Abstract
Niosomes fall into the category of vesicular drug delivery systems as they have lamellar structures that are formed due to the self-assembly of nonionic surfactants. Compared to other vesicular carriers, such as liposomes, niosomes are less toxic due to their nonionic composition. Other added advantages include lesser cost, better stability under wide varieties of pH and better drug carrier capacity. Although developed initially to be used in the cosmeceutical industry, niosomes enter into pharmaceutics, which can be mainly attributed to their multidrug carrier ability. However, niosomes are also associated with shortcomings, some of them being lower drug encapsulation efficiency, non-uniform-sized vesicles and drug leakage during synthesis. In this chapter, an attempt is being made to give the reader an overall idea about the synthesis and characterization of niosomes and the factors affecting its morphology and stability. In addition, the progress made in the research studies and the applications of niosomes is also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 Introduction
Even though liposomes can encapsulate the various types of drugs and are capable of sustained release, they are also associated with several disadvantages like high formulation cost, brief shelf life, low stability of their aqueous suspensions at different pH levels and toxicity. These factors led to diverting the focus on developing vesicular carriers that were more biocompatible and could nullify the abovementioned shortcomings. Hence, niosomes came into being. They were first formulated by the cosmetic company L’Oréal, and the first commercial product containing niosomes was released in 1987 (Singh and Sharma 2016).
Niosomes possess most of the physical and structural properties of liposomes having unilamellar, bilamellar or multilamellar structure enclosing an aqueous core. Their ability to self-assemble easily in an aqueous medium during preparation is because of the high interfacial tension between water and the hydrocarbon portion of the surfactant involved. In addition, the presence of strong steric or ionic repulsion between the head groups of the surfactant molecule makes sure that these groups always stay in contact with water. These two strong contradicting forces impart strong structural strength to niosomes (Uchegbu and Florence 1995). The formation of stable niosomes is also governed by membrane-stabilizing agents, primarily cholesterol (Abdelkader et al. 2014). The property of high-interfacial activity also gives the niosomes the ability to simultaneously load many hydrophilic and hydrophobic drugs, for example, doxorubicin and curcumin (anticancer drugs) (Mahale et al. 2012) . Moreover, the presence of nonionic surfactants enhances the fluidity of biological membrane at the delivery site, hence making niosomes an ideal drug delivery vehicle for a variety of drugs via the transdermal route. It has also been observed that the stability of peptide-based drugs is more when encapsulated in niosomes. However, niosomes are also associated with disadvantages like drug leakage and its hydrolysation in aqueous suspensions, and they also exhibit more irritability than liposomes (Moghassemi and Hadjizadeh 2014; Singh and Sharma 2016).
This chapter aims to provide a comprehensive account of niosomes, including their structural properties, synthesis, characterization techniques used and administration routes and an overview of their applications.
16.2 Advantages of Niosomes
When a comparative study is done, niosomes have significant advantages over liposomes which can be summarized as follows:
-
1.
The risk of toxicity posed by niosomes is way less when compared to liposomes.
-
2.
Niosomes are more chemically stable and offer a longer shelf life than liposomes.
-
3.
They are capable of being degraded within any biological system and pose no threat of severe immune response by the body in which they have been administered.
-
4.
Because of the high fluidity exhibited by niosomes, they can be administered effectively and safely to the body via many routes like oral, transdermal, pulmonary as well as ocular with better patient compliance.
-
5.
The presence of high-interfacial activity in niosomes gives them the ability to perform multidrug delivery mainly as a combination.
-
6.
The use of nonionic surfactants for synthesizing niosomes gives the advantage of higher biocompatibility than liposomes (Rajera et al. 2011; Chandu et al. 2012; Moghassemi and Hadjizadeh 2014; Verma and Utreja 2019).
A variety of substances ranging from simple and complex proteins like bovine serum albumin (BSA), α-interferon, immunosuppressants like cyclosporine A, hormones like vasopressin, insulin and luteinizing releasing hormone and antigens for influenza virus have been encapsulated effectively and delivered in a targeted and sustained mode using niosomes. Moreover, as niosomes can provide high drug concentration and bioavailability, many pharmaceutical drugs also have been encapsulated in niosomes for various applications like anti-leishmanial, anti-inflammatory, anti-tubercular and hormonal and anticancer therapy (Kumar and Rajeshwarrao 2011; Mahale et al. 2012).
16.3 Structure of Niosomes
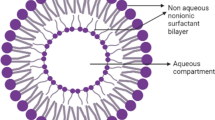
Niosomes have vesicular or micellar structure, generally with a hydrophilic core and hydrophobic outer shell as shown in Fig. 16.1. The formation of micellar structures mainly is by the self-assembly of the nonionic surfactants, which can be ascribed mainly to the hydrophobic and hydrophilic interactions at the interfacial level at the time of aggregation. The hydrophobic interactions take place at the hydrocarbon tail portion of the nonionic surfactant molecule. The hydrophilic interactions are between the hydrophilic heads and the aqueous medium. They tend to remain in constant contact with the aqueous medium in which the niosomes are prepared. Hence, these two opposing forces tend to decrease (hydrophobic) the interfacial area per surfactant molecule, while the hydrophilic force tends to increase the interfacial area (Abdelkader et al. 2014). Normally, hydrophilic drugs get encapsulated in the aqueous core, while hydrophobic drugs get entrapped at the hydrophobic shell (Abdelkader et al. 2014; Moghassemi and Hadjizadeh 2014).
Structure of a niosome. (Adapted from Mahale et al. 2012)
However, the morphology of niosomes depends not only on the nonionic surfactant involved but also on other factors like stabilizing agents like cholesterol and other formulation parameters, which will be discussed in the following sections.
16.4 Factors Affecting the Structure and Properties of Niosomes
16.4.1 Nature of the Nonionic Surfactant Involved
Nonionic surfactants have a polar (hydrophilic) head and non-polar (hydrophobic) tail. Since they are amphiphilic and do not carry a net charge, they exhibit lesser toxicity, lesser haemolytic activity, more stability and lesser irritation when compared to other class of surfactants. They also show more potent inhibition towards P-proteins which enhance their drug absorption capacity. They also maintain an almost stable physiological pH in the solution. The inhibition of nonionic surfactants towards the multidrug resistant p-glycoprotein allows adsorption and thereby targeting of a variety of cancer drugs (doxorubicin, curcumin, morusin), steroids (hydrocortisone) and cardiovascular drugs (beta-blockers) (Agarwal et al. 2018).
Some of the significant nonionic surfactants include polyglycerol alkylethers, glucosyl dialkyl ethers, crown ethers, polyoxyethylene ethers and esters, such as series of Brijs, Spans and Tweens (Shilpa et al. 2011). A new class of surfactants known as Gemini surfactants which have two hydrophobic chains and two hydrophilic head groups linked with spacers have emerged as ideal candidates for niosomes. Another class of surfactants called bolaamphiphiles contains bipolar amphiphiles with two polar heads connected by one or two long hydrophobic spacers that are also being used for niosomal formulation (Pardakhty et al. 2007; Mahale et al. 2012).
The chain length and the size of the hydrophilic head group play a crucial role in the entrapment efficiency of the niosomes. Long stearyl chains increase entrapment efficiency, while shorter lauryl chains decrease entrapment efficiency. Longer alkyl group chain with extended hydrophilic head group favours entrapment of hydrophilic drugs, while shorter alkyl chain length with long hydrophilic head group is better suited for entrapment of hydrophobic drugs (Nasseri 2005). Some of the commonly used nonionic surfactants for formulating niosomes have been shown in Table 16.1 (Raymond et al. 2006; Kumar and Rajeshwarrao 2011; Ag Seleci et al. 2016; Gharbavi et al. 2018).
The other significant properties of the nonionic surfactants which determine the nature of niosomes formed are:
-
(a)
Hydrophilic-lipophilic balance (HLB) value
-
(b)
Critical packing parameter
-
(a)
Hydrophilic-lipophilic balance (HLB) value
The HLB value is a number is a ratio of the hydrophilic and lipophilic groups in a surfactant molecule. Its value ranges from 0 to 20 for nonionic surfactant molecules. Generally, as a thumb rule, surfactants with values greater than 10 have a greater affinity for water (hydrophilic), while those with a value less than 10 have an affinity towards oil (lipophilic). An HLB number ranging from 4 to 8 is highly favourable for vesicle formation (Uchegbu and Florence 1995). Attributing to their higher aqueous solubility, hydrophilic surfactants cannot form free hydrated units (vesicles), but instead, they aggregate and undergo coalescence forming lamellar-like structures (Jousma et al. 1988, 1989). On the other hand, surfactants with a higher HLB value ranging between 14 and 17 do not usually form niosomes (Shahiwala and Misra 2002). When it comes to surfactants with an HLB value of around 10, cholesterol plays a critical role in forming niosomes (Tavano et al. 2011). Table 16.2 summarizes the effect of HLB value on niosomal formation (Shahiwala and Misra 2002).
-
(b)
Critical packing parameter
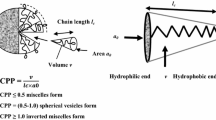
Another important factor that determines niosomal formation is the critical packing parameter (CPP) of the monomer. CPP value primarily determines the type of aggregates formed. Figure 16.2 shows the influence of CPP on the morphology of niosomes formed. It is given by the expression, CPP = V/( a0 x lc), where V corresponds to the molecule tail volume, a0 corresponds to the surface area per molecule at the hydrocarbon-water interface and lc corresponds to the critical span of a molecular chain in fluid when in aggregation. For better understanding, the diagrammatic representation of above-mentioned parameters is shown in Fig. 16.2.
Diagrammatic representation of critical packing parameter (CPP). (Adapted from Kumar and Rajeshwarrao 2011)
However, the values of CPP and HLB alone cannot be reliable in foretelling the shape of the micelles formed. One more factor known as AC is the effective area per lipid chain, i.e. the cross-sectional of the molecule tail. If the value of Ac is above 0.43 nm2, then a micellar structure is formed; if it is slightly below, then bilayer vesicles are formed; and if the value is way less than 0.43 nm2, then multilamellar structures are formed (Israelachvili et al. 1976; Tavano et al. 2011). A point to be noted is that during niosomal preparation, addition of a water-insoluble nonionic surfactant (as a co-surfactant) to water-soluble nonionic surfactant can result in formation of large-sized niosomes with lesser entrapment efficiency. This can be attributed to the competitive interaction between the hydrophobic co-surfactant and cholesterol during bilayer structure formation, resulting in forming a weak membrane, and hence drug leakage occurs (Yeo et al. 2019).
