Abstract
Nonsymmetric bis-spiropyran derivatives based on 1,3-dihydroxy-6-oxo-6H-benzo[c]chromene-2,4-dicarbaldehyde were synthesized. The obtained compounds with substituents R = H, Cl at positions 5,5′ of the indoline fragment in DMSO solution exist in a fully open merocyanine form. Their change for the electron-acceptor group NO2 results to the existence of the corresponding bis-spiropyran in the form of a tautomeric mixture of spirocyclic and merocyanine forms. The resulting compounds in DMSO solution undergo photoinduced cyclization. The nitro derivative demonstrates both positive and negative photochromism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Spiro derivatives of pyran are the most well-known and studied class of photochromic compounds due to the possibility of targeted modification of their structure and spectral-luminescent properties in a wide range [1–4], the creation of optical information recording devices, molecular switches, bio- and chemosensors, as well as the possibility of targeted drug delivery [5–9].
Compounds with two spiropyran fragments have been investigated to a much lesser extent; however, the presence of two potentially nonequivalent photoactive centers provides an opportunity for obtaining multifunctional photochromic systems [10–13]. Compounds 3a–3c were obtained by condensation of 3Н-indolium iodides 1a–1c [14] with 1,3-dihydroxy-6-oxo-6H-benzo[c]chromene-2,4-dicarbaldehyde 2 [15] in the presence of triethylamine (Scheme 1).
According to the 1H NMR spectroscopic data, compounds 3a, 3b are in the open merocyanine form 3M. In solutions in DMSO-d6, two six-proton singlet signals are observed at 1.68‒1.75 ppm and two three-proton singlet signals at 3.53–3.56 ppm. The proton signals of the diene bridges appear as two doublets at 7.90– 7.98 and 8.39–8.44 ppm. Compound 3c exists as a tautomeric mixture of spirocyclic S and merocyanine M forms. In the upfield region, along with signals at 1.18, 1.26 and 2.78, 2.86 ppm corresponding to two pairs of magnetically nonequivalent geminal methyl and N-methyl groups of the indoline fragment in the spirocyclic tautomer, there are two signals of the same groups in the merocyanine form at 1.74, 1.77 and 3.55, 3.56 ppm. Based on the signal intensity, the ratio of the spirocyclic S and merocyanine M forms is 3 : 1.
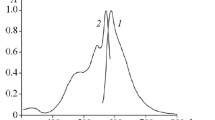
The solutions of compounds 3a–3c in DMSO are marked by intense electronic absorption bands characteristic of merocyanine forms with maxima at 477–479, 528–531, and 561–562 nm (Table 1, Fig. 1). In compound 3b, the electron-withdrawing Cl substituent at 5 and 5′ positions of the indoline fragment decreases the absorption intensity by ~1.6 times compared to unsubstituted compound 3а. For the nitro derivative 3c, the equilibrium in DMSO solution is strongly shifted towards the spirocyclic form, which is confirmed by a maximum at 367 nm and the almost complete absence of bands in the visible spectral diapason. The long-wavelength maxima of compound 3c are shifted bathochromically compared to compounds 3a, 3b.
The spectra of compounds 3а, 3b solutions in DMSO do not change upon UV light irradiation, which is associated with the mainly presence of merocyanine tautomers in the solutions, according to 1H NMR data. Compound 3c exists in equilibrium of spirocyclic and merocyanine tautomer forms in solutions (Fig. 2) and have positive photochromism. In compound 3c DMSO-solution, a photoreaction occurs with the formation of colored merocyanine tautomers upon UV light irradiation (λ 365 nm) (Fig. 2). The system returns to its initial equilibrium state after the ending of irradiation.
For compounds 3a–3c solutions, negative photochromism is observed upon irradiation with visible light in the absorption bands of merocyanine tautomers. Upon light irradiation (λ 546 nm), the solutions become discolored due to the occurrence of photoinitiated cyclization to the spiro form S (Fig. 3). The intensity decreasing of the absorption band at 561 nm of the merocyanine tautomer (compound 3a) is accompanied by the appearance of a band in the short-wavelength spectrum region (λ 338 nm), which is characteristic for spirocyclic pyran derivatives [16]. The initial absorption spectra regenerate after the irradiation ending.
