Abstract
Vesicular drug delivery system are novel means to improve the bioavailability of the encapsulated drug along with numerous advantages over conventional drug delivery systems. Liposomes were first in such type of delivery systems but it was not so successful due to their numerous drawbacks. Niosomes or non ionic surfactant vesicles are formed from self assembly of hydrated surfactant monomers. They are formulated by non ionic surfactants but various ionic amphiphiles like di cetyl phosphate, sodium deoxycholate and stearylamine etc. are also incorporated inorder to achieve a stable vesicular suspension by inducing negative or positive charge. The most striking feature of such delivery system is that it can be used to encapsulate both hydrophobic as well as hydrophilic drug. The emphasis of this type of vesicular drug delivery system is placed over the slow release of drug in a controlled manner, resulting into sustained activity, reduced toxicity, protection of active moiety, targeting, modification in distribution profile of drugs and enhancing the bioavailability of encapsulated drug. This article summarizes the merits of niosomal drug delivery system over conventional drug therapy, its structural components, factors affecting its formation, method of preparation, evaluation techniques, therapeutic applications and patents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For many decades, treatment of dreadful disease or a chronic illness has been accomplished by administering drugs to the patients through various conventional pharmaceutical dosage forms like tablets, capsules, pills, creams, ointments, liquids, aerosols, injectables and suppositories as carriers. But the main problem with such type of drug therapy was to maintain the plasma drug concentration within the therapeutic range for prolonged period of time without fluctuation.
To overcome these problems novel drug delivery system came into existence which delivers drug at rate dictated by the need of the body over the period of treatment and channels the active entity solely to the site of action, these include niosomes, liposomes, nanoparticles, microspheres, microemulsions, floating tablets, impalatable pumps and magnetic microcapsules etc.
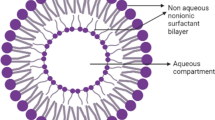
Among different carriers liposomes and niosomes are well documented drug delivery systems. Structurally, niosomes are similar to liposomes and possess similar physical properties but due to deteriorative susceptibility and high cost of lipids it has been proposed that niosomes could be used as an alternative version of liposomes, compared to liposomes the non ionic surfactants used to formulate niosomes are chemically more stable and cheaper in cost. Niosomes are non ionic surfactant based unilamellar or multilamellar vesicles which enclose an aqueous solution of solute within a membrane resulting from organization of surfactant macro molecules as bilayer.
There size ranges between 10 and 1000 nm. These are ampiphillic in nature, which allows entrapment of hydrophilic drug in the core cavity and hydrophobic drugs in the non-polar region present within the bilayer hence both hydrophilic and hydrophobic drugs can be incorporated into niosomes. Handling and storage of surfactants does not require any special conditions. The characteristics and the performance of the prepared niosomes can be controlled by altering the composition, concentration of various additives, size, lamellarity and surface charge of vesicles (Khan et al. 2011).
Merits of niosomes
Niosomal drug delivery system have number of benefits over convention drug delivery system which are enumerated below.
-
1.
Niosomes can act as novel drug dosage form for drug molecules having a wide range of solubility as their infrastructure consists of hydrophilic and hydrophobic part (Biju et al. 2006; Vyas and Khar 2002; Indhu et al. 2004).
-
2.
Targeted drug delivery can also be achieved using niosomes, the drug is delivered directly to the body part where the therapeutic effect is required. Thereby reducing its dosage (Mujoriya and Bodla 2011; Kazi et al. 2010).
-
3.
As vesicle suspension is water based vehicle hence provide better patient compliance than oil based dosage forms.
-
4.
They improve oral bioavailability of poorly absorbed drugs, by delaying clearance from the circulation and by protecting the drug from biological environment.
-
5.
They are osmotically active, stable and also increase the stability of entrapped drug.
-
6.
Oral, parenteral, ocular as well as topical routes can be adopted for their administration with improved bioavailability.
-
7.
The vesicles may act as a depot, releasing the drug in a controlled manner providing sustained release of the enclosed drug.
-
8.
The biodegradable, biocompatible and nonimmunogenic surfactants are used in preparation of niosomes and also handling and storage of surfactants requires no special conditions (Biju et al. 2006; Vyas and Khar 2002; Indhu et al. 2004).
