Abstract
The role of jute, Chorchorus capsularis (cv. Sonali; JRC-321), leaf physicochemical cues in the form of cuticular surface ultrastructures and wax chemicals mainly n-alkanes and free fatty acids (FFAs) on attraction and oviposition preference of Diacrisia casignetum Kollar (Lepidoptera: Arctiidae) was studied under laboratory conditions. The GC–MS and GC-FID analyses of mature jute leaf surface wax indicated the presence of 257.04 and 171.36 µg/leaf n-alkanes and FFAs, respectively. Eighteen n-alkanes from n-C16 to n-C36 and 13 FFAs from C12:0 to C20:0 were detected in the leaf surface wax. The cuticular surface ultrastructures and its chemicals have been demonstrated in this work to serve as cues for eliciting attraction and oviposition responses of the adults to mature jute leaves. The synthetic combination mixture mimicking the natural surface wax components of 4 n-alkanes (n-C17, n-C18, n-C27, n-C29) and 5 FFAs (C16:0, C16:1, C18:1, C18:2, C18:3) was most attractive to D. casignetum adults, whereas same mixtures excluding 2 n-alkanes (n-C27, n-C29) indicated significantly optimum oviposition preference at leaf equivalent (µg/leaf) concentrations that may be used for this pest management program as baited trap. The present study will also ensure sustainability of success in integrated pest management (IPM) in the form of green pest management (GPM) in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The expanding field of insect–plant interactions has long been focused on the process by which phytophagous insects find and accept their host plants [9, 20, 23, 38]. The interactions are primarily mediated by a set of physical and chemical cues specific to the plants [5, 17]. The sensory cues that elicit or inhibit attraction, oviposition and feeding clearly play crucial role in survival of most phytophagous insects, particularly in lepidopterans, because their neonates are often relatively immobile and thus depend on the judicious choice of host plant by the adult females [20, 36]. The host finding and oviposition site selection require a set of sensory modalities or cues (visual, olfactory, tactile and gustatory) of the females before laying eggs [20, 33]. After an insect flight on a plant, both physical and chemical cues of leaf surface become paramount in determining the suitability for attraction and oviposition [20, 39].
The leaf surfaces are covered with a thin layer of hydrophobic wax constituents, and it differs among plant species as well as within a plant during development [13, 24, 27, 29]. The leaf surface provides an enormous variety of microstructures, unicellular and multicellular outgrowths from the epidermis which provide an epic signature for host selection by insects [13, 29, 34]. The surface wax consists of alkanes, fatty acids, alkyl esters, alcohols, etc., and serves many physiological functions for protecting plants as well as in insect–plant interactions [13, 24, 27, 30,31,32, 38]. The importance of plant leaf alkanes and fatty acids as allelochemicals has been demonstrated in different insects in insect–plant interactions such as olfactory attractant [16, 18, 24, 27, 28, 30,31,32] and or oviposition attractant [15, 19].
The polyphagous pest, Diacrisia casignetum (Lepidoptera: Arctiidae), is one of the major limiting factors as defoliator (Fig. 1) in the successful cultivation of several economic crops including jute (Chorchorus capsularis), a natural fiber crop after cotton, in India and many other Asian countries [21, 22, 24,25,26,27]. Still in the era of modern agriculture, insects are controlled by chemical insecticides having detrimental effect to human health and environment [2, 7, 8].
So, it has become imperative now to have a precise knowledge on the physical and chemical cue-mediated interactions between the crop plant (jute) and the phytophagous insect (D. casignetum) for their ecologically sustainable green management. Hence, it is my considerable interest to find out the role of physical features of the mature jute leaf along with their surface wax chemicals (n-alkanes and FFAs) in behavioral responses (attraction and oviposition) of D. casignetum which may help in monitoring this pest as well as their green control strategy in the near future.