16.4.2 Addition of Cholesterol
Cholesterol is an inherent part of biological membranes that play a key role in the membrane properties like aggregation, fusion processes, ion permeability and elasticity and the size and shape of the vesicles. The addition of cholesterol during niosomal formulation mainly enhances the fluidity and rigidity of the niosomes (Liu et al. 2007). As shown in Fig. 16.3, cholesterol strengthens the structural integrity of the niosome by hydrogen bond formation with the surfactant involved (span 60) (Moghassemi and Hadjizadeh 2014). The addition of cholesterol is also dependent on the HLB value of the surfactant involved. When the surfactant has an HLB value of above 10, a minimum addition of cholesterol is necessary to compensate larger head groups (Bandyopadhyay and Johnson 2007). Cholesterol also improves the cohesion among the areas of non-polar portions of the vesicular bilayer formed (Manconi et al. 2006). Its action also sees the stabilizing effect of cholesterol on niosomes of inhibiting the destabilizing effects of serum and plasma components, decreasing the vesicle permeability (ROGERSON et al. 1988). Cholesterol also imparts better stability to the niosomes by improving the gel to the liquid transition temperature of the vesicles formed. Moreover, cholesterol addition helps more hydrophobic surfactants in vesicle formation by inhibiting aggregate formation (Kumar and Rajeshwarrao 2011).
Morphology of niosomes formed according to CPP value. (Adapted from Marianecci et al. 2014)
Generally on a quantitative basis, the maximum amount of the surfactant and lipids like cholesterol used is in the range of 1 to 2.5% (w/w). Changing the surfactant/cholesterol ratio can drastically affect the drug entrapment efficiency, the viscosity and, upon hydration, the assembly of the niosomes formed (Uchegbu and Vyas 1998; Kumar and Rajeshwarrao 2011). Studies have shown that the best entrapment efficiency is for those niosomes having 60% of cholesterol (Carafa et al. 2002; Moghassemi et al. 2015, 2017). Cholesterol also plays a key role in affecting or regulating the niosomal release profile of the entrapped drug.
16.4.3 Addition of Charge-Inducing Agents and Phase Transition Temperature
Charge-inducing agents are added for the purpose to impart a net charge on the surface of the niosomes for attaining bilayer stabilization and enhance mobility in an electrophoretic sense, as observed in the case of erythrocytes. However, adding such agents beyond a specific amount will inhibit niosomal formation (Waddad et al. 2013). Particle aggregation is also prevented due to electrostatic repulsion between like-charged vesicles. Charge-inducing agents also improve drug encapsulation efficiency in some cases and improve chances of skin permeability and chances of hybrid niosomal complex formation (Oh et al. 2006; Cametti 2008). A few examples of charge-inducing agents include dicetyl phosphate (negative charge inducing), phosphatidic acid (negative charge) and stearylamine (positive charge) (Uchegbu and Vyas 1998; Rajera et al. 2011; Abdelkader et al. 2014; Moghassemi and Hadjizadeh 2014).
Another essential factor that decides the entrapment efficiency of the surfactant involved during formulation will be the phase transition temperature of the surfactant. Phase or gel transition temperature is termed as the temperature that is required to change the inherent closely packed state of the surfactant to a more randomly oriented state with increased fluidity. An example where gel transition temperature plays a deciding factor is in the surfactant span 60. Span 60 has a trend of having higher entrapment efficiency because of its high gel transition temperature (Kumar and Rajeshwarrao 2011; Key et al. 2018). Studies conducted for combined delivery of hydrophobic and hydrophilic anticancer drugs (curcumin and doxorubicin, respectively) have shown that Tween 60 has a better entrapment efficiency than span 60 Tween 60 a longer hydrophobic and hydrophilic chains of than span 60 (Naderinezhad et al. 2017). Some of the commonly used nonionic surfactants for formulating niosomes have been shown in Table 16.1 (Manosroi et al. 2003; Kumar and Rajeshwarrao 2011; Ag Seleci et al. 2016; Gharbavi et al. 2018).
16.4.4 Hydration Medium
The type of hydration medium used to form niosomes is primal. Phosphate buffer solution (PBS) of various pH levels has been used as a hydration medium. The pH of the buffer medium is decided based on the solubility of the drug that has to be encapsulated. An increase in entrapment efficiency occurs when the hydration medium is acidic (pH=5.5), and it decreases under alkaline conditions (pH=6.8) (Kumar and Rajeshwarrao 2011). It has been observed that entrapment efficiency increases when hydration time is prolonged, but at the same time, the entrapment efficiency decreases when the volume of the hydration medium is increased (Kumar and Rajeshwarrao 2011; Mahale et al. 2012).
The temperature of the hydration medium should be maintained at a level above the gel to the liquid phase transition of the surfactant involved. The temperature determines the possibility of the aggregation or assembly of the surfactant into vesicles and the shape of the vesicles formed (Azmin et al. 1985). The duration of hydration time also influences entrapment efficiency and vesicular size, with shorter durations producing large-sized vesicles with lesser entrapment efficiency. It has been recommended that the optimal time for hydration will be 60 mins, with a volume of hydration medium being 5 ml (Yeo et al. 2019). Vesicles of smaller size are produced when hydration time is prolonged. The morphology or structural properties occur whenever a change is induced in the hydration temperature; this can also affect vesicle formation (Uchegbu and Vyas 1998; Rajera et al. 2011). In research conducted by Maryam, it was found that upon increasing the temperature of the hydration medium (from 25 °C to 55 °C) and hydration time (from 10 to 25 mins), the population of large-sized multilamellar vesicles increased (Homaei 2016). In another study, earlier formed polyhedral vesicles were transformed into spherical vesicles when the temperature was increased from 25 °C to 48 °C, and upon reverse cooling from 55 °C to 35 °C, polyhedral vesicles retained. During the transition stage, spherical vesicles of smaller size in a clustered state are formed at 49 °C (Arunothayanun et al. 2000).
16.4.5 Nature of the Drug to be Encapsulated
The degree of encapsulation of the concerned drug depends on its various properties like molecular weight and structural properties, whether hydrophilic or lipophilic (Mozafari 2007). Its subsequent interaction with the surfactant involved can affect vesicle size and entrapment efficiency. As far as entrapment of hydrophilic drugs is concerned, the efficiency is normally within 10–20% (Peltonen et al. 2002; Ferreira et al. 2004). But it has been observed if a charge interaction occurs between the surfactant and the drug, there is an enhancement in the entrapment efficiency. This was seen in a study conducted on transdermal delivery of a hydrophilic drug gallidermin in which interaction that occurred between the positive and negative charges of the drug and niosomes, respectively, brought up the entrapment efficiency up to 45% (Manosroi et al. 2010).
When it comes to the entrapment efficiency of hydrophobic drugs, an inverse relationship exists, i.e. entrapment efficiency decreases as the concentration of the drug increases as that would interfere with the vesicle formation (Marianecci et al. 2010). The process of drug entrapment ceases once the bilayer structural saturation occurs.
16.4.6 Method of Niosomal Preparation
The method of niosomal preparation can influence the vesicle size and drug entrapment efficiency. A study conducted concerning prednisolone-loaded ethoniosomes (ethanol-based niosomes) showed that the vesicles prepared by thin-film hydration method have better entrapment efficiency than those prepared by the ethanol injection method. But niosomes produced by the ethanol injection method were smaller in size (Gaafar et al. 2014). The niosomes formulated by the transmembrane pH method have high drug entrapment efficiency (Parthasarathi et al. 1994; Kazi et al. 2010; Rajera et al. 2011).
16.5 Methods of Preparation of Niosomes
16.5.1 Thin-Film Hydration Technique
Also called the “Hand shaking method,” this is the most commonly used preparation method. As shown in Fig. 16.4, initially, the involved nonionic surfactant, hydrophobic drug and cholesterol taken in appropriate quantities are dissolved in a suitable organic solvent and introduced in a round bottom flask. The organic solvent is then evaporated preferably in a rotary evaporator leading to the formation of a thin layer or film, which, when wetted by a suitable hydration medium like phosphate buffer (in which hydrophilic drug is dissolved), results in the formation of multilamellar vesicles (Rajera et al. 2011; Gandhi et al. 2012; Moghassemi and Hadjizadeh 2014; Ag Seleci et al. 2016). It can be seen that a large variety of hydrophilic and hydrophobic drugs can be encapsulated in niosomes using this technique, for example, paclitaxel (Bayindir and Yuksel 2010), zidovudine (Ruckmani and Sankar 2010), green tea extract (Isnan and Jufri 2017), lornoxicam (Bini et al. 2012) and gallidermin (Manosroi et al. 2003).
Thin-film hydration technique or “Hand shaking method”. (Adapted from Ge et al. 2019)
16.5.2 Ether Injection Method
An organic solvent like diethyl ether is used to mix the surfactant and cholesterol together, and then the mixture gets introduced into an aqueous solution containing the drug. It has to be made sure the temperature of the aqueous medium should be maintained at a temperature above 60 °C (Moghassemi and Hadjizadeh 2014). The solvent is evaporated, which leads to the formation of unilamellar vesicles of diameter ranging from 50 to 1000 μm (Fig. 16.5) (Uchegbu and Vyas 1998; Verma et al. 2010). This method has been used to prepare stavudine-loaded niosomes (Shreedevi et al. 2016).
Ether injection method. (Adapted from Chen et al. 2019)
16.5.3 Reverse-Phase Evaporation Method
After mixing the surfactant and cholesterol in an organic solvent, an aqueous solution of the preferred hydrophilic drug is added to the organic mixture, leading to the formation of a two-phase system (Fig. 16.6). After homogenization, the organic phase is then removed in negative pressure mode. This method forms large unilamellar vesicles (LUVs) and has been used to prepare niosomal formulations containing bovine serum albumin (Moghassemi et al. 2017), ellagic acid (Junyaprasert et al. 2012) and isoniazid (Singh et al. 2011).