Due to the equilibrium establishment between the spirocyclic S and merocyanine M isomers under normal conditions under UV irradiation, additional coloration of compound 3c solutions occurs and a photostationary state comes. After turning off the irradiation source, the system relaxes to its initial equilibrium state, demonstrating positive photochromism. Irradiation with visible light on the absorption band of merocyanine isomers initiates a cycle of negative photochromism, leading to the color change of the solution until a new photostationary state is established. The system relaxes to the initial equilibrium state after the irradiation ending. Consequently, compound 3c solutions demonstrate the property of so-called photochromic “balance,” when the photoinduced shift of the merocyanine isomer part to one side or another is compensated by opposite thermal processes [17].
Thus, the obtained bis-spyro compounds based on 1,3-dihydroxy-6-oxo-6H-benzo[c]chromene-2,4-dicarbaldehyde exist as a tautomeric mixture of spirocyclic and merocyanine forms, their solutions in DMSO undergo photoinduced cyclization, and the nitro derivative exhibits the properties of both positive and negative photochromism.
EXPERIMENTAL
The 1H NMR spectra were recorded on a Bruker DPX-250 (250 MHz) in DMSO-d6 relative to the residual non-deuterated solvent signals. The vibration spectra were recorded on a Varian Excalibur 3100 FT-IR device by attenuated total reflectance method with using a ZnSe crystal. The electron absorption spectra were registered on an Agilent 8453 spectrophotometer with a sample thermostating option. Photolysis of solutions was carried out under irradiation by a Newport system with a 200 W mercury lamp with a set of interference filters (Newport system). We used DMSO of spectral purity (Aldrich) for solutions preparing. Melting points were determined in glass capillaries using a PTP (M) apparatus. Elemental analysis was performed by the classical method [18].
General procedure for the synthesis of compounds 3a–3c. To the solution of 2 mmol of 2,3,3-trimethylindolium iodine 1a–1c and 1 mmol of 1,3-dihydroxy-6-oxo-6H-benzo[c]chromene-2,4-dicarbaldehyde 2 in 50 mL isopropanol, 0.7 mmol (0.1 mL) of triethylamine was added at heating. The mixture was refluxed for 5 h and then cooled, spilled in water (50 mL), and extracted with chloroform several times. The extract was dried under CaCl2 and evaporated to volume 10–15 mL. The residue was purified by column chromatography on Al2O3 (eluent—CHCl3) and recrystallized from isopropanol.
2,4-Bis[2-(1,3,3-trimethyl-1,3-dihydroindol-2-ylidene)ethylidene]-1Н-benzo[c]chromene-1,3,6(2Н,4Н)-trione (3a). Yield 54%, mp 260‒262°С (i-PrOH). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 1.68 s (6H, Me), 1.71 s (6H, Me), 3.53 s (3H, NMe), 3.54 s (3H, NMe), 7.17‒7.54 m (8НAr + 1Нcoumarin), 7.83‒7.85 m (1Hcoumarin), 7.90 d (1H, H1, J = 14.3), 7.98 d (1H, H1, J = 13.8), 8.13 t (1Нcoumarin, J = 9.2), 8.41 d (1H, H1, J = 13.8), 8.72 d (1H, H2, J = 14.1), 9.57 d (1Нcoumarin, J = 8.2). Found, %: C 78.65; Н 5.66; N 7.85. C39H34N2O4. Calculated, %: C 78.77; Н 5.76; N 4.71.