Materials and Methods
Jute Leaves Collection and Insect Mass Culture
Fresh mature (1–3 weeks old) jute leaves were randomly collected from the field near Chinsurah Rice Research Center (22°53′N, 88°23′E), Hooghly, West Bengal, India, for the experiment as in Roy [21, 22]. Insect mass culture was conducted at 27 ± 1 °C, 12 L:12 D with light intensity of 1500 lux, 65 ± 5% relative humidity (RH) in a biochemical oxygen demand (BOD) incubator, and from fourth generation onward, the bioassay was conducted for their behavioral responsiveness in the laboratory conditions as described by Roy and Barik [24,25,26,27].
Scanning Electron Microscopy (SEM) of Normal and De-waxed Leaves
Both abaxial and adaxial surfaces of a fresh and de-waxed mature leaves were separately mounted on aluminum holders (stabs) and coated with gold–palladium (2 nm thickness) to observe the ultrastructure of each surfaces of the leaf using Hitachi-made Scanning Electron Microscope (Model: S 530 with IB 2 ion cotter, Japan) as described by Adati and Matsuda [1] and Roy et al. [29].
Extraction, Identification and Quantification of Leaf Surface Wax
Freshly collected mature jute leaves of 200 g [fresh weight 612 ± 4 mg/leaf or 36 ± 0.53 cm2/leaf (Mean ± SE)] were dipped in 2 L n-hexane for 1 min at room temperature for extraction of surface wax from the leaves which yielded a straw-colored extract without trace of chlorophyll [18, 29, 31, 32]. The crude extract was passed through Whatman No. 41 (Maidstone, UK) filter paper and was evaporated at room temperature (27 °C) to dryness. The extraction was repeated three times separately, and the dry extract yields were 216 ± 10 mg/200 g leaves. Each crude extract was then dissolved in 20 mL n-hexane and divided into four equal portions (equivalent to 50 g of leaves). The first and second ones were used for identification and quantification of n-alkanes and FFAs, and the remaining third and fourth ones after purification were used for attraction and oviposition bioassay, respectively. All solvents used were of analytical grade and purchased from E. Merck (Mumbai, India). All standard n-alkanes and fatty acids (FAs) (> 99% purity) were purchased from Sigma-Aldrich, Germany. Isolation, identification and quantification of n-alkanes and FFAs were done through TLC, IR spectroscopy, GC–MS and GC-FID in specific protocols as well as programming as in Sarkar et al. [31], Roy et al. [29], Sarkar et al. [32] and Roy and Barik [27], respectively.
Bioassay Experiments
Preparation of Surface Wax n-Alkanes and FFAs for Bioassay
Both natural n-alkanes and FFAs isolated from the mature jute leaf surface wax were prepared in leaf equivalent (μg/leaf/mL) amount dissolving in petroleum ether for different types of bioassay (attraction and oviposition) experiments. The petroleum ether was used as the control solvent because D. casignetum adults were neither attracted nor deterred by it in preliminary bioassay. To prepare the synthetic individual n-alkanes, FAs and their mixtures mimicking the natural leaf wax, the equivalent (μg/leaf/mL) amount was prepared by the same procedure as in naturally isolated chemicals.
Preparation of D. casignetum Adults for Bioassay
The F4 onward generations of D. casignetum adults were collected from the mass culture for different bioassay experiments in the laboratory condition at 27 ± 1 °C, 60 ± 5% RH and light intensity of 1500 lux. Newly emerged females were provisioned with water and starved for 12 h prior to use in olfactory attraction, and only 10% sucrose solution provided as food during oviposition bioassays in different conditions. Females were used in bioassay because for oviposition they are guided by different physicochemical cues in suitable host finding for their future neonates. All the bioassay experiments were conducted with six replications.
Adult Attraction for n-Alkanes and FFAs
The effectiveness of n-alkanes and FFAs as olfactory attractants was evaluated in different conditions as they are low volatile substances that act as close range allelochemicals [14, 16, 24, 27, 28, 30]. The behavioral responses of adult females were investigated in a Y-tube olfactometer [20-cm-long (l) stem and arms, 8 cm diameter (d), 60° Y angle] (Fig. 2 in supplementary material) as described by Roy and Barik [24, 27]. Experiments with synthetic individual n-alkanes, FAs, mixtures of synthetic n-alkanes and FAs, mixtures of natural n-alkanes and FFAs and natural cuticular wax were conducted in the same manner as described by Roy and Barik [24, 27] and Sarkar et al. [31, 32].