Schematic diagram of reverse phase evaporation method. (Adapted from Durak et al. 2020)
16.5.4 Transmembrane pH Gradient
This method involves dissolving equal proportions of the surfactant and cholesterol in an organic solvent like chloroform, followed by removing the organic solvent under reduced pressure. A thin film like lipid layer gets formed, hydrated by vortexing using an acidic solution (usually citric acid). Once the mixture is freeze-thawed, an aqueous solution of the desired drug is added and mixed well by vortexing. The pH of the final mixture is then adjusted to the desired value using disodium hydrogen solution (Fig. 16.7). Remote drug loading can happen using this method (Kazi et al. 2010; Kumar and Rajeshwarrao 2011; Durak et al. 2020).
Schematic diagram of transmembrane pH gradient. (Adapted from Chen et al. 2019)
16.5.5 The “Bubble” Method
This method is devoid of using an organic solvent. A layered system comprising of a flask having three necks is used. In the flask, proper mixing of the surfactant, cholesterol and phosphate buffer is ensured. A water bath is used to maintain the temperature of the flask at the desired temperature. At one neck of the flask, a thermometer is placed to note the temperature. Nitrogen gas is supplied through the second neck, while the final neck has an attachment for reflux cooling. After dispersing the mixture at a temperature of 70 °C and homogenizing for 15 s, nitrogen gas is supplied (Fig. 16.8) (Moghassemi and Hadjizadeh 2014). Large unilamellar vesicles formed by this method are required to undergo a suitable size reduction (Kazi et al. 2010; Mahale et al. 2012).
Schematic diagram of the “Bubble” method. (Adapted from Yeo et al. 2017)
16.5.6 Microfluidization Method
In this method, a mixture of the desired drug and the surfactant upon dissolving in a reservoir is pumped under pressure to an interaction chamber maintained at low temperatures (using ice packs) because the resulting interaction produces heat. The solution is passed through a cooling loop for further cooling (Fig. 16.9). Comparatively, much uniformly smaller-sized niosomes get produced by this method (Sorgi and Huang 1996; Kazi et al. 2010; Kumar and Rajeshwarrao 2011).
Schematic diagram of microfluidization method. (Adapted from Chen et al. 2019)
16.5.7 Heating Method
In this method, the surfactant (and other additives) and cholesterol are hydrated separately with buffer at room temperature under an inert nitrogen atmosphere for about 1 h. The buffer solution of cholesterol is heated to 120 °C for an hour to ensure proper cholesterol dissolving. After cooling the solution to about 60 °C, a buffer solution of the surfactant and other ingredients are added while the solution is continuously stirred, leading to the formation of niosomes. The niosomes are first kept at room temperature for about half an hour, followed by storage at 4–5 °C in nitrogen atmosphere (Fig. 16.10) (Moghassemi and Hadjizadeh 2014; Ag Seleci et al. 2016).
Schematic diagram of a heating method. (Adapted from Moghassemi and Hadjizadeh 2014)
16.5.8 Multi-membrane Extrusion Technique or Single-Pass Technique
This method involves passing a lipid-containing drug from a porous device through a nozzle. Passing through the nozzle allows forming uniformly sized niosomes with about 50–500 nm (Gharbavi et al. 2018).
16.5.9 Dehydration-Rehydration Method
In this method, niosomes are first prepared by thin-film hydration method followed by overnight freeze-drying using liquid nitrogen, which results in the formation of powdered niosomes. The powered niosomes are then hydrated using phosphate buffer saline of pH 7.4 at 60 °C. Niosomes containing the drug naltrexone for ocular delivery have been formulated using this method (Abdelkader et al. 2012).
16.5.10 Freeze and Thaw Method
This method is somewhat similar to the method mentioned above, but the niosomes prepared by thin-film hydration undergo freezing cycles. The niosomes are first frozen using liquid nitrogen for a minute, followed by 1-min thawing in a water bath at 60 °C. This cycle is done five times. This alternate freeze method and thaw helps reduce the size of the niosomes formed, but the entrapment efficiency of the niosomes is compromised. This method produces multilamellar vesicles (Moghassemi and Hadjizadeh 2014; Bartelds et al. 2018).
16.5.11 Sonication Method
This method is just probe sonication of a mixture of the surfactant and cholesterol and a buffer solution of the drug at 60 °C, which produces multilamellar vesicles. Unilamellar vesicles can be further obtained using ultrasonication. This method was used to formulate niosomes containing the drug cefdinir (Bansal et al. 2013).
16.5.12 The Handjani-Vila Method
In this method, a mix of the surfactant and cholesterol is added to an aqueous solution of the desired drug followed by agitation or ultracentrifugation for the purpose of homogenization. Throughout the process, the temperature has to be maintained (Palani 2010).
16.5.13 Supercritical Carbon Dioxide Fluid
In this method, the supercritical carbon dioxide apparatus used to prepare niosomes is a view cell with two glass windows on both sides equipped with a high-pressure pump for carbon dioxide gas feeding. An appropriate amount of the surfactant mixed with cholesterol and the drug dissolved in Dulbecco’s phosphate buffer solution is added to the view cell. The view cell is maintained at high pressure (about 200 bar) and temperature (about 60 °C), and carbon dioxide is introduced into the view cell. The mixture undergoes magnetic stirring for about 30 min, and once equilibrium is achieved, the pressure is released, and niosome dispersions are obtained. Throughout the process, the temperature has to be maintained constant (Manosroi et al. 2008).
16.5.14 Microfluidic Hydrodynamic Focusing
In this method, two miscible liquids undergo mixing in microchannels in a rapid but controlled manner. This method is very effective in producing niosomes with better size distribution than other regularly used methods. The parameters like surfactant chemical structure, the material used for microchannel fabrication and microfluid mixing conditions affect the assembly of the niosomes. When the flow rate ratio is increased, there is a subsequent decrease in the diffusive mixing time, hence producing small-sized niosomes. However, when a microchannel of a wider size is used, diffusive mixing time increases, thereby producing large-sized niosomes (Lo et al. 2010).
16.6 Type of Niosomes
Broadly, niosomes formed are of three types, as shown in Table 16.3 and Fig. 16.11.
Besides this general classification, there are some special niosomes formed as discussed below which was shown in Table 16.4.
16.6.1 Proniosomes
These are niosomes in the dehydrated state which, upon hydration in the aqueous medium, form dispersions. Since being in the dry form, the chances of the niosomes to aggregate, fuse or cake are less than average niosomes, and hence the problems of transportation and distribution are minimized (Rajera et al. 2011; Gharbavi et al. 2018). Proniosomes, once hydrated, can be used for transdermal delivery of drugs. Their percutaneous absorption capacity is much better than currently used semisolid preparations (Touitou et al. 2000; Touitou and Godin 2007).
16.6.2 PEGylated Niosomes
These are niosomes that have undergone modification with polyethylene glycol (PEG), and they can evade the phagocytosis by mononuclear cells, thereby increasing the circulation time of the drug-encapsulated niosomes in the body (Moghassemi and Hadjizadeh 2014). When monostearate was attached to the hydrophilic tail of PEG, it could be incorporated better into the hydrophobic lipid vesicular core (He et al. 2017). Moreover, it has been observed that PEGylation helps in improving the solubility and bioavailability of certain drugs, for example, gambogenic acid. In this case, span 60 was the surfactant used along with cholesterol and diacetyl phosphate as additives, and niosomes were prepared by ether injection method followed by modification with PEG (Lin et al. 2013). Dual drug delivery (paclitaxel and curcumin) also is possible with PEGylated niosomes (Alemi et al. 2018).
16.6.3 Elastic Niosomes
These do come under the class of niosomes, but a nonionic surfactant is used along with ethyl alcohol and water. So we can say that it’s a hybrid version of ethosomes. They are very suitable for transdermal drug delivery because of their ability to quickly move through the tight junction pores at the stratum corneum (Kumar and Rajeshwarrao 2011). They can be loaded with drugs independent of molecular weight and have a longer duration of action when compared to conventional niosomes. An example of a drug loaded in elastic niosomes is papain used for treating scars. The component of the drug carrier was Tween 61 along with cholesterol and methanol which are taken in the ratio 1:1 (Manosroi et al. 2013a).
16.6.4 Discomes
They are, as the name suggests, large disc-shaped niosomes (11–60 μm in size) formed after spherical niosomes undergo a morphological transformation when kept incubated in Solulan 24 of varied proportions at 74 °C and shaken for 1 h (Uchegbu et al. 1992; Abdelkader et al. 2014). They are preferably used for drug delivery via the ocular route (Abdelkader et al. 2014; Gharbavi et al. 2018), and the drug naltrexone has been delivered using discomes (Abdelkader et al. 2012).
16.6.5 Bola-Surfactant Niosomes
Bola-surfactant or bola-amphiphiles, unlike single-headed amphiphiles, have two hydrophilic groups attached to both ends of a long hydrophobic carbon chain and can aggregate or undergo self-assembly to form vesicles under certain conditions. Niosome formation has been reported to be formed when the bola-surfactant omega-hexadecyl-bis-(1-aza-18-crown-6), span 80 and cholesterol were taken in the molar ratio (2:3:1).
16.6.6 Aspasomes
Aspasomes are lamellar made from a mix of ascorbyl palmitate in water. Cholesterol and diacetyl phosphate are added for enhancing rigidity and improving stabilization, respectively. Apasomes are suitable for drug delivery via the transdermal route because of their excellent permeation properties and hence used in various cosmeceutical and pharmaceutical preparations. Moreover, they possess excellent anti-oxidative properties and hence are used in treatments involving reactive oxygen species (Rajera et al. 2011; Gandhi et al. 2012; Gharbavi et al. 2018; Aboul-Einien et al. 2020).