Structural components of niosomes
It is necessary to understand the role of the basic structural components of niosomes before preparation. These components include most importantly the nonionic amphiphiles/surfactants and the hydration medium.
Nonionic amphiphiles/surfactants
Nonionic surfactants are the most common type of surface active agent used in preparing vesicles due to the superior benefits they impart with respect to stability, compatibility and toxicity compared to their anionic, amphoteric or cationic counterparts (Jiao 2008; Zografi 1995; Hall 1987). They are generally less toxic, less hemolytic and less irritating to cellular surfaces and tend to maintain near physiological pH in solution. They have many functions including acting as solubilizers, wetting agents, emulsifiers and permeability enhancers. They are also strong P-glycoprotein inhibitors, a property useful for enhancing drug absorption and for targeting to specific tissues (Zhang and Morris 2005).
Nonionic surfactants are comprised of both polar and non-polar segments and possess high interfacial activity. The formation of bilayer vesicles instead of micelles is dependent on the hydrophilic–lipophilic balance (HLB) of the surfactant, the chemical structure of the components and the critical packing parameter (CPP). On the basis of the CPP of a surfactant, the type of vesicle, which it will form, can be predicted as shown in Fig. 1. The method of calculating CPP from the volume of the hydrophobic group, area of the hydrophilic head group and length of the lipophilic alkyl chain of the surfactant is also shown in Fig. 1. The chain length and size of the hydrophilic head group of the nonionic surfactant affect the entrapment efficiency of drug. Nonionic surfactants with stearyl (C18) chains show higher entrapment efficiency than those with lauryl (C12) chains. The Tween series of surfactants bearing a long alkyl chain and a large hydrophilic moiety in combination with cholesterol in a 1:1 ratio have the highest entrapment efficiency of water soluble drugs (Uchegbu and Vyas 1998; Uchegbu and Florence 1995; Arunothayanun et al. 2000).
Critical packing parameter (CPP) of an amphiphile where ν is the hydrophobic group volume, lc the critical hydrophobic group length and a 0 the area of the hydrophilic head group (Kumar and Rajeshwarrao 2011)
The HLB value of a surfactant plays a key role in controlling drug entrapment of the vesicle it forms. A surfactant with an HLB value in the range 14–17 is not suitable to produce niosomes whereas one with an HLB value of 8.6 gives niosomes with the highest entrapment efficiency. Entrapment efficiency decreases as the HLB value decreases from 8.6 to 1.7 (Lawrence et al. 1996; Biswal et al. 2008; Shahiwala and Misra 2002) or HLB > 6, cholesterol must be added to the surfactant in order to form a bilayered vesicle and for lower HLB values cholesterol enhances stability of vesicles. The entrapment efficiency is affected by the phase transition temperature (Tc) of the surfactant. Thus Span 60 with a high Tc exhibits the highest entrapment efficiency (Biswal et al. 2008). The most common nonionic amphiphiles used for vesicle formation are alkyl ethers, alkyl esters, alkyl amides and esters of fatty acids (Uchegbu and Vyas 1998).
Cholesterol
Steroids bring about changes in fluidity and permeability of the bilayer and are thus important components. Cholesterol is usually added to the non-ionic surfactants to provide rigidity and orientational order. Cholesterol is an amphiphilic molecule; it orients its OH group towards aqueous phase and aliphatic chain towards surfactant’s hydrocarbon chain. Rigidization is provided by alternative positioning of rigid steroidal skeleton with surfactant molecules in the bilayer by restricting the movement of carbons of hydrocarbon. Cholesterol is also known to prevent leakage by abolishing gel to liquid phase transition (Dahiya et al. 2011).
Charge inducers
Charge inducers increase the stability of the vesicles by induction of charge on the surface of the prepared vesicles. It act by preventing the fusion of vesicles due to repulsive forces of the same charge and provide higher values of zeta potential. The commonly used negative charge inducers are dicetyl phosphate (Dcp), dihexadecyl phosphate, sodium deoxycholate and lipoamine acid and positive charge inducers are sterylamine and cetylpyridinium chloride (Shan et al. 2008).
Hydration medium
Phosphate buffer at various pHs is the most commonly used hydration medium for preparation of niosomes. The actual pH of the hydration medium depends on the solubility of the drug being encapsulated.