Both natural n-alkanes and FFAs isolated from the mature jute leaf surface wax were prepared in leaf equivalent (μg/leaf/mL) amount dissolving in petroleum ether for the bioassay experiment. Adults were neither attracted nor deterred by petroleum ether in preliminary assays so it was used as the control solvent. To prepare the synthetic individual n-alkanes, FAs and their mixtures mimicking the natural leaf equivalent (μg/leaf/mL) amount were prepared by the same procedure as in naturally isolated chemicals. The stem of the olfactometer (Fig. 2 in supplementary material) was connected to a porous glass vial [8.0 cm (d) × 20.0 cm long (l)] in which test insects were released. Each arm of the olfactometer was connected to a glass-made micro-kit adapter fitted into a [4.0 cm (d) × 4.0 cm (l)] glass vial (Fig. 2 in supplementary material). The membrane pump producing an air flow of 450 mL/min was first purified by passing through charcoal filter, and the flow of purified air was adjusted to 150 mL/min which led into left and right glass vials through the micro-kit adapters. All the connections between different parts of the setup consisted of silicon tubing. One mL of solvent bearing leaf equivalent (μg/leaf/mL) amount of identified natural n-alkanes and FFAs was applied separately to the Whatman No. 41 filter paper pieces (2 × 2 cm2) used as volatile cues and another one with solvent (petroleum ether) used as control and allowed to evaporate the solvent in open space (1 min) under laboratory condition, and these filter papers were introduced into the glass vials attached with the olfactometer as described by Roy and Barik [24, 27]. The dual choice tests were conducted for both natural and synthetic n-alkanes and FAs individually and in mixtures. One adult female, D. casignetum, was introduced into the porous glass vial attached with the olfactometer to measure the attractiveness as described by Roy and Barik [24, 27]. The behavior of each female was observed for 3 min in the Y-tube because increasing the experimental time did not increase the number of responding insects. A decision line was located in each side of the Y-tube, and an individual crossing the line within 3 min from release with at least half the body was counted as a response. If no line was crossed after the experimental time had run out, the experiment was counted as “no response.” To eliminate traces from previous trials, the tube was cleaned with petroleum ether followed by acetone and dried before a new individual was tested. Each experiment with one volatile sample was conducted until a total of 72 (12 × 6) females had responded and after testing 6 insects the olfactometer setup and the position of the two arms were systematically changed (rotated 180°) in order to avoid positional bias. Experiments with synthetic individual n-alkanes, FAs, mixtures of synthetic n-alkanes and FAs, mixtures of natural n-alkanes and FFAs and natural cuticular wax were conducted in the same manner.
Adult Oviposition Bioassay
Oviposition preference was assessed by using newly emerged 12 pairs of male and female D. casignetum in a group with 6 replications (12 × 6=72 pairs) for the total of 20 different conditions in egg-laying chambers. The dual choice test with single jute leaf freshly excised from the plant, de-waxed leaf and or a similar leaf-shaped filter paper (36 cm2) was conducted for different treatments accordingly in each glass jar (5 L) covered with nylon net, and the data were collected up to 96 h with 24-h interval. The leaves and or filter papers were watered during the observation period to prevent shrinkage. For the choice experiments with filter paper, each leaf-shaped filter paper (36 cm2) was marked to create two halves vertically. One half was treated with the test compound in leaf equivalent (μg/leaf/mL) amount, and the other half of the paper was kept as a control treated with control solvent. Each compound was spread over the selected half filter paper in leaf equivalent (μg/leaf/mL) amount per test, and after evaporating the solvent, one pair of newly emerged adult moths (1:1 sex ratio) were released in each glass jar. Each jar was provided with 10% honey solution as food and then kept in a BOD incubator as in mass culture. The paper sheet of each replicate having egg masses was detached from the jar, and eggs deposited on treated and untreated surfaces were counted at the black head stage. The oviposition preference index (OPI%) was determined using the formula [(T − C)/(T + C)] × 100] where T is the number of eggs laid in various treatments or normal leaf and C is the number of eggs laid in controls [35]. The oviposition preference [OA% = T/(T + C) × 100] and oviposition deterrence [OD% = C/(T + C) × 100]) were also calculated [35].