16.7 Characterization of Niosomes
16.7.1 Morphology, Size, Size Distribution and Other Structural Properties of Niosomes
Microscopic techniques like SEM (scanning electron microscopy) and TEM (transmission electron microscopy) are usually used to examine and the size and shape of niosomes formed (Moghassemi and Hadjizadeh 2014; Agarwal et al. 2018). AFM (atomic force microscopy), X-ray spectroscopy and NMR can determine the number of lamellae present in the niosomal structure (Madhav and Saini 2011; Rajera et al. 2011; Marianecci et al. 2014). X-ray scattering technique can be used for examining the thickness of the bilayer structure with the help of energy-dispersive X-ray diffraction (Liu and Guo 2007a, b; Domenici et al. 2009; Di Marzio et al. 2011). Fluorescence probe as a function of temperature can be used for determining the membrane rigidity of niosomes (Singh et al. 2004; Rajera et al. 2011).
To have a proper understanding of the niosomal membrane packing structure, fluorescence polarization is used, which is also used to determine the microviscosity of the membrane (Manosroi et al. 2008; Marianecci et al. 2014). Confocal laser scanning microscopy (CLSM) can be used to understand the location of entrapped entities (dyes). Usually, the hydrophilic substance gets embedded in the core of the niosomes and the hydrophobic substance in the outer shell of the niosomes as shown in Fig. 16.12. In Fig. 16.12, the dyes used were Nile red (hydrophobic) and FITC-dextran (hydrophilic). Nile red gets deposited at the niosome hydrophobic shell (Fig. 16.12a), while FITC-dextran gets encapsulated in the aqueous core (Fig. 16.12b). From Fig. 16.12c we can visualize the ability of niosomes to load both hydrophobic and hydrophilic entities simultaneously.
CLSM image of niosomes showing the distribution of the Nile red and FITC-dextran. (a) Green channel (FITC-dextran), (b) red channel (Nile red) and (c) overlapped image of red and green channels. Scale bar = 5 μm. (Adapted from Sharma et al. 2015)
Average particle size is usually determined by the dynamic light scattering (DLS) method, but the actual particle size of the vesicle on an individual basis can be determined by TEM (Ma et al. 2018). Determining the overall or net charge on niosomes is essential to evaluate the stability of niosomes. Zeta potential is calculated to determine the stability of niosomes and is done using zeta potential analyser, DLS instrument, Mastersizer, pH-sensitive fluorophores, microelectrophoresis and high-performance capillary electrophoresis (Shilpa et al. 2011). A niosomal system, having a negative value of zeta potential within the range of −41.7 to −58.4 mV, is considered stable with enough electrostatic repulsion between the particles (Bayindir and Yuksel 2010).
16.7.2 Entrapment Efficiency (EE)
It is usually calculated by subtracting the unloaded drug from the total amount of drug added (Kumar and Rajeshwarrao 2011). The quantity of unloaded drug is determined by filtration, chromatographic techniques like HPLC and gel chromatography and centrifugation, while the concentration of loaded drugs can be determined by dissolving the niosomes in a solvent like 0.1% TritonX-100 or 50% n-propanol and determining the assay of the solution using any specific method (Vyas et al. 2005; Manvi et al. 2012; Rinaldi et al. 2018).
The equation determines the entrapment efficiency (usually mentioned in percentage):
% Entrapment Efficiency = (Quantity of drug-loaded in the niosome/ Total quantity of drug taken) X 100
16.7.3 Drug Release Studies (In Vitro and In Vivo)
The dialysis membrane method is the most preferred to examine the in vitro drug release from niosomes. The niosomal suspension, after being put in a dialysis bag, is placed in a container having the dissolution media (usually buffer at the desired value of pH). The whole assembly is then placed on a magnetic stirrer, and the temperature is maintained at 37 °C (to simulate normal human body temperature) (Fig. 16.13). A sample is drawn at specific time intervals from the receptor, and the concentration of the drug is determined usually by spectroscopic methods like UV-Vis spectroscopy (Manosroi et al. 2010; Kumar and Rajeshwarrao 2011; Rajera et al. 2011).
The dialysis method, a prevalent method used to study the in vitro drug release from niosomes. (Adapted from Durak et al. 2020)
In another in vitro method, about 15 mg of the niosomal preparation is dissolved in 15 ml of phosphate buffer of pH 4.5 and 7.4. The pH of 4.5 and 7.4 was chosen to mimic the intracellular environment and blood plasma pH conditions. The samples are taken in Eppendorf tubes and continuously kept under rotation for a specific period of days. The tubes are removed at specific time intervals and centrifuged at high speeds of about 15000 rpm, and the resultant supernatant is analysed for determining the drug content by spectroscopic methods (Agarwal et al. 2018).
The in vivo study depends on the chosen route of drug administration or delivery, concentration of the drug taken and the presence or circulation time of the drug inside the body tissues, namely, the liver, spleen, lung and bone marrow (Verma et al. 2010; Moghassemi and Hadjizadeh 2014). Usually animal models like laboratory strain or species of rats, mice and guinea pigs are used to study the distribution of the drugs in the previously mentioned tissues. The distribution pattern is studied by sacrificing the drug administered animals, and its various tissues (as mentioned before) are removed, are washed with buffer and underwent homogenization and centrifugation. The drug concentration is analysed from the supernatant (Rajera et al. 2011).
16.7.4 Stability Studies
To check on the degree of drug leakage from the niosomal suspension, the stability studies are conducted at various temperatures like refrigeration temperature (4 °C), room temperature and elevated temperature (45 °C) (Kopermsub et al. 2011; Moghassemi and Hadjizadeh 2014). The usual parameters determined periodically during stability studies include entrapment efficiency, size and shape of the niosomes. The determination of these parameters is usually conducted after exposing the niosomal suspension to various conditions of humidity and irradiation with light of desired frequency range. In one study, the effect on niosomal stability under different body enzymes like trypsin, chymotrypsin and pepsin was studied, and it was discovered that the niosomes protect the encapsulated drug (paclitaxel) from these gastrointestinal enzymes (Bayindir and Yuksel 2010).
16.8 Routes of Niosomal Administration
Some of the most frequently used routes of administration for niosomes are discussed in this section. The route selection depends on the drug properties, the site of drug delivery and the concerned disease (Fig. 16.14).
16.8.1 Intravenous
The main advantage of the intravenous route is that the niosomes have a direct entry into systemic circulation, and niosomes ensure that the stability of the drug is maintained and its duration of being present in the blood is prolonged. To ensure that the prepared niosomes evade the monophagocytic system, the niosomes can undergo PEGylation in the synthesis stage. PEGylation also enhances the stability and bioavailability of niosomes. Morin hydrate and paeonol are two medications that were administered via an intravenous method include morin hydrate and paeonol (Waddad et al. 2013; He et al. 2017).
16.8.2 Oral
Generally, the oral route is the most preferred non-invasive route for any drug administration. A setback of oral administration is the destruction of the drug by the highly acidic environment and enzymes of the gastrointestinal system and, hence, a decrease in the drug’s bioavailability (Bayindir and Yuksel 2010). However, it has been observed that niosomes can protect the drug and get adsorbed into the gastric mucosa, increasing oral bioavailability. Some of the drugs with the proven increase in oral bioavailability by niosomal delivery are cefdinir (Bansal et al. 2013), paclitaxel (Bayindir and Yuksel 2010) and ginkgo biloba (Onochie et al. 2013). In another study, by coating the insulin-loaded niosomes with trimethyl chitosan, there was enhancement in the permeation ability of insulin across the intestinal membrane (Moghassemi et al. 2015).
16.8.3 Ocular
When administered via the ocular route, the low bioavailability of a drug can be attributed to a loss in the precorneal portion because of tear formation and a short period of stay in the conjunctival sac (Biswas and Majee 2017). A study found that during the ocular delivery of the drug naltrexone, anionic niosomes had better permeation ability than neutral niosomes (Abdelkader et al. 2012). Functionalizing the niosomes with specific agents will improve their corneal permeation ability and hence prolong their availability. This was observed in coating niosomes (loaded with tacrolimus) with hyaluronic acid, which decreased its lipophilicity and hence better permeation (Zeng et al. 2016). Hence using suitable agents for surface charge modification and inducing functionalization help in improving the chances of the niosomes to be better candidates for ocular drug delivery.
16.8.4 Nasal
Nasal administration is a suitable route for drug delivery if the objective is to escape the gastric and hepatic metabolism, thereby improving the efficacy of delivering drugs to the brain. However, nasal administration has some shortcomings, including short residence time in the nasal cavity because if airflow obstruction occurs, the niosomes can be eliminated by the mucociliary presence. Moreover, the susceptible nature of the nasal mucosa can drastically reduce the drug bioavailability and permeation. The bioavailability of the drug diltiazem has increased when loaded in niosomes and faced fewer chances of elimination by the nasal mucosa (Ammar et al. 2017).
16.8.5 Pulmonary
The pulmonary route for drug administration shows better permeation, targeting, sustained or prolonged drug delivery and, hence, better therapeutically. Ciprofloxacin-loaded niosomes exhibited a lesser minimal inhibitory concentration than the free drug. In the study involving MTT assay on human carcinoma lung cell line (A549), the niosomal suspension showed higher cytotoxicity compared to the free drug form of ciprofloxacin, and the nebulization was also higher. These results can show that niosomes can be considered for effective pulmonary drug delivery (Moazeni et al. 2010).
16.8.6 Transdermal
The transdermal route is always preferred if the delivery of the drug is superficial and localized. The most significant benefit of this route is that the concerned drug does not undergo pre-systemic metabolism resulting in better bioavailability and better patient compliance. The primary challenging part for transdermal drug delivery is crossing the barrier made by the stratum corneum (SC). One of the primary reasons is that SC is tightly connected, limiting the drug permeation (Alexander et al. 2012). It has been observed that vesicular systems are ideal vehicles for transdermal drug delivery because of their malleable nature giving them better penetration ability through the SC and hence better bioavailability and lesser side effects. The sustained or prolonged release of drug is also ensured when loaded into niosomes.