Factors affecting formation of niosomes
Surfactant and lipid amount
The maximum amount of surfactant/lipid that can be used to prepare niosomes is generally 10–30 mmol/L (1–2.5 %, w/w). As the surfactant/lipid level increases, the amount of drug to be encapsulated also increases leading to an increase in the viscosity of the system (Uchegbu and Vyas 1998).
Nature of encapsulated drug
The physico-chemical properties of encapsulated drug influence charge and rigidity of the niosome bilayer. The drug interacts with surfactant head groups and develops the charge that creates mutual repulsion between surfactant bilayers and hence increases vesicle size (Stafford et al. 1988).
Another factor to be considered is whether the drug to be encapsulated is amphiphilic. Doxorubicin when encapsulated in niosomes, aggregation occurred and was overcome by the addition of a steric stabilizer (Uchegbu and Florence 1995). The solubility of certain poorly soluble drugs can be increased by formulation in niosomes but only up to a certain limit above which drug precipitation will occur (Balakrishnan et al. 2009). An increase in the encapsulation of flurbiprofen due to saturation of drug in the hydration medium has been reported (Mokhtar et al. 2008). However, when niosomes were prepared using higher amounts of minoxidil, optical microscopy revealed minoxidil crystals dispersed in between the niosomal particles (Balakrishnan et al. 2009).
Cholesterol content and charge
An increase in cholesterol content of the bilayers resulted in a decrease in the release rate of encapsulated material, and therefore an increase in the rigidity of the resulting bilayers. The interlamellar distance between successive bilayers in multilamellar vesicle tends to increase due to presence of charge and leads to greater overall entrapped volume (Hunter et al. 1988).
Temperature of hydration
Hydration temperature influences the shape and size of the niosome, temperature change of niosomal system affects assembly of surfactants into vesicles by which induces vesicle shape transformation. Ideally the hydration temperature for niosome formation should be above the gel to liquid phase transition temperature of system (Uchegbu and Vyas 1998).Along with the above mentioned factors, volume of hydration of medium and time of hydration of niosomes are also critical factors. Improper selection of these factors may result in formation of fragile niosomes or creation of drug leakage problems (Biswal et al. 2008).
Effect of pH of the hydration medium
Entrapment efficiency of niosomes is greatly affected by the pH of the hydration medium. High entrapment of flurbiprofen was reported at acidic pH with a maximum encapsulation efficiency of 94.6 % at pH 5.5. At lower pH, the proportion of unionized flurbiprofen increases and partitions more readily into the lipid bilayer than the ionized species (Mokhtar et al. 2008). However at lower pH, niosome formulations should be examined by optical microscopy for the presence of drug precipitates both before and after centrifugation and washing. This will help to determine the concentration of drug in the hydration medium giving optimum encapsulation in niosomes.
Method of preparation of niosomes
Niosomes widely differ in their properties depending on the method used for production which include following methods.
Sonication
It is a typical method of production of the vesicles, illustrated in Fig. 2 in which a 10-ml glass vial drug solution in buffer is added to the surfactant/cholesterol mixture. Then the mixture is probe sonicated at 60 °C for 3 min using a sonicator with titanium probe to yield niosomes. The resulting vesicles are small and unilamellar (Baillie et al. 1986).
Micro fluidization
It is a recent technique based on submerged jet principle. In this two fluidized streams interact at ultra high velocities and move forward through precisely defined micro channel within the interaction chamber shown in Fig. 3. The impingement of thin liquid sheet along a common front is arranged such that the energy supplied to the system remains within the area of niosomes formation which results in a greater uniformity, smaller size and better reproducibility of niosomes formed (Khandare et al. 1994).
Ether injection method
This method provide means of making niosomes by slowly introducing a solution of surfactant dissolved in diethyl ether into warm water maintained at 60 °C. The surfactant mixture in ether is injected through 14-gauge needle into an aqueous solution of material. Vaporization of ether leads to formation of single layered vesicles as depicted in Fig. 4. Depending upon the conditions used, the diameter of the vesicle range from 50 to 1000 nm. (Rogerson et al. 1988).