Data Analyses
The data on total amounts of n-alkanes and FFAs were analyzed by one-way ANOVA followed by Tukey HSD multiple-comparison test. The data obtained on responses of D. casignetum bioassay to surface waxes, individual compounds and combination of synthetic compounds were analyzed by Chi-square (χ2) test based on the null hypothesis whether the ratio of individuals choosing the stimulus over the control solvent is differed significantly from the ratio of 1:1 [16, 18, 24, 27, 28, 30,31,32, 38, 40]. Individuals that did not respond to any one of the treatments in respective bioassay experiments were excluded from the analyses. All the statistical analysis was conducted by using SPSS software (SPSS 16.0; SPSS Inc., Chicago, IL, USA).
Results
Leaf Surface Ultrastructure
The waxy deposition on adaxial leaves surface revealed obscure cellular configurations in mature jute leaves. Ribs and furrows were distinctly visible. Both adaxial and abaxial surface showed undulations conspicuous with ridges of variable height and wide due to irregular waxy depositions (Fig. 1a, b). Undulated ridges and furrows reduced due to de-waxing of the leaf (Fig. 2c, d). The study on the relationship of the ultrastructure of both normal and de-waxed leaves with behavioral responses of the insect pest was conducted by oviposition preference test.
Surface Wax Composition
The n-hexane extracts of 100 g mature jute leaves yielded 108 ± 3 mg (mean ± SE) surface wax out of which n-alkanes and FFAs represented 42 ± 0.4 and 28 ± 0.1 mg, respectively, with the balance consisting of unidentified surface wax compounds. The identified n-alkanes of a single leaf represented 257.038 µg n-alkanes with the balance consisting of unidentified branched-chain alkanes (Table 1). Total 18 different n-alkanes were identified between n-C16 and n-C36 and expressed in leaf equivalent amount (µg/leaf) and in mol% (Table 1). Among them nonacosane (n-C29) was the predominant (58.058 µg/leaf or 22.587 ± 0.109 mol%), whereas hexadecane (n-C16) was detected in the lowest amount (1.301 µg/leaf or 0.506 ± 0.028 mol%). All the n-alkanes displayed significantly different patterns in the leaf surface waxes with few exceptions (F17,51= 1699.32, P < 0.0001). The identified FFAs of a single leaf represented 171.359 µg FFAs with the balance consisting of unidentified fatty acids (Table 2). Thirteen (9 saturated and 4 mono-unsaturated) FFAs between C12:0 and C20:0 were detected in the jute leaf and expressed in leaf equivalent amount (µg/leaf) and in mol% (Table 2). Among them trioctadecanoin acid (C18:1) was the predominant one accounting 58.058 µg/leaf (6.122 ± 0.160 mol%), whereas least abundant (2.142 µg/leaf or 1.767 ± 0.038 mol%) was dodecanoic acid (C12:0). All the FFAs were significantly differed (F12,36= 26,201.20, P < 0.0001) in the leaf surface wax with few exceptions.
Adult Attraction
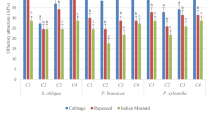
A series of olfactometric tests (15 treatments) examining the effectiveness (minimum 60%) of synthetic n-alkanes and FAs individually, and their mixtures, as well as natural n-alkanes, FFAs and their mixtures in leaf equivalent amount (µg/leaf) on adult D. casignetum, are presented in Table 3. Among the natural chemicals, n-alkanes (257.04 µg/leaf), FFAs (171.36 µg/leaf) and surface wax (660.95 µg/leaf) attraction gradually increased (65.799 ± 2.672, 74.985 ± 4.436 and 75.197 ± 2.211%, respectively) in respective treatments. Within the identified chemicals, 4 n-alkanes [n-C17(a), n-C18(b), n-C27(c), n-C28(d)] and 5 FFAs [C16:0 (p), C16:1 (q), C18:1 (r), C18:2 (s), C18:3 (t)] individually, in mixture and in their combined mixture, elicited significantly positive responses to D. casignetum. Among the natural and synthetic chemicals, the highest positive attraction (81.893 ± 2.350%) was elicited by synthetic combined mixture (279.14 µg/leaf) of 4 n-alkanes and 5 FFAs (a + b+c + d+p + q+r + s+t) with highly significant differences (χ2 = 25.825, df = 1, P < 0.00001), whereas the lowest attractiveness (63.225 ± 2.544%) was found in n-C17 (11.74 µg/leaf) with low significant differences (χ2 = 2.906, df = 1, P < 0.05). All the responses significantly differed within the 15 treatments in both chemicals (F14,84= 14.005, P < 0.001) and control solvent (F14,84= 36.837, P < 0.001).