Usually, ethosomes are preferred over niosomes for transdermal drug delivery. The predominant presence of ethanol in high concentration in ethosomes imparts them the ability to modify the highly dense alignment of the SC’s lipid bilayers, thereby ensuring deeper penetration. However, niosomal gel loaded with the drug lopinavir showed better permeation and more site deposition than ethosomal gel loaded with the same drug (Patel et al. 2012). Moreover, the use of proniosomes helps in improving chances of the drug delivery. This was seen in the case of the anti-fungal drug fluconazole. The proniosomal formulation had a better drug deposition and more localization when compared to the available marketed formulation of the drug (Sandeep et al. 2014). The use of penetration enhancers can help in achieving sustained drug delivery. A study was conducted on the effect of chemical enhancers on the penetrating ability of ellagic acid. The potential of a penetration enhancer of two chemicals, namely, dimethyl sulphoxide (DMSO) and N-methyl-2 pyrrolidone, was examined. It was found that niosomes with DMP are better suited for dermal delivery, while DMSO niosomes were ideally better for epidermal drug delivery of ellagic acid (Junyaprasert et al. 2013).
16.9 Applications of Niosomes
Niosomes have been found to be versatile drug delivery carrier. As mentioned before, they are more stable and less cytotoxic than liposomes, and loading multiple drugs onto a single carrier and stimuli-responsive drug release have been possible with niosomes. Niosomes have been found to be effective in delivering drugs via different routes, and some of the applications in recent years are being discussed in this section (Fig. 16.15).
16.9.1 Delivery of Anticancer Drugs
One of the vast and established applications of niosomes is their ability to deliver anticancer drugs. Normally for anticancer targeted drug delivery, targeting on the tumour is of two types:
-
Passive, in which the drug carrier (in this case niosomes) gets deposited on the tumour cells on account of tumour cells having unique properties than normal cells (e.g. the enhanced permeability of tumour cell membranes allows better drug deposition in the tumour cells) (Attia et al. 2019).
-
Active, in which drug targeting is achieved because of the abnormal presence of certain receptors on tumour cells. Active targeting involves the attachment of niosomes with specific ligands. For attaching ligands, either PEG or cholesterol conjugation of niosomes is done (Kim et al. 2012; Oswald et al. 2017; Gharbavi et al. 2018).
Sustained and more stable delivery of gambogenic acid for anticancer activity was possible with PEGylation of niosomes (Lin et al. 2013). The bioavailability of paclitaxel when orally delivered increased by loading them in niosomes. The simultaneous multidrug delivery for anticancer activity is also possible; this has been seen in the case of niosomes loaded with doxorubicin (hydrophilic) and curcumin (niosomes). This strategy increased the bioavailability of curcumin, and release happened for 7 days with effective cytotoxic activity on HeLa cell lines, while the release of doxorubicin was only for 2 days (Sharma et al. 2015) which shows further research has to be conducted on enhancing niosomal uptake and release of hydrophilic drugs. Another example of dual-drug delivery for anticancer activity is combined delivery of paclitaxel and curcumin and showed enhanced anti-tumour activity (Alemi et al. 2018). A stimuli-responsive drug release was seen in the case of morusin-loaded niosomes. Here the release of the drug from the niosomes was pH-sensitive with the higher release of the drug in an acidic environment (pH=4.5) than in a neutral or alkaline environment (pH=7.4) (Agarwal et al. 2018).
16.9.2 Delivery of Proteins- and Peptide-Based Drugs and Vaccines
The oral delivery of protein- and peptide-based drugs has been challenging because of the drug destruction by the highly acidic environment and enzymatic activity in the gastrointestinal tract. Nevertheless, studies have shown that they are better protected by niosomal loading of such drugs and hence better bioavailability (Kumar and Rajeshwarrao 2011). To enhance the bioavailability and permeation of insulin at the intestinal mucosa, a study was conducted by loading insulin in niosomes and then coating them with trimethyl chitosan. It was seen that the uptake of such coated niosomes was four times more than insulin alone in Caco-2 cell monolayer (Moghassemi et al. 2015). Another study involving oral delivery of peptides was an investigation done by H. Yoshida (Yoshida et al. 1992). The peptide/protein 9-desglycinamide 8-arginine vasopressin (DGAVP) was encapsulated in stable niosomes made of polyoxyethylene alkylethers (C18EO3). The in vitro release studies conducted on the rat intestinal loop model showed that the release of DGAVP from the niosomes was much higher than DGAVP solution alone. This study shows the role of the surfactant used in formulating the niosomes as penetration enhancers in the intestinal tract.
Bilosomes are niosomes formulated by including bile salt in the vesicle bilayers during the preparation of niosomes. These bilosomes have been found to be effective for oral delivery of vaccines because of their ability to protect antigens from being degraded by the proteolytic enzymes of the gastrointestinal tract (Wilkhu et al. 2013). Hence, niosomes can be considered for non-invasive administration of vaccines which will promote better patient compliance. Niosomes or proniosomes are highly permeable to oxygen and hence can be used as a haemoglobin carrier, and niosomes can be used to treat anaemia by making the presence of haemoglobin more in the blood (Radha et al. 2013).
16.9.3 Delivery of anti-HIV Drugs
Lopinavir is an anti-HIV drug that acts as a protease inhibitor. However, its high lipophilicity, vulnerability to P-glycoprotein efflux transporters and cytochrome sensitivity make the systemic bioavailability of the drug poor, and hence oral administration is least preferred. Transdermal delivery of the drug was possible by niosomal gel, and its properties were compared with that of an ethosomal gel. Even though in the comparative study, ethosomal gel had a better drug deposition, the niosomal gel showed better and deeper penetration, and the drug release profile was more sustained (Patel et al. 2012). Another example of increasing the bioavailability of an anti-HIV drug administered orally by niosomal delivery is the case of the anti-HIV drug zidovudine (Ruckmani and Sankar 2010).
The dual-drug delivery ability of niosomes has been found to an ideal candidate for targeted delivery of anti-HIV drugs. In a study, mannosylated niosomes were used as a dual-drug delivery system loaded with gold nanoparticles (GNPs) and the anti-retroviral medication efavirenz (Malik et al. 2018). Efaverinz being lipophilic got loaded in the hydrophobic vesicle membrane, while GNPs got encapsulated in the aqueous core of the niosomes. The niosomes were functionalized with a mannate appendage to enhance their targeting ability via receptor-based drug delivery. HIV host cells have receptors for the oligosaccharide mannan, and these functionalized niosomes can interact better with the target and improve the bioavailability of the drug in question. GNPs can effectively inhibit the entry of HIV into cells. Moreover, the GNPs also played an essential role in developing this niosomal formulation into a thermoresponsive gel, assuring non-invasive drug administration. This study shows one more example of how surface modification of niosomes helps in improving its targeting ability.
16.9.4 Treatment of Parasitic Diseases
Niosomes can also be used to treat a parasitic disease like leishmaniasis. Leishmaniasis mainly affects the liver and spleen, and loading the antimonials (drugs for treating leishmaniasis) in niosomes can improve the drug uptake by the liver and hence cause lesser side effects on other organs. Selenium is an important element necessary in our body for cancer prevention and anti-inflammatory and anti-oxidant effects and also has predatory action against Leishmania major and Leishmania tropica and Leishmania infantum. Vesicular delivery is one of the best approaches to deliver selenium, providing sufficient bioavailability. A dual-loaded niosomal formulation of selenium and glucantime was synthesized, which showed very effective lethality in vitro activity against leishmaniasis (Mostafavi et al. 2019).
Schistosomiasis is a disease caused by schistosomes which are parasitic flatworms. Praziquantel is the drug recommended by WHO for the early treatment of schistosomiasis. However, its low bioavailability when administered orally can affect its efficacy and bioavailability. In a study, niosomes were loaded with the drug to overcome these shortcomings (Zoghroban et al. 2019). The niosomes were further modified using Peceol to improve their absorption. The in vivo studies were conducted on rats, and the formulation was administered orally. The use of niosomal formulation resulted in death of 50% of the adult parasites in the rats, while the drug, when administered alone, only showed 10% death. Hence, encapsulation of the drug into niosomes enhanced its absorption and anti-parasitical activity as well.
16.9.5 Delivery of Drugs to the Brain
Functionalization of niosomes by surface modification can help in enhancing their delivery to the brain to treat neurological disorders like Alzheimer’s disease. The drug pentamidine was able to cross the BBB when they were loaded in niosomes modified with chitosan-glutamate coating (Rinaldi et al. 2018). The niosomes were delivered intranasally which helped the drug in bypassing the hepatic metabolism The specific targeting of the oral alkylating agent temozolomide to treat glioblastoma was possible by surface modification of niosomes with the peptide cholorotoxin (Mamelak and Jacoby 2007).
During the onset of neurological disorders, the blood-brain barrier (BBB) deteriorates, and the membrane loses its highly selective permeability property. The presence of specific receptors also becomes more at the BBB, making receptor-mediated drug delivery possible. The process of PEGylation of niosomes helps in the association of ligands with niosomes. One way is to attach the concerned ligand to PEG chains at their distal end if it’s PEGylated niosomes. The other way is direct incorporation of a conjugate made of cholesterol, PEG and the ligand into niosomes during the formulation or synthesis step (Kim et al. 2012; Oswald et al. 2017). Some examples that show ligand attachment to niosomes have been beneficial: the delivery of the neuropeptide vasoactive intestinal peptide (VIP) to the brain using niosomes containing glucose (Dufes et al. 2004).
16.9.6 Niosomes as Diagnostic Agents
The ability of niosomes to load a hydrophilic and hydrophobic compound simultaneously has been beneficial in developing niosomes as a diagnostic imaging agent. The dyes or contrast agents can be incorporated either into the vesicle layer or in the aqueous compartment of niosomes or on the surface of niosomes by conjugation (Masotti 2013). The conjugation of gadobenate dimeglumine-loaded niosomes with n-palmitoylglucosamine (NPG) and PEG 4400 helped improve the targeting and imaging capability of gadolinium associated with MR imaging. This study again shows the importance of PEG surface modification of niosomes, improving their functionality (Luciani et al. 2004).
For a multifunctional approach, theranostic nanoparticles are garnering significant interest in recent times, which can also act as imaging agents to visualize active cellular uptake of these nanoparticles. With this aim in mind, in one study, gadolinium nanoparticles and protoporphyrin IX were simultaneously loaded in niosomes (Barlas et al. 2016). The multifunctionality role of niosomes is the combined “radio therapy and photodynamic therapy” along with MRI and fluorescence imaging by gadolinium and protoporphyrin IX, respectively. As far as the anticancer effect of the agents was considered, they showed the excellent therapeutic effect on HeLa cells (human cervical cancer cell lines) and A549 cells (human alveolar type II). The imaging capabilities of the niosomes are shown in Fig. 16.16 (fluorescence imaging due to the presence of protoporphyrin IX) and Fig. 16.17 (MR imaging with the assistance of gadolinium nanoparticles).
