual charge from a processed wafer. Advanced micro-fabrication equipment. PCT Int, Appl 20100271744
Liang Y (2009) Organic optoelectronic device and method for manufacturing the same. PCT Int, Appl 20090208776
Pechmann HV (1884) New buildings blocks of coumarin. Synthesis of daphnetins. Chem Ber 17:929–936
Faler GR, Lynch JC (1987) Preparation of spiro biindane bisphenol. U.S. 4701566
Jean B, Jacques P, Bernard V (1993) Ion-responsive fluorescent compounds. Effect of cation binding on the photophysical properties of a coumarin linked to monoaza and diaza-crown ethers. J Phys Chem 97:4552–4557
Duff JC, Bills EJ (1932) Reactions between hexamethylenetetramine and phenolic compounds. Part I. A new method for the preparation of 3 and 5-aldehydosalicylic acids. J Chem Soc 3:1987–1988
Reimer K, Tiemann F (1876) Formation of phenolic aldehydes from phenols, chloroform and alkali. Ber 9:1268–1285
Vilsmeier A, Haack A (1927) Vilsmeier reagent (chloromethylene) dimethyl ammonium chloride or DMF/POCl3, or DMF/oxalyl chloride. Ber 60:119–123
Knoevenagel E (1898) Berichte der deutschen chemischen Gesellschaft. Chem Ber 31:2596–2619
Hyo SJ, Pil SK, Jeong WL, Jae IK, Chang SH, Jong WK, Shihai Y, Jin YL, Jung HL, Taiha J, Jong SK (2009) Coumarin-derived Cu2+-selective fluorescence sensor: synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131:2008–2012
Pavol H, Jana D, Henrieta S, Anton G (2010) Influence of polarity of solvents on the spectral properties of bichromophoric coumarins. Molecules 15:8915–8932
Jing L, Xiang GL, Shi RW (2002) Photo luminescent and theoretical studies of 3-acetyl-8-methoxycoumarin. Chin J Struct Chem 31:1003–1007
Azuma K, Uchiyama SS, Kajiro T, Santa T, Imai KA (2003) Study of the relationship between the chemical structures and the fluorescence quantum yields of coumarins, quinoxalinones and benzoxazinones for the development of sensitive fluorescent derivatization reagents. Photochem Photobiol Sci 2:443–449
Morimoto A, Yatsuhashi T, Shimada T, Biczok L, Tryk DA, Inoue H (2001) Radiation less deactivation of an intramolecular charge transfer excited state through hydrogen bonding: effect of molecular structure and hard-soft anionic character in the excited state. J Phys Chem A 105:10488–10496
Retsek JL, Gentemann S, Medforth CJ, Smith KM, Chirvony VS, Fajer J, Holten D (2000) Photo induced evolution on the conformational landscape of non planar dodecaphenylporphyrin: picosecond relaxation dynamics in the 1(π, π*) excited state. J. Phys Chem. B 104:6690–6693
Acknowledgments
HSPR thanks UGC, UGC-SAP, CSIR and DST-FIST for financial assistance. VST thanks CSIR for fellowship. We thank IISc, Bangalore for recording MS spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, H.S.P., Tangeti, V.S. Synthesis and Photochromic Properties of Spirobiindane Based Bis-coumarins. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 85, 41–49 (2015). https://doi.org/10.1007/s40010-014-0179-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-014-0179-8