Oviposition Responses
The oviposition bioassay conducted by total of 20 treatments and having OA (%) > 50% was included, whereas below levels were excluded from the experiment, respectively (Table 4). Within the treatments the highest OPI (46.999 ± 5.120%) was found in synthetic combined mixture of 2 n-alkanes and 5 FFAs (a + b+p + q+r + s+t) treated normal leaf (163.44 µg/leaf) having OA (%) of 73.499 ± 2.560%, while lowest OPI was found in n-C17 (5.288 ± 0.254%) having OA (%) of 52.644 ± 0.127%. All the treatments showed significant differences in all the three (OPI, OA and OD %) parameters (F19,114= 30.908, P < 0.001) calculated from the oviposition responses. This type of responsiveness toward the normal leaf over the other treatments was due to the intact surface ultrastructure as well as applied synthetic principal surface wax components.
Discussion
The host selection is a challenging task for mating, oviposition and feeding in lepidopterans. They require unique set of recognition cues for their complex behavioral process [10]. Complex stimuli have to be extracted from the environment and translated into a relevant behavioral output [33]. There are limitations in extraction of information from host volatiles due to noise of non-host volatiles in the ecological context. Specially, for a polyphagous pest, broader diet increases risk of oviposition on non-host or poor host along with evaluation time [3]. To resolve these questions, I have tested D. casignetum females in a series of behavioral assay for attraction and oviposition responses. There are a handful of studies that investigate antennal reaction to host plant volatiles [38] as well as olfactory cues with other stimuli [12]. Even though such findings are often discussed in other cases, unequivocal evidence for physicochemical cues used in navigation of lepidopterans has so far been scarce.
In this study, the result confirmed that D. casignetum females are able to use olfactory attraction in host plant selection for oviposition. In this study, the olfactometer tests demonstrated that the principal surface wax components as well as their synthetic analogs provide olfactory cues to attract adult female moths. In oviposition bioassay test in different conditions was also proved the use of physicochemical cues for their host or even oviposition site selection. Role of olfaction is well documented in moths due to their typical nocturnal lifestyle [6]. Even female moths can regulate navigation to make fine-tuned decisions for host selection and oviposition by visual [37, 39], olfactory [24, 27], tactile [11] and gustatory [4] cues separately or in combinations with each other.
The surface ultrastructure of the mature jute leaves represented epic pattern of wax deposition on both adaxial and abaxial sides like uniqueness of other plants [13, 24, 27, 29]. In the cuticular wax 18 types of n-alkanes from n-C16 to n-C36 and 13 types of FFAs from C12:0 to C20:0 were detected as major components with significant variations in their respective quantity (mol% and µg/leaf) as in other plants [16, 18, 24, 27, 29, 31, 32]. The highest olfactory attractiveness of D. casignetum females was found toward 4 n-alkanes (n-C17, n-C18, n-C27, n-C29) and 5 FFAs (C16:0, C16:1, C18:1, C18:2, C18:3) in combination mixture than individual compounds or their separate mixture or even natural wax components. The responsiveness helps the females in judicious host selection for oviposition. In oviposition bioassay the gravid females of D. casignetum were mostly preferred intact leaf rather than the other but their degree of preference can be amplified when the intact leaf pretreated with the same combined synthetic mixture as in olfactory attraction excluding two n-alkanes (n-C27 and n-C29).The finding of both olfactory and oviposition bioassay in different conditions can explain the clue how D. casignetum females choose their oviposition site in such a perfect passion through host physicochemical cues for better survival and growth of their neonates. It is apparent from my study that understanding the potential semiochemicals in the behavioral responses of insects could play a significant role in any biointensive pest management strategy if standardized protocols for identification of the semiochemicals and their appropriate delivery systems for field applications are available.