Fluorescence imaging of niosomes loaded with protoporphyrin IX. (Adapted from Barlas et al. 2016)
MR imaging of niosomes loaded with gadolinium nanoparticles. (Adapted from Barlas et al. 2016)
Similarly, in another study, with one more aim of improving the targeting ability of niosomes, folic acid was attached to niosomes in which GNPs and the photosensitizer protoporphyrin IX were encapsulated. The tagging of folic acid helped in receptor-mediated delivery to the cancer cell lines used in this study: HeLa cells (human cervical cancer cell lines) and A549 cells (human alveolar type II). The GNPs and protoporphyrin IX were simultaneously loaded to provide both radio therapy and photodynamic therapy, respectively. GNPs, due to surface plasmon resonance (SPR), allowed live imaging and tracking of the niosomal entry into the cancer cells (Demir et al. 2018). So what we see here is that by surface modification of niosomes and loading them with multifunctional agents, we can make niosomal assemblies just like assembling “building blocks” which can perform a variety of functions.
16.9.7 Patents on Niosomes
The release of patented and marketed formulations of niosomes has occurred, showing that the global acceptance of niosomes for drug delivery is also expanding. The bioavailability of doxorubicin has significantly increased with the help of niosomal formulations. A patented niosomal gel loaded with doxorubicin is used to treat cancers associated with the skin (Ramanunny et al. 2020). Another example of a patent is a dental gel developed for transdermal drug delivery for treating periodontal diseases (Samoylenko 2016). In another patent, the targeting ability of anticancer drug chlorotoxin increased when incorporated into a niosomal formulation, and thermal responsive drug release was possible when the niosomes were incorporated into the chitosan hydrogel network.
16.10 Conclusions
The main attractive feature of niosomes is their ability to encapsulate both hydrophilic and hydrophobic compounds simultaneously. Their ease of synthesis, lesser toxicity and cheaper cost than liposomes make them a better candidate for vesicular drug delivery. Hence, niosomes have been used to encapsulate many drugs and enzymes, peptides, vaccines, genes and imaging agents. The surface modification of niosomes with particular entities helps them in protecting the encapsulated drug from the highly acidic environment of the gastrointestinal tract and improves the drug absorption in the intestinal mucosa. This showcases the potential of niosomes being developed as a primal source for oral administration (the most preferred and non-invasive mode of drug administration) for many drugs. Since most niosomal formulations are in suspension, they can be easily ingested, which can be a great relief for those who have a phobia of swallowing tablets. The loading of niosomes with agents like metallic nanoparticles (gold and gadolinium) and photosensitizers (protoporphyrin IX) has paved the path of developing multifunctional vesicular assemblies for cancer treatment and imaging.
However, the increase in entrapment efficiency of hydrophilic drugs in niosomes remains a challenge, and surface modification by coating with suitable agents might be a solution. The method of ligand attachment on niosomes to improve their targeting ability remains unexplored to a great extent. The surge in the number of patents and marketed niosomal formulations proves that niosomes are getting much attention globally and are on the brink of being recognized as a well-established and universal pharmaceutical formulation for the non-invasive administration of drugs.
References
Abdelkader H, Wu Z, Al-Kassas R, Alany RG (2012) Niosomes and discomes for ocular delivery of naltrexone hydrochloride: morphological, rheological, spreading properties and photo-protective effects. Int J Pharm 433:142–148
Abdelkader H, Alani AWG, Alany RG (2014) Recent advances in non-ionic surfactant vesicles (niosomes): Self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Deliv 21:87–100. https://doi.org/10.3109/10717544.2013.838077
Aboul-Einien MH, Kandil SM, Abdou EM et al (2020) Ascorbic acid derivative-loaded modified aspasomes: formulation, in vitro, ex vivo and clinical evaluation for melasma treatment. J Liposome Res 30:54–67
Aburahma MH, Abdelbary GA (2012) Novel diphenyl dimethyl bicarboxylate provesicular powders with enhanced hepatocurative activity: preparation, optimization, in vitro/in vivo evaluation. Int J Pharm 422:139–150
Ag Seleci D, Seleci M, Walter J-G et al (2016) Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J Nanomater 2016:7372306. https://doi.org/10.1155/2016/7372306
Agarwal S, Mohamed MS, Raveendran S et al (2018) Formulation, characterization and evaluation of morusin loaded niosomes for potentiation of anticancer therapy. RSC Adv 8:32621–32636. https://doi.org/10.1039/c8ra06362a
Alemi A, Reza JZ, Haghiralsadat F et al (2018) Paclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacy. J Nanobiotechnol 16:1–20
Alexander A, Dwivedi S, Ajazuddin et al (2012) Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release 164:26–40. https://doi.org/10.1016/j.jconrel.2012.09.017
Ammar HO, Haider M, Ibrahim M, El Hoffy NM (2017) In vitro and in vivo investigation for optimization of niosomal ability for sustainment and bioavailability enhancement of diltiazem after nasal administration. Drug Deliv 24:414–421
Arunothayanun P, Bernard M-S, Craig DQM et al (2000) The effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether. Int J Pharm 201:7–14
Aranya, Manosroi Paveena, Wongtrakul Jiradej, Manosroi Hideki, Sakai Fumio, Sugawara Makoto, Yuasa Masahiko, Abe (2003) Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids and Surfaces B: Biointerfaces 30(1-2) 129-138 10.1016/S0927-7765(03)00080-8
Attia MF, Anton N, Wallyn J et al (2019) An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol 71:1185–1198. https://doi.org/10.1111/jphp.13098
Azmin MN, Florence AT, Handjani-Vila RM et al (1985) The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methotrexate in mice. J Pharm Pharmacol 37:237–242. https://doi.org/10.1111/j.2042-7158.1985.tb05051.x
Bandyopadhyay P, Johnson M (2007) Fatty alcohols or fatty acids as niosomal hybrid carrier: Effect on vesicle size, encapsulation efficiency and in vitro dye release. Colloids Surf B Biointerfaces 58:68–71. https://doi.org/10.1016/j.colsurfb.2007.01.014
Bansal S, Aggarwal G, Chandel P, Harikumar SL (2013) Design and development of cefdinir niosomes for oral delivery. J Pharm Bioallied Sci 5:318
Barlas FB, Demir B, Guler E et al (2016) Multimodal theranostic assemblies: double encapsulation of protoporphyrine-IX/Gd3+ inniosomes. RSC Adv 6:30217–30225. https://doi.org/10.1039/c5ra26737d
Bartelds R, Nematollahi MH, Pols T et al (2018) Niosomes, an alternative for liposomal delivery. PLoS One 13:e0194179
Bayindir ZS, Yuksel N (2010) Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci 99:2049–2060. https://doi.org/10.1002/jps.21944
Bini KB, Akhilesh D, Prabhakara P, Kamath JV (2012) Development and characterization of non-ionic surfactant vesicles (niosomes) for oral delivery of lornoxicam. Int J Drug Dev Res 4:147–154
Biswas GR, Majee SB (2017) Niosomes in ocular drug delivery. Eur J Pharm Med Res 4:813–819
Cametti C (2008) Polyion-induced aggregation of oppositely charged liposomes and charged colloidal particles: the many facets of complex formation in low-density colloidal systems. Chem Phys Lipids 155:63–73
Carafa M, Santucci E, Lucania G (2002) Lidocaine-loaded non-ionic surfactant vesicles: characterization and in vitro permeation studies. Int J Pharm 231:21–32. https://doi.org/10.1016/S0378-5173(01)00828-6
Chandu VP, Arunachalam A, Jeganath S et al (2012) International journal of novel trends in pharmaceutical sciences niosomes : a novel drug delivery system. Int J Nov Trends Pharm Sci 2:25–31
Chen S, Hanning S, Falconer J et al (2019) Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur J Pharm Biopharm 144:18–39. https://doi.org/10.1016/j.ejpb.2019.08.015
Davarpanah F, Khalili Yazdi A, Barani M et al (2018) Magnetic delivery of antitumor carboplatin by using PEGylated-Niosomes. Daru 26:57–64. https://doi.org/10.1007/s40199-018-0215-3
Demir B, Barlas FB, Gumus ZP et al (2018) Theranostic niosomes as a promising tool for combined therapy and diagnosis: “all-in-one” approach. ACS Appl Nano Mater 1:2827–2835. https://doi.org/10.1021/acsanm.8b00468
Di Marzio L, Marianecci C, Petrone M et al (2011) Novel pH-sensitive non-ionic surfactant vesicles: comparison between Tween 21 and Tween 20. Colloids Surf B Biointerfaces 82:18–24. https://doi.org/10.1016/j.colsurfb.2010.08.004
Domenici F, Panichelli D, Castellano AC (2009) Alamethicin-lipid interaction studied by energy dispersive X-ray diffraction. Colloids Surf B Biointerfaces 69:216–220. https://doi.org/10.1016/j.colsurfb.2008.11.029
Dufes C, Gaillard F, Uchegbu IF et al (2004) Glucose-targeted niosomes deliver vasoactive intestinal peptide (VIP) to the brain. Int J Pharm 285:77–85. https://doi.org/10.1016/j.ijpharm.2004.07.020
Durak S, Rad ME, Yetisgin AA et al (2020) Niosomal drug delivery systems for ocular disease—recent advances and future prospects. Nanomaterials 10:1–29. https://doi.org/10.3390/nano10061191