Thin film hydration
All vesicles forming Components i.e. surfactant, cholesterol and charge inducers are dissolved in a volatile organic solvent like diethyl ether, methanol or chloroform etc. in a round bottom flask. Using rotary evaporator the organic solvent is evaporated at room temperature forming a thin dry film of dissolved components. The dried thin film is hydrated with aqueous phase with gentle agitation which leads to formation of niosomes as shown in Fig. 5. The drug can be added to the aqueous phase if hydrophilic and can be dissolved in organic solvent with other components if hydrophobic (Baillie et al. 1986; Palozza et al. 2006).
Reverse phase evaporation method
The ingredients are dissolved in a mixture of volatile organic solvents (ether and chloroform) and drug is dissolved in aqueous phase. Water in oil emulsion is formed of the two phases in a bath sonicator. The basic principle involves evaporation of organic solvent to form niosomes. This emulsion is dried in a rotary evaporator at 40 °C to form a semi solid gel of large vesicles. Small quantity of buffer is added and the semi solid form is sonicated at 4–5 °C to form small unilamellar vesicles (Guinedi et al. 2005).
Formation of niosomes from proniosomes
In this method of producing niosomes a water-soluble carrier such as sorbitol is coated with surfactant resulting in the formulation of dry formulation in which each water soluble particle is covered with a thin film of dry surfactant. This preparation is termed “Proniosomes”. Then proniosome powder is filled in a screw capped vial, and mixed with water or saline at 80 °C by vortexing (shown in Fig. 6), followed by agitation for 2 min results in the formation of niosomal suspension (Blazek Welsh and Rhodes 2001).
Transmembrane pH gradient method
Equal proportions of surfactant and cholesterol are dissolved in chloroform and evaporated under reduced pressure to produce a thin lipid film on the wall of a round bottomed flask. The film is hydrated with a solution of an acidic compound (generally citric acid) by vortex mixing. The resulting product is subjected to freeze–thaw cycles after which an aqueous solution of drug is added and the mixture vortexed. The pH of the sample is then raised to 7–7.2 using a disodium hydrogen phosphate solution. Niosomes using this method reported an entrapment efficiency of 87.5 %(Kumar and Rajeshwarrao 2011).
The bubble method
In this method nitrogen gas is passed through a sample of homogenized surfactants to give large unilamellar vesicles. These are then subjected to size reduction to give small unilamellar vesicles (Chauhan and Luorence 1989).
Multiple membrane extrusion method
Mixture of surfactant, cholesterol and dicetyl phosphate in chloroform is made into thin film by evaporation. The film is hydrated with aqueous drug solution and the resultant suspension extruded through polycarbonate membranes which are placed in series for up to 8 passages as illustrated in Fig. 7. It is a good method for controlling niosome size (Khandare et al. 1994; Nasseri and Florence 2003).
Characterization of niosomes
Niosomes are characterized chiefly for their size, morphology, charge, rigidity, homogeneity, percentage recovery, drug entrapment capacity, and drug release.
Vesicle morphology
Vesicle structure and shape can be characterized by various types of microscopy such as optical (Vyas and Khar 2002; Wadhe et al. 2009), freezefracture, electron (Marianecci et al. 2010), surface electron, scanning electron, negative staining transmission electron, cryo-electron, fluorescence and confocal (Bibi et al. 2011).
Vesicle size
Niosome size can range from around 10 nm to around 50 µm. Several techniques can be used to determine vesicle size and size distribution. Large niosomes, those with diameters over 1 µm, can be adequately measured by light microscopy and the Coulter counter which offers the possibility of collecting information on particle shape. For vesicles in the submicron range, size can be assessed by zeta sizer or electron microscopic analysis or by light scattering techniques.
Vesicle charge
The vesicle surface charge can play an important role in the behavior of niosomes in vivo. In order to obtain an estimate of the surface potential, the zeta potential of individual niosomes can be measured by microelectrophoresis. An alternative approach is the use of pH-sensitive fluorophores. More recently, dynamic light scattering have been used to measure the zeta potential of niosomes (Bhaskaran and Lakshmi 2009).