2,4-Bis[2-(1,3,3-trimethyl-5-chloro-1,3-dihydroindol-2-ylidene)ethylidene]-1Н-benzo[c]chromene-1,3,6(2Н,4Н)-trione (3b). Yield 40%, mp 275‒277°С (i-PrOH). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): 1.71 s (12H, Me), 3.53 s (6Н, NMe), 7.19‒7.54 m (4НAr + 1Нcoumarin), 7.70‒7.75 m (2НAr), 7.84‒7.99 m (3Нcoumarin, Н1, Н2), 7.92 d (1H, H1, J = 14.1), 8.17 t (1Hcoumarin, J = 6.0), 8.42 d (1H, H2, J = 14.1), 9.58 d (1Н, Hcoumarin, J = 8.1). Found, %: C 70.74; Н 5.02; N 4.40. C39H32Cl2N2O4. Calculated, %: C 70.59; Н 4.86; N 4.22.
2,6-Dispiro(1,3,3-trimethyl-5-nitro-1,3-dihydroindol-2′)-2Н,6Н,10Н-benzo[c]dipyrano[2,3-f:2,3-h]chromene-10-one (3c). Yield 40%, mp 250‒252°С (i-PrOH). 1H NMR spectrum (DMSO-d6), δ, ppm (J, Hz): S form, 1.14 s (3H, Me), 1.18 s (3H, Me), 1.26 s (3H, Me), 1.32 s (3H, Me), 2.78 s (3H, NMe), 2.86 s (3H, NMe), 5.85 d (1H, H3′, J = 10.5), 5.87 d (1H, H13′, J = 10.5), 6.83 m (2H, Н7, H7′′), 6.88 d (1Н, H4′, J = 10.6), 7.13 t (1Н, Hcoumarin, J = 7.8), 7.34 s (1Н, H14′, J = 10.3), 7.38 d (1Hcoumarin, J = 7.8), 7.88 d (1Hcoumarin, J = 7.8), 8.05 s (1H, Н4), 8.07 s (1H, Н4′′), 8.14–8.17 m (3Н, Н6, H6′′, Hcoumarin); M form, 1.74 s (6H, Me), 1.77 s (6H, Me), 3.55 s (3Н, NMe), 3.56 s (3Н, NMe), 7.39 d (2H, Н7, H7′′, J = 7.0), 7.13 t (1Hcoumarin, J = 7.8), 7.39 t (1Hcoumarin, J = 7.8), 7.88 d (1Hcoumarin, J = 7.8), 8.21 d (2Н, Н6, H6′′, J = 8.1), 8.25 s (1H, Н4), 8.29 s (1H, Н4′′), 8.54 d (1H, H1, J = 13.8), 8.59 d (1H, H2, J = 14.4), 8.64–8.71 m (2H, H1, Н2), 9.50 m (1Hcoumarin). Found, %: C 68.26; Н 4.85; N 8.25. C39H32N4O8. Calculated, %: C 68.41; Н 4.71; N 8.18.
REFERENCES
Bertelson, R.C., Organic Photochromic and Thermochromic Compounds, Crano, J.C. and Guglielmetti, R., New York: Kluwer Academic Publishers, 1999, vol. 1, p. 11.