In summary, the bioassay (olfactory attraction and oviposition) experiments proved that how host physicochemical cues were used by D. casignetum to sharpen their decision for attraction and ultimate oviposition site selection on their preferred host plants or rather plant parts. So, for management of the notorious pest, D. casignetum, on different economic crops like sunflower, safflower, green gram, castor, sesame, including jute, through adopting and mimicking the combination mixture of synthetic semiochemicals will be an alternative, ecofriendly and sustainable strategy over the traditional pest management strategies. The present study also ensures the application of the chemical combination mixture for further research on this notorious defoliator pest in integrated pest management (IPM) model in the form of green pest management (GPM) or rather ecological pest management (EPM) in the near future.
References
Adati T, Matsuda K (2000) The effect of leaf surface wax on feeding of the strawberry leaf beetle, Galerucella vittaticollis, with reference to host plant preference. Tohoku J Agric Res 50:57–61
Aktar MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12. https://doi.org/10.2478/v10102-009-0001-7
Bernays EA (2001) Neural limitations in phytophagous insects: implications for diet breadth and evolution of host affiliation. Ann Rev Entomol 46:703–727
Chapman RF (2003) Contact chemoreception in feeding by phytophagous insects. Ann Rev Entomol 48:455–484
Chapman RF, Bernays EA (1989) Insect behavior at the leaf surface and learning as aspects of host plant selection. Cell Mol Life Sci 45:215–222
Cunningham JP, Zalucki MP, West SA (1999) Learning in Helicoverpa armigera (Lepidoptera: Noctuidae): a new look at the behaviour and control of a polyphagous pest. Bull Entomol Res 89:201–207
Desneux N, Decourtye A, Delpuech J-M (2007) The sublethal effects of pesticides on beneficial arthropods. Ann Rev Entomol 52:81–106. https://doi.org/10.1146/annurev.ento.52.110405.091440
Egan JF, Bohnenblust E, Goslee S, Mortensen D, Tooker J (2014) Herbicide drift can affect plant and arthropod communities. Agric Ecosyst Environ 185:77–87. https://doi.org/10.1016/j.agee.2013.12.017
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Finch S, Collier RH (2000) Host-plant selection by insects—a theory based on ‘appropriate/inappropriate landings’ by pest insects of cruciferous plants. Entomol Exp Appl 96:91–102
Foster SP, Howard AJ (1998) Influence of stimuli from Camellia japonica on ovipositional behavior of generalist herbivore Epiphyas postvittana. J Chem Ecol 24:1251–1275
Heinz CA (2008) Host plant odor extracts with strong effects on oviposition behavior in Papilio polyxenes. Entomol Exp Appl 128:265–273
Jetter R, Schaffer S, Riederer M (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23:619–628
Koschier EH, Kogel WJD, Visser JH (2000) Assessing the attractiveness of volatile plant compounds to western flower thrips Frankliniella occidentalis. J Chem Ecol 26:2643–2655
Li G, Ishiwaka Y (2006) Leaf epicuticular wax chemicals of the Japanese knotweed Fallopia japonica as oviposition stimulant for Ostrinia latipennis. J Chem Ecol 32:595–604
Malik U, Barik A (2015) Free fatty acids from the weed, Polygonum orientale leaves for attraction of the potential biocontrol agent, Galerucella placida (Coleoptera: Chrysomelidae). Biocontrol Sci Technol 25:593–607
McCallum EJ, Cunningham JP, Lücker J, Zalucki MP, De Voss JJ, Botella JR (2011) Increased plant volatile production affects oviposition, but not larval development, in the moth Helicoverpa armigera. J Exp Biol 214:3672–3677