Niosomal recovery % and entrapment efficiency
To determine drug loading and encapsulation efficiency, the niosomal aqueous suspension was ultracentrifuged, supernatant was removed and sediment was washed twice with distilled water to remove the unentrapped drug. The niosomal recovery was calculated as follows:
The drug entrapment can be calculated by taking appropriate amount of vesicles and digested using isopropanol, triton X 100 or any other organic solvent for complete vesicle disruption. The resultant solution is analysed using suitable analytical technique and the percentage drug entrapment can be calculated by using the following formula (Kumar et al. 2011).
In vitro release
In-vitro release studies are carried out by dialysis through a semi permeable membrane. A niosome preparation is incorporated in an open end dialysis membrane and placed in a receptor compartment containing buffer. Samples are periodically collected and analyzed using a suitable analytical method (Martin 1990).
Stability
The main problems associated with storage of vesicles are aggregation, fusion and leakage of drug. (Ammar et al. 2011). defined stable formulations of tenoxicam as those showing high entrapment efficiency (>60 %) and retention (>90 %) over a period of months. At the end of each month, only stable formulations were selected to continue for another month. It was found that there was no significant change in the mean size of vesicles after 90 days when compared with those of freshly prepared sucrose stearate niosomes. However, the entrapment efficiency was affected (10 %) following storage (Abd-Elbary et al. 2008).
Therapeutic applications of niosomes
The application of niosomal technology is widely varied and can be used to treat a number of diseases.
-
1.
Niosomes can be used for pulmonary delivery of drugs such beclomethasone dipropionate which provided sustained and targeted delivery, improved mucus permeation and amplified therapeutic effect (Terzano et al. 2005).
-
2.
Niosomes have also been used as carriers for iobitridol, a diagnostic agent used for X ray imaging (Ruckmani et al. 2000).
-
3.
Many drugs like acyclovir, fluconazole, Griseofulvin, Rifampicin Gatifloxacin etc. can be effectively delivered through oral route which enhanced the oral bioavailability and provided sustained release for prolonged periods.(Chauhan et al. 2009; Jadon et al. 2009; Attia et al. 2007; Pavala et al. 2010).
-
4.
Transdermal niosomal formulations of ketorolac, nimesulide, Flurbiprofen NSAIDS etc. performed better than the tradition formulations. They may serve as a solubilization matrix, as local depot for sustained release of dermally active compounds, as permeation enhancers, or as a rate-limiting membrane barrier for the modulation of systemic absorption of drugs via the skin (Schreier and Bouwstra 1994).
-
5.
The delivery of proteins by oral administration can be effectively done by its niosomal formulation e.g. oral administration of recombinant human insulin in a niosomal formulation protected it against proteolytic activity of α-chymotrypsin, trypsin and pepsin in vitro (Pardakhty et al. 2007).
-
6.
Niosomes, decreased the rate of proliferation of tumor and with decreased side effects. Some experimentally evaluated niosomal formulations identified in literature are: Daunorubicin HCl, Doxorubicin, Methotrexate, Bleomycin and Vincristine etc. (Parthasarathi et al. 1994).
-
7.
Zidovudine is commonly used to treat patients with AIDS but is limited by its toxicity and low potency. (Ruckmani and Sankar 2010) concluded that zidovudine loaded niosomes would provide sustained delivery of drug and a more effective AIDS therapy.
-
8.
Sodium stibogluconate (anti-leishmanial drug) loaded niosomes showed that it was possible to administer higher levels of the drug without the triggering of the side effects, and thus allowed greater efficacy in treatment (Baillie et al. 1986; Hunter et al. 1988).
-
9.
A number of surfactants have immune stimulatory properties and have been used as vaccine adjuvants (Hilgers et al. 1989).
-
10.
Ophthalmic niosomal preparations of Acyclovir, Gentamycin, Ofloxacin and brimonidine were therapeutically effective with a long duration of action due to slow and prolonged zero order release of drug.
-
11.
Due to their immunological selectivity, low toxicity and greater stability; niosomes are being used to study the nature of the immune response provoked by antigens.
-
12.
Niosomes can be used as carriers for haemoglobin within the blood. The niosomal vesicle is permeable to oxygen and hence can act as a carrier for haemoglobin in anemic patients (Mujoriya and Bodla 2011).
-
13.
Targeting of bioactive agents to reticulo endothelial system (RES) and to organs other than RES (Yoshioka et al. 1994).