El-Laithy HM, Shoukry O, Mahran LG (2011) Novel sugar esters proniosomes for transdermal delivery of vinpocetine: preclinical and clinical studies. Eur J Pharm Biopharm 77:43–55
Ferreira LS, Ramaldes GA, Nunan EA, Ferreira LAM (2004) In vitro skin permeation and retention of paromomycin from liposomes for topical treatment of the cutaneous leishmaniasis. Drug Dev Ind Pharm 30:289–296
Gaafar PME, Abdallah OY, Farid RM, Abdelkader H (2014) Preparation, characterization and evaluation of novel elastic nano-sized niosomes (ethoniosomes) for ocular delivery of prednisolone. J Liposome Res 24:204–215
Gandhi A, Sen SO, Paul A (2012) Abstract : introduction : structural components of niosomes 2:339–353
Ge X, Wei M, He S, Yuan WE (2019) Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 11. https://doi.org/10.3390/pharmaceutics11020055
Gharbavi M, Amani J, Kheiri-Manjili H et al (2018) Niosome: a promising nanocarrier for natural drug delivery through blood-brain barrier. Adv Pharmacol Sci 2018:6847971. https://doi.org/10.1155/2018/6847971
D, Gopinath D, Ravi B.R, Rao S.S, Apte D, Renuka D, Rambhau (2004) Ascorbyl palmitate vesicles (Aspasomes): formation characterization and applications. International Journal of Pharmaceutics 271(1-2) 95-113 10.1016/j.ijpharm.2003.10.032
He R-X, Ye X, Li R et al (2017) PEGylated niosomes-mediated drug delivery systems for paeonol: preparation, pharmacokinetics studies and synergistic anti-tumor effects with 5-FU. J Liposome Res 27:161–170. https://doi.org/10.1080/08982104.2016.1191021
Homaei M (2016) Preparation and characterization of giant niosomes
Isnan AP, Jufri M (2017) Formulation of niosomal gel containing green tea extract (Camellia sinensis L. Kuntze) using thin-layer hydration. Int J Appl Pharm 9:38–43. https://doi.org/10.22159/ijap.2017.v9s1.23_28
Israelachvili JN, Mitchell DJ, Ninham BW (1976) Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc Faraday Trans 2 72:1525. https://doi.org/10.1039/f29767201525
Jousma H, Joosten JGH, Junginger HE (1988) Mesophases in mixtures of water and polyoxyethylene surfactant: Variations of repeat spacing with temperature and composition. Colloid Polym Sci 266:640–651. https://doi.org/10.1007/BF01411505
Jousma H, Joosten JGH, Gooris GS, Junginger HE (1989) Changes of mesophase structure of BRIJ 96/water mixtures on addition of liquid paraffin. Colloid Polym Sci 267:353–364. https://doi.org/10.1007/BF01413630
Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D (2012) Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int J Pharm 423:303–311
Junyaprasert VB, Singhsa P, Jintapattanakit A (2013) Influence of chemical penetration enhancers on skin permeability of ellagic acid-loaded niosomes. Asian J Pharm Sci 8:110–117. https://doi.org/10.1016/j.ajps.2013.07.014
Kazi KM, Mandal AS, Biswas N et al (2010) Niosome: a future of targeted drug delivery systems. J Adv Pharm Technol Res 1:374
Key A, Sch T, Pharm AJ, et al (2018) Scholars Academic Journal of Pharmacy (SAJP) Ethosomes: The Novel Drug Delivery Carriers. https://doi.org/10.21276/sajp.2018.7.6.9
Kim TH, Jo YG, Jiang HH et al (2012) PEG-transferrin conjugated TRAIL (TNF-related apoptosis-inducing ligand) for therapeutic tumor targeting. J Control Release 162:422–428. https://doi.org/10.1016/j.jconrel.2012.07.021
Kopermsub P, Mayen V, Warin C (2011) Potential use of niosomes for encapsulation of nisin and EDTA and their antibacterial activity enhancement. Food Res Int 44:605–612
Kumar GP, Rajeshwarrao P (2011) Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharm Sin B 1:208–219. https://doi.org/10.1016/j.apsb.2011.09.002
Li Y, Xu F, Li X et al (2020) Development of curcumin-loaded composite phospholipid ethosomes for enhanced skin permeability and vesicle stability. Int J Pharm:119936. https://doi.org/10.1016/j.ijpharm.2020.119936
Lin T, Fang Q, Peng D et al (2013) PEGylated non-ionic surfactant vesicles as drug delivery systems for Gambogenic acid. Drug Deliv 20:277–284
Lin H, Lin L, Choi Y, Michniak-Kohn B (2020) Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int J Pharm 581:119278. https://doi.org/10.1016/j.ijpharm.2020.119278
Liu T, Guo R (2007a) Investigation of PEG 6000/Tween 80/Span 80/H 2 O niosome microstructure. Colloid Polym Sci 285:711–713
Liu T, Guo R (2007b) Structure and transformation of the niosome prepared from PEG 6000/Tween 80/Span 80/H2O lamellar liquid crystal. Colloids Surf A Physicochem Eng Asp 295:130–134. https://doi.org/10.1016/j.colsurfa.2006.08.041
Liu T, Guo R, Hua W, Qiu J (2007) Structure behaviors of hemoglobin in PEG 6000/Tween 80/Span 80/H2O niosome system. Colloids Surf A Physicochem Eng Asp 293:255–261. https://doi.org/10.1016/j.colsurfa.2006.07.053
Lo CT, Jahn A, Locascio LE, Vreeland WN (2010) Controlled self-assembly of monodisperse niosomes by microfluidic hydrodynamic focusing. Langmuir 26:8559–8566. https://doi.org/10.1021/la904616s
Luciani A, Olivier JC, Clement O et al (2004) Glucose-receptor mr imaging of tumors: study in mice with pegylated paramagnetic niosomes. Radiology 231:135–142. https://doi.org/10.1148/radiol.2311021559
Ma H, Guo D, Fan Y et al (2018) Paeonol-loaded ethosomes as transdermal delivery carriers: design, preparation and evaluation. Molecules 23:1756
Madhav NVS, Saini A (2011) Niosomes: a novel drug delivery system. Int J Res Pharm Chem 1:498–511
Mahale NB, Thakkar PD, Mali RG et al (2012) Niosomes: novel sustained release nonionic stable vesicular systems –an overview. Adv Colloid Interface Sci 183–184:46–54. https://doi.org/10.1016/j.cis.2012.08.002
Mahmood S, Mandal UK, Chatterjee B (2018) Transdermal delivery of raloxifene HCl via ethosomal system: Formulation, advanced characterizations and pharmacokinetic evaluation. Int J Pharm 542:36–46. https://doi.org/10.1016/j.ijpharm.2018.02.044
Malik T, Chauhan G, Rath G et al (2018) Efaverinz and nano-gold-loaded mannosylated niosomes: a host cell-targeted topical HIV-1 prophylaxis via thermogel system. Artif Cells Nanomed Biotechnol 46:79–90. https://doi.org/10.1080/21691401.2017.1414054
Mamelak AN, Jacoby DB (2007) Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert Opin Drug Deliv 4:175–186. https://doi.org/10.1517/17425247.4.2.175
Manconi M, Vila AO, Sinico C et al (2006) Theoretical and experimental evaluation of decypolyglucoside vesicles as potential drug delivery systems. J Drug Deliv Sci Technol 16:141–146. https://doi.org/10.1016/s1773-2247(06)50021-8
Manosroi A, Chutoprapat R, Abe M, Manosroi J (2008) Characteristics of niosomes prepared by supercritical carbon dioxide (scCO2) fluid. Int J Pharm 352:248–255. https://doi.org/10.1016/j.ijpharm.2007.10.013
Manosroi A, Khanrin P, Lohcharoenkal W et al (2010) Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm 392:304–310
Manosroi A, Chankhampan C, Manosroi W, Manosroi J (2013a) Transdermal absorption enhancement of papain loaded in elastic niosomes incorporated in gel for scar treatment. Eur J Pharm Sci 48:474–483
Manosroi A, Chankhampan C, Ofoghi H et al (2013b) Low cytotoxic elastic niosomes loaded with salmon calcitonin on human skin fibroblasts. Hum Exp Toxicol 32:31–44. https://doi.org/10.1177/0960327112454892
Manvi SR, Gupta VRM, Srikanth K, Devanna N (2012) Formulation and evaluation of candesartan niosomal suspension. Res J Pharm Technol 5:1570–1572
Marianecci C, Paolino D, Celia C et al (2010) Non-ionic surfactant vesicles in pulmonary glucocorticoid delivery: characterization and interaction with human lung fibroblasts. J Control Release 147:127–135
Marianecci C, Di Marzio L, Rinaldi F et al (2014) Niosomes from 80s to present: The state of the art. Adv Colloid Interface Sci 205:187–206. https://doi.org/10.1016/j.cis.2013.11.018
Masotti A (2013) Niosomes as candidate bioconjugates for imaging and pH-sensitive drug delivery nanocarriers for rare pediatric tumors. J Drug Deliv Sci Technol 23:22–24. https://doi.org/10.1016/S1773-2247(13)50003-7
Meng S, Chen Z, Yang L et al (2013) Enhanced transdermal bioavailability of testosterone propionate via surfactant-modified ethosomes. Int J Nanomedicine 8:3051–3060. https://doi.org/10.2147/IJN.S46748
Moazeni E, Gilani K, Sotoudegan F et al (2010) Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J Microencapsul 27:618–627. https://doi.org/10.3109/02652048.2010.506579
Moghassemi S, Hadjizadeh A (2014) Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release 185:22–36
Moghassemi S, Parnian E, Hakamivala A et al (2015) Uptake and transport of insulin across intestinal membrane model using trimethyl chitosan coated insulin niosomes. Mater Sci Eng C 46:333–340
Moghassemi S, Hadjizadeh A, Omidfar K (2017) Formulation and characterization of bovine serum albumin-loaded niosome. Aaps Pharmscitech 18:27–33
Mostafavi M, Khazaeli P, Sharifi I et al (2019) A novel niosomal combination of selenium coupled with glucantime against leishmania tropica. Korean J Parasitol 57:1–8. https://doi.org/10.3347/kjp.2019.57.1.1
Mozafari MR (2007) Nanomaterials and nanosystems for biomedical applications. Springer
Naderinezhad S, Amoabediny G, Haghiralsadat F (2017) Co-delivery of hydrophilic and hydrophobic anticancer drugs using biocompatible pH-sensitive lipid-based nano-carriers for multidrug-resistant cancers. RSC Adv 7:30008. https://doi.org/10.1039/c7ra01736g