Kortekaas, L. and Browne, W.R., Chem. Soc. Rev., 2019, vol. 48, p. 3406. https://doi.org/10.1039/C9CS00203K
Klajn, R., Chem. Soc. Rev., 2014, vol. 43, p. 148. https://doi.org/10.1039/C3CS60181A
Minkin, V.I., Russ. Chem. Rev ., 2013, vol. 82, p. 1. https://doi.org/10.1070/RC2013v082n01ABEH004336
Bercovic, G., Krongauz, V., and Weiss, V., Chem. Rev., 2000, vol. 100, no. 5, p. 1741. https://doi.org/10.1021/cr9800715
Pianowski, Z.L., Chem. Eur. J., 2019, vol. 25, no. 20, p. 5128. https://doi.org/10.1002/chem.201805814
Zhang, X., Chen, L., Lim, R.H., Gonuguntla, S., Lim, K.W., Pranantyo, D., Yong, W.P., Yam, W.J.T., Low, Z., Teo, W.J., Nien, H.P., Loh, Q.W., and Soh, S., Adv. Mater., 2019, vol. 31, no. 11. Article 1804540. https://doi.org/10.1002/adma.201804540
Sahoo, P.R., Prakash, K., and Kumar, S., Coord. Chem. Rev., 2018, vol. 357, p. 18. https://doi.org/10.1016/j.ccr.2017.11.010
Cardano, F., Del Canto, E., and Giordani, S., Dalton Trans., 2019, vol. 48, p. 15537. https://doi.org/10.1039/c9dt02092
Ozhogin, I.V., Mukhanov, E.L., Chernyshev, A.V., Pugachev, A.D., Lukyanov, B.S., and Metelitsa, A.V., J. Mol. Struct., 2020, vol. 1221. Article 128808. https://doi.org/10.1016/j.molstruc.2020.128808
Nikolaeva, O.G., Karlutova, O.Yu., Cheprasov, A.S., Metelitsa, A.V., Dorogan, I.V., Dubonosov, A.D., and Bren, V.A., Chem. Heterocycl. Compd., 2015, vol. 51, p. 229. https://doi.org/10.1007/s10593-015-1689-2
Ozhogin, I.V., Tkachev, V.V., Lukyanov, B.S., Mukhanov, E.L., Chernyshev, A.V., Komissarova, O.A., Minkin, V.I., and Aldoshin, S.M., Doklady Chem., 2016, vol. 471, p. 378. https://doi.org/10.1134/S0012500816120090
Mukhanov, E.L., Alekseenko, Yu.S., Dorogan, I.V., Tkachev, V.V., Lukyanov, B.S., Aldoshin, S.M., Bezuglyi, S.O., Minkin, V.I., Utenyshev, A.N., and Ryashchin, O.N., Chem. Heterocycl. Compd., 2010, vol. 46, p. 279. https://doi.org/10.1007/s10593-010-0503-4
Pottier, E., Sergent, M., Phan Tan Luu, R., and Guglielmetti, R., Bull. Soc. Chim. Fr., 1992, vol. 101, no. 8, p. 719. https://doi.org/10.1002/bscb.19921010810
Nikolaeva, O.G., Karlutova, O.Yu., Dubonosov, A.D., Bren, V.A., and Minkin, V.I., Russ. J. Gen. Chem., 2020, vol. 90, no. 12, p. 2219. https://doi.org/10.1134/S1070363220120014
Nikolaeva, O.G., Kostyrina, O.Yu., Shepelenko, E.N., Tsukanov, A.V., Metelitsa, A.V., Borodkin, G.S., Dubonosov, A.D., Bren, V.A., and Minkin, V.I., Russ. J. Org. Chem., 2011, vol. 47, no. 9, p. 1370. https://doi.org/10.1134/S1070428011090193
Metelitsa, A., Chernyshev, A., Voloshin, N., Solov’eva, E., Rostovtseva, I., Dorogan, I., Gaeva, E., and Guseva, A., Dyes and Pigments, 2021, vol. 186. Article 109070. https://doi.org/10.1016/j.dyepig.2020.109070
Gel’man, N.E., Terent’eva, N.A., Shanina, G.M., Kiparenko, L.M., and Rezl, V., Metody kolichestvennogo organicheskogo elementnogo mikroanaliza (Methods for Quantitative Organic Elemental Microanalysis), Moscow: Khimiya, 1987.
Funding
The study was financially supported by the Ministry of Science and Higher Education of the Russian Federation in the scope of the state task in the field of scientific activity no. 0852-2020-0019, as well as in the scope of the state task of The Southern Scientific Center of the Russian Academy of Sciences (no. 01201354239, A.D. Dubonosov).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 4, pp. 544–549 https://doi.org/10.31857/S0044460X21040089.
Rights and permissions
About this article
Cite this article
Nikolaeva, O.G., Karlutova, O.Y., Gaeva, E.B. et al. Synthesis and Photochromic Properties of Bis-Spirocyclic Compounds Based on 1,3-Dihydroxy-6-oxo-6H-benzo[c]chromene-2,4-dicarbaldehyde. Russ J Gen Chem 91, 626–630 (2021). https://doi.org/10.1134/S1070363221040083
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221040083