Patents
Because niosomes are becoming increasingly popular, the number of patents for niosomal formulations is increasing enormously. A short summary of some of the important patents related to niosomes is indicated in Table 1.
Conclusion
Niosomes are novel and efficient approach to drug delivery which offer a variety of advantages over conventional and other vesicular delivery systems, namely targeted delivery, reduction of dose, stability, enhanced bioavailability, compatibility of non-ionic surfactants, ease of modification, delayed clearance, suitability for a wide range of Active Pharmaceutical Agents etc. Being composed of nonionic surfactants, cholesterol, charge inducers and active agent, these can be prepared easily and economically without requiring any special conditions for storage, protection, handling, or industrial manufacturing. They can be administered effectively by various routes of administration namely oral, ocular, parenteral and transdermal. From the above compilation of work it can be concluded that niosomes have suitability to serve as tool for novel drug delivery and for targeting wide variety of therapeutically active moieties. Although the technology utilized in niosomes is greatly in its infancy but has evolved for treatment of cancer and many dreadful infectious diseases efficiently with reduced side effects and better patient compliance. The delivery system is already in use in cosmetic industry but it requires further exploration and research to bring out other commercially available products as they have a great potential to perform better than conventional drug delivery system.
References
Abd-Elbary A, El-laithy HM, Tadros MI (2008) Sucrose stearate-based proniosome-derived niosomes for the nebulisable delivery of cromolyn sodium. Int J Pharm 357(1–2):189–198. doi:10.1016/j.ijpharm.2008.01.056
Alcantar N, Dearborn K, Van Auker M, Toomey R, Hood E (2008) Niosome hydro gel drug delivery. US patent number US2008/0050445 A1
Alcantar N, Williams EC, Toomey R (2010) Niosome hydrogel drug delivery. US patent number US2010/0068264 A1
Ammar HO, Ghorab M, El-Nahhas SA, Higazy IM (2011) Proniosomes as a carrier system for transdermal delivery of tenoxicam. Int J Pharm 405:142–152. doi:10.1016/j.ijpharm.2010.11.003
Arunothayanun P, Bernard MS, Craig DQ, Uchegbu IF, Florence AT (2000) The effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether. Int J Pharm 201:7–14
Attia IA, El-Gizawy SA, Fouda MA, Donia AM (2007) Influence of a niosomal formulation on the oral bioavailability of acyclovir in rabbits. AAPS PharmSciTech. doi:10.1208/pt0804106
Baillie AJ, Coombs GH, Dolan TF, Laurie J (1986) Non-ionic surfactant vesicles, niosomes, as a delivery system for the anti-leishmanial drug, sodium stibogluconate. J Pharm Pharmacol 38:502–505
Balakrishnan P, Shanmugam S, Lee WS, Lee WM, Kim JO et al (2009) Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm 377:1–8. doi:10.1016/j.ijpharm.2009.04.020
Bhaskaran S, Lakshmi PK (2009) Comparative evaluation of niosome formulations prepared by different techniques. Acta Pharm Sci 51:27–32
Bibi S, Kaur R, Henriksen-Lacey M, Mc Neil SE, Wilkhu J, Lattmann E (2011) Microscopy imaging of liposomes: from cover- slips to environmental SEM. Int J Pharm 417:138–150
Biju SS, Telegaonar S, Mishra PR, Khar RK (2006) Vesicular system: an overview. Indian J Pharm Sci 68:141–153
Biswal S, Murthy PN, Sahu J, Sahoo P, Amir F (2008) Vesicles of non-ionic surfactants (niosomes) and drug delivery potential. Int J Pharm Sci Nanotechnol 1(1):1–8
Blazek Welsh AI, Rhodes DG (2001) SEM imaging predicts quality of niosomes from maltodextrin-based proniosomes. Pharm Res 18(5):656–661
Chauhan S, Luorence MJ (1989) The preparation of polyoxyethylene containing non-ionic surfactant vesicles. J Pharm Pharmacol 41:1–6