Nasr M (2010) In vitro and in vivo evaluation of proniosomes containing celecoxib for oral administration. Aaps Pharmscitech 11:85–89
Nasseri B (2005) Effect of cholesterol and temperature on the elastic properties of niosomal membranes. Int J Pharm 300:95–101. https://doi.org/10.1016/j.ijpharm.2005.05.009
Oh Y-K, Kim MY, Shin J-Y et al (2006) Skin permeation of retinol in Tween 20-based deformable liposomes: in-vitro evaluation in human skin and keratinocyte models. J Pharm Pharmacol 58:161–166. https://doi.org/10.1211/jpp.58.2.0002
Onochie ITO, Nwakile CD, Umeyor CE et al (2013) Formulation and evaluation of niosomes of benzyl penicillin. J Appl Pharm Sci 3:66
Oswald M, Geissler S, Goepferich A (2017) Targeting the Central Nervous System (CNS): a review of rabies virus-targeting strategies. Mol Pharm 14:2177–2196. https://doi.org/10.1021/acs.molpharmaceut.7b00158
G., Parthasarathi N., Udupa P., Umadevi G., Pillai (2008) (1994) Niosome Encapsulated of Vincristine Sulfate: Improved Anticancer Activity with Reduced Toxicity in Mice. Journal of Drug Targeting 2(2) 173-182 10.3109/10611869409015907
Palani S (2010) Niosomes in targeted drug delivery: some recent advances. Int J Pharm Sci Res 1:1–8
Paolino D, Muzzalupo R, Ricciardi A et al (2007) In vitro and in vivo evaluation of Bola-surfactant containing niosomes for transdermal delivery. Biomed Microdevices 9:421–433. https://doi.org/10.1007/s10544-007-9046-6
Pardakhty A, Varshosaz J, Rouholamini A (2007) In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int J Pharm 328:130–141. https://doi.org/10.1016/j.ijpharm.2006.08.002
Patel KK, Kumar P, Thakkar HP (2012) Formulation of niosomal gel for enhanced transdermal lopinavir delivery and its comparative evaluation with ethosomal gel. AAPS PharmSciTech 13:1502–1510. https://doi.org/10.1208/s12249-012-9871-7
Peltonen L, Koistinen P, Karjalainen M et al (2002) The effect of cosolvents on the formulation of nanoparticles from low-molecular-weight poly (I) lactide. Aaps Pharmscitech 3:52
Radha GV, Rani TS, Sarvani B (2013) A review on proniosomal drug delivery system for targeted drug action. J Basic Clin Pharm 4:42–48. https://doi.org/10.4103/0976-0105.113609
Rajera R, Nagpal K, Singh SK, Mishra DN (2011) Niosomes: a controlled and novel drug delivery system. Biol Pharm Bull 34:945–953
Ramanunny AK, Wadhwa S, Gulati M et al (2020) Nanocarriers for treatment of dermatological diseases: principle, perspective and practices. Eur J Pharmacol 173691. https://doi.org/10.1016/j.ejphar.2020.173691
Raymond CR, Paul JS, Siân CO (2006) Handbook of pharmaceutical excipients, 5th edn. Pharmaceutical Press, London
Rinaldi F, Hanieh PN, Chan LKN et al (2018) Chitosan glutamate-coated niosomes: a proposal for nose-to-brain delivery. Pharmaceutics 10:38
Rita, Muzzalupo Fiore Pasquale, Nicoletta Sonia, Trombino Roberta, Cassano Francesca, Iemma Nevio, Picci (2007) A new crown ether as vesicular carrier for 5-fluoruracil: Synthesis characterization and drug delivery evaluation. Colloids and Surfaces B: Biointerfaces 58(2) 197-202 10.1016/j.colsurfb.2007.03.010
Rogerson A, Cummings J, Willmott N, Florence AT (1988) The distribution of doxorubicin in mice following administration in niosomes. J Pharm Pharmacol 40:337–342. https://doi.org/10.1111/j.2042-7158.1988.tb05263.x
Ruckmani K, Sankar V (2010) Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech 11:1119–1127. https://doi.org/10.1208/s12249-010-9480-2
Sahoo RK, Biswas N, Guha A, Kuotsu K (2014) Maltodextrin based proniosomes of nateglinide: bioavailability assessment. Int J Biol Macromol 69:430–434
Samoylenko VA (2016) The results of clinical testing of photodynamic therapy in the complex treatment of gingivitis, complicating orthodontic treatment with bracket systems. Medicni Perspekt (Medical Perspect 21). https://doi.org/10.26641/2307-0404.2016.2.72164
Sandeep G, Vasavi Reddy D, Devireddy SR (2014) Formulation and evaluation of fluconazole pro-niosomal gel for topical administration. J Appl Pharm Sci 4:98–104. https://doi.org/10.7324/JAPS.2014.40717
Shahiwala A, Misra A (2002) Studies in topical application of niosomally entrapped nimesulide. J Pharm Pharm Sci a Publ Can Soc Pharm Sci Soc Can des Sci Pharm 5:220–225
Sharma V, Anandhakumar S, Sasidharan M (2015) Self-degrading niosomes for encapsulation of hydrophilic and hydrophobic drugs: an efficient carrier for cancer multi-drug delivery. Mater Sci Eng C 56:393–400. https://doi.org/10.1016/j.msec.2015.06.049
Shilpa S, Srinivasan BP, Chauhan M (2011) Niosomes as vesicular carriers for delivery of proteins and biologicals. Int J Drug Deliv 3:14–24. https://doi.org/10.5138/ijdd.2010.0975.0215.03050
Shreedevi HM, Nesalin JAJ, Mani TT (2016) Development and evaluation of stavudine niosome by ether injection method. Int J Pharma Sci Res 7:38–46
Singh TG, Sharma N (2016) Nanobiomaterials in cosmetics: current status and future prospects. Elsevier Inc.
Singh A, Dally WJ, Gupta AK, Towles B (2004) Adaptive channel queue routing on K-Ary n-cubes. In: Proceedings of the sixteenth annual ACM symposium on parallelism in algorithms and architectures. Association for Computing Machinery, New York, pp 11–19
Singh G, Dwivedi H, Saraf SK, Saraf SA (2011) Niosomal delivery of isoniazid-development and characterization. Trop J Pharm Res 10
Sorgi FL, Huang L (1996) Large scale production of DC-Chol cationic liposomes by microfluidization. Int J Pharm 144:131–139
Tavano L, Alfano P, Muzzalupo R, De Cindio B (2011) Niosomes vs microemulsions: new carriers for topical delivery of Capsaicin. Colloids Surf B Biointerfaces 87:333–339. https://doi.org/10.1016/j.colsurfb.2011.05.041
Touitou E, Godin B (2007) Ethosomes for skin delivery. J Drug Deliv Sci Technol 17:303–308
Touitou E, Dayan N, Bergelson L et al (2000) Ethosomes – novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release 65:403–418. https://doi.org/10.1016/S0168-3659(99)00222-9
Uchegbu IF, Florence AT (1995) Non-ionic surfactant vesicles (niosomes): physical and pharmaceutical chemistry. Adv Colloid Interface Sci 58:1–55. https://doi.org/10.1016/0001-8686(95)00242-I
Uchegbu IF, Vyas SP (1998) Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm 172:33–70. https://doi.org/10.1016/S0378-5173(98)00169-0
Uchegbu IF, Bouwstra JA, Florence AT (1992) Large disk-shaped structures (discomes) in nonionic surfactant vesicle to micelle transitions. J Phys Chem 96:10548–10553
Veerareddy PR, Bobbala SKR (2013) Enhanced oral bioavailability of isradipine via proniosomal systems. Drug Dev Ind Pharm 39:909–917
Verma S, Utreja P (2019) Vesicular nanocarrier based treatment of skin fungal infections: Potential and emerging trends in nanoscale pharmacotherapy. Asian J Pharm Sci 14:117–129
Verma S, Singh SK, Syan N et al (2010) Nanoparticle vesicular systems: a versatile tool for drug delivery. J Chem Pharm Res 2:496–509
Vyas SP, Singh RP, Jain S et al (2005) Non-ionic surfactant based vesicles (niosomes) for non-invasive topical genetic immunization against hepatitis B. Int J Pharm 296:80–86
Waddad AY, Abbad S, Yu F et al (2013) Formulation, characterization and pharmacokinetics of Morin hydrate niosomes prepared from various non-ionic surfactants. Int J Pharm 456:446–458. https://doi.org/10.1016/j.ijpharm.2013.08.040
Wilkhu JS, McNeil SE, Anderson DE, Perrie Y (2013) Characterization and optimization of bilosomes for oral vaccine delivery. J Drug Target 21:291–299. https://doi.org/10.3109/1061186X.2012.747528
Yeo PL, Lim CL, Chye SM et al (2017) Niosomes: a review of their structure, properties, methods of preparation, and medical applications. Asian Biomed 11:301–313. https://doi.org/10.1515/abm-2018-0002
Yeo LK, Chaw CS, Elkordy AA (2019) The effects of hydration parameters and co-surfactants on methylene blue-loaded niosomes prepared by the thin film hydration method. Pharmaceuticals 12:46
Yoshida H, Lehr CM, Kok W et al (1992) Niosomes for oral delivery of peptide drugs. J Control Release 21:145–153. https://doi.org/10.1016/0168-3659(92)90016-K
Zeng W, Li Q, Wan T et al (2016) Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: Mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Colloids Surf B Biointerfaces 141:28–35. https://doi.org/10.1016/j.colsurfb.2016.01.014
Zoghroban HS, El-Kowrany SI, Aboul Asaad IA et al (2019) Niosomes for enhanced activity of praziquantel against schistosoma mansoni: in vivo and in vitro evaluation. Parasitol Res 118:219–234. https://doi.org/10.1007/s00436-018-6132-z
Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2018R1A6A1A03025582). This work was supported by the Technology development Program (S2911350) funded by the Ministry of SMEs and Startups(MSS, Korea)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alle, M., Samed, N., Kim, JC. (2021). Niosomes: A Smart Drug Carrier Synthesis, Properties and Applications. In: Kim, JC., Alle, M., Husen, A. (eds) Smart Nanomaterials in Biomedical Applications. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-84262-8_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-84262-8_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84261-1
Online ISBN: 978-3-030-84262-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)