Chauhan M, Sharma SK, Anilkumar N (2009) Span-60 niosomal oral suspension of fluconazole: formulation and in vitro evaluation. J Pharm Res Health Care 1(2):142–156
Dahiya NK, Rao R, Nanda S (2011) Preparation and characterization techniques in niosomal vesicular systems—a review. J Pharm Biomed Sci 5:1–8
Gail S, Shenoy DB, Lee RW (2010) Adjuvant and vaccine compositions. US patent number US2010/0226932 A1
Guinedi AS, Mortada ND, Mansour S, Hathout RM (2005) Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int J Pharm 306(1–2):71–82. doi:10.1016/j.ijpharm.2005.09.023
Hall DG (1987) Thermodynamics of micelle formation. In: Schick MJ (ed) Nonionic surfactants: physical chemistry, surfactant science series, vol 23. Marcel Dekker, New York, pp 233–296
Hilgers LA, Zigterman GJWJ, Snippe H (1989) Immunomodulating properties of amphiphilic agents. In: Kammuller ME, Bioksma N, Sienen W (eds) Autoimmunity and toxicology. Elsevier, Amsterdam, pp 294–306
Hood E, Strom JA, Van Auker M (2006) Ultrasound enhancement of drug release across non ionic surfactant membranes. US patent number US2006/0292211 A1
Hunter CA, Dolan TF, Coombs GH, Baillie AJ (1988) Vesicular system (niosomes and liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J Pharm Pharmacol 40(3):161–165
Indhu PK, Garg A, Anil KS, Aggarwal D (2004) Vesicular system in ocular drug delivery. Indian J Pharm Sci 269:1–14
Jadon PS, Gajbhiye V, Jadon RS, Gajbhiye KR, Ganesh N (2009) Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech 10(4):1186–1192. doi:10.1208/s12249-009-9325-z
Jiao J (2008) Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv Drug Deliv Rev 60(15):1663–1673. doi:10.1016/j.addr.2008.09.002
Kazi KM, Mandal AS, Biswas N, Guha A, Chatterjee S, Behera M, Kuotsu K (2010) Niosome: a future of targeted drug delivery systems. J Adv Pharm Technol Res 1(4):374–380. doi:10.4103/0110-5558.76435
Khan A, Sharma PK, Visht S, Malviya R (2011) Niosomes as colloidal drug delivery system: a review. J Chronother Drug Deliv 2(1):15–21
Khandare JN, Madhavi G, Tamhankar BM (1994) Niosomes novel drug delivery system. East Pharm 37:61–64
Kumar GP, Rajeshwarrao P (2011) Nonionic surfactant vesicular systems for effective drug delivery-an overview. Acta Pharm Sin B 1(4):208–219. doi:10.1016/j.apsb.2011.09.002
Kumar A, Pal JL, Jaiswal A, Singh V (2011) Review on niosomes as novel drug delivery system. Int Res J Pharm 2(5):61–65
Lawrence J, Chauhan S, Lawrence SM, Barlow D (1996) The formation, characterization and stability of non-ionic surfactant vesicles. STP Pharma Sci 6(1):49–60
Marianecci C, Paolino D, Celia C, Fresta M, Carafa M, Alhaique F (2010) Non-ionic surfactant vesicles in pulmonary glucocorticoid delivery: characterization and interaction with human lungfibro-blasts. J Control Release 147(1):127–135. doi:10.1016/j.jconrel.2010.06.022
Martin JF (1990) Pharmaceutical manufacturing of liposomes. In: Tyle P (ed) Specialized drug delivery systems manufacturing and production technology. Marcel Dekker, New York, pp 267–314
Mokhtar M, Sammour OA, Hammad MA, Megrab NA (2008) Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int J Pharm 361(1–2):104–111. doi:10.1016/j.ijpharm.2008.05.031
Mujoriya RZ, Bodla RB (2011) Niosomes—challenge in preparation for pharmaceutical scientist. Int J Appl Pharm 3:11–15
Nasseri B, Florence AT (2003) Microtubules formed by capillary extrusion and fusion of surfactant vesicles. Int J Pharm 266(1–2):91–98. doi:10.1016/S0378-5173(03)00385-5
Palozza P, Muzzalupo R, Trombino S, Valdannini A, Picci N (2006) Solubilization and stabilization of beta-carotene in niosomes: delivery to cultured cells. Chem Phys Lipids 139(1):32–42. doi:10.1016/j.chemphyslip.2005.09.004
Pardakhty A, Varshosaz J, Rouholamini A (2007) In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int J Pharm 328:130–141. doi:10.1016/j.ijpharm.2006.08.002
Parthasarathi G, Udupa N, Umadevi P, Pillai GK (1994) Niosome encapsulated vincristine sulphate: improved anticancer activity with reduced toxicity in mice. J Drug Target 2:173–182. doi:10.3109/10611869409015907
Pavala RN, Suriyaprakash TNK, Senthamarai R (2010) Formulation and evaluation of rifampicin and gatifloxacin niosomes on logarithmic-phase cultures of Mycobacterium tuberculosis. Int J Pharm Biol Sci 1:379–387
Rogerson A, Cummings J, Willmott N, Florence AT (1988) The distribution of doxorubicin in mice following administration in niosomes. J Pharm Pharmacol 40:337–342
Ruckmani K, Sankar V (2010) Formulation and optimization of Zidovudine niosomes. AAPS PharmSciTech 11:1119–1127. doi:10.1208/s12249-010-9480-2
Ruckmani K, Jayakar B, Ghosal SK (2000) Nonionic surfactant vesicles (niosomes) of cytarabine hydrochloride for effective treatment of leukemias: encapsulation, storage, and in vitro release. Drug Dev Ind Pharm 26(2):217–222
Schreier H, Bouwstra J (1994) Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release 30:1–15. doi:10.1016/0168-3659(94)90039-6
Shahiwala A, Misra A (2002) Studies in topical application of niosomally entrapped nimesulide. J Pharm Pharm Sci 5:220–225
Shan W, Liu H, Shi J, Yang L, Hu N (2008) Self-assembly of electroactive layer-by-layer films of heme proteins with anionic surfactant dihexadecyl phosphate. Biophys Chem 134:101–109
Stafford S, Ballie AJ, Florence AT (1988) Drug effect on the size of chemically defined nonionic surfactant vesicles. J Pharm Pharmacol 40:26
Terzano C, Allegra L, Alhaique F, Marianecci C, Carafa M (2005) Non-phospholipid vesicles for pulmonary glucocorticoid delivery. Eur J Pharm Biopharm 59:57–62. doi:10.1016/j.ejpb.2004.06.010
Uchegbu IF, Florence AT (1995) Non-ionic surfactant vesicles (niosomes): physical and pharmaceutical chemistry. Adv Colloid Interface Sci 58:1–55
Uchegbu IF, Vyas SP (1998) Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm 172:33–70. doi:10.1016/S0378-5173(98)00169-0
Van Auker M, Plaas A, Hood E (2007) Immuno targeting of non ionic surfactant vesicles. US patent number US2007/0172520 A1
Vyas SP, Khar RK (2002) Controlled drug delivery—concepts and advances. CBS Publisher and Distributor, New Delhi
Wadhe K, Kalsait R, Umekar M (2009) Alternate drug delivery system: recent advancement and future challenges. Arch Pharm Sci Res 1:97–105
Yang CC, Le YC, Liu CC (2005) Compositions and methods of enhanced transdermal delivery of steroid compounds and preparation methods. US patent number US2005/0239747 A1
Yoshioka T, Sternberg B, Florence AT (1994) Preparation and properties of vesicles (niosomes) of sobitan monoesters (Span 20, 40, 60, and 80) and a sorbitan triester (Span 85). Int J Pharm 105:1–6. doi:10.1016/0378-5173(94)90228-3
Zhang S, Morris ME (2005) Efflux transporters in drug excretion. In: Wang B, Siahaan T, Soltero R (eds) Drug delivery: principles and applications. Wiley, Hoboken, pp 381–398
Zografi G (1995) Interfacial phenomena. In: Gennaro AR (ed) Remington: the science and practice of pharmacy, 17th edn. Mark Publishing, Pennsylvania, pp 241–251
Acknowledgments
The authors are thankful to the Institute of Pharmacy, Bundelkhand University, Jhansi, U.P. to provide facility to compile this review. The authors of this publication receive no funding for this research work, the authors Rizwana Khan and Raghuveer Irchhaiya have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, R., Irchhaiya, R. Niosomes: a potential tool for novel drug delivery. Journal of Pharmaceutical Investigation 46, 195–204 (2016). https://doi.org/10.1007/s40005-016-0249-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0249-9