Mukherjee A, Sarkar N, Barik A (2014) Long-chain free fatty acids from Momordica cochinchinensis leaves as attractants to its insect pest, Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae). J Asia-Pacific Entomol 17:229–234
Parr MJ, Tran BMD, Simmonds MSJ, Kite GC, Credland PF (1998) Influence of some fatty acids on oviposition by the bruchid beetle, Callosobruchus maculatus. J Chem Ecol 24:1577–1593
Renwick JAA, Chew FS (1994) Oviposition behavior in lepidoptera. Ann Rev Entomol 39:377–400
Roy N (2014) Role of Chorchorus capsularis phytochemicals on the feeding dynamics of Diacrisia casignetum Kollar (Lepidoptera: Arctiidae). J Entomol Zool Stud 2:227–236
Roy N (2015) Life table and population parameters of Diacrisia casignetum Kollar (Lepidoptera: Arctiidae) on jute, Chorchorus capsularis (cv. Sonali; JRC-321), leaves. Int J Fauna Biol Stud 2:23–29
Roy N, Barik A (2010) The role of volatiles in tritrophic interactions. Environ Ecol 28:352–355
Roy N, Barik A (2012) Alkanes used for host recognition by the arctiid moth, Diacrisia casignetum Kollar. J Entomol Res 36:345–350
Roy N, Barik A (2012) The impact of variation in foliar constituents of sunflower on development and reproduction of Diacrisia casignetum Kollar (Lepidoptera: Arctiidae). Psyche. https://doi.org/10.1155/2012/812091
Roy N, Barik A (2013) Influence of four host plants on feeding, growth and reproduction of Diacrisia casignetum (Lepidoptera: Arctiidae). Entomol Sci 16:112–118
Roy N, Barik A (2014) Long-chain fatty acids: semiochemicals for host location by the insect pest, Diacrisia casignetum. J Kansas Entomol Soc 87:22–36
Roy N, Chattopadhyay C, Barik A (2013) Assessing the attractiveness of odorous esterified fatty acids to arctiid moth, Diacrisia casignetum Kollar. Ecoscan 3:87–91
Roy N, Laskar S, Barik A (2012) Determination of n-alkane profile through developmental state of sunflower leaves. South Pacific J Nat Appl Sci 30:72–76
Roy N, Laskar S, Barik A (2012) The attractiveness of odorous esterified fatty acids to the potential biocontrol agent, Altica cyanea. J Asia-Pacific Entomol 15:277–282
Sarkar N, Mukherjee A, Barik A (2013) Long-chain alkanes: allelochemicals for host location by the insect pest, Epilachna dodecastigma (Coleoptera: Coccinellidae). Appl Entomol Zool 48:171–179
Sarkar N, Mukherjee A, Barik A (2013) Olfactory responses of Epilachna dodecastigma (Coleoptera: Coccinellidae) to long-chain fatty acids from Momordica charantia leaves. Arth-Plant Int 7:339–348
Schäpers A, Carlsson MA, Gamberale-Stille G, Janz N (2015) The role of olfactory cues for the search behavior of a specialist and generalist butterfly. J Insect Behav 28:77–87
Schoonhoven LM, Van Loon JJA, Dicke M (2005) Insect-plant biology. Oxford University Press, Oxford
Singh R, Koul O, Rup PJ, Jindal J (2011) Oviposition and feeding behavior of the maize borer, Chilo partellus, in response to eight essential oil allelochemicals. Entomol Exp Appl 138:55–64
Srinivasan R, Su FC, Huang SS (2013) Oviposition dynamics and larval development of Helicoverpa armigera on a highly preferred unsuitable host plant, Solanum viarum. Entomol Exp Appl 147:217–224
Wadhera D, Capaldi-Phillips ED (2014) A review of visual cues associated with food on food acceptance and consumption. Eat Behav 15:132–143
Yang G, Zhang Y-N, Gurr GM, Vasseur L, You M-S (2016) Electroantenogram and behavioral responses of Cotesia plutellae to plant volatiles. Insect Sci 23:245–252
Yi X, Liu J, Wang P, Hu M, Zhong G (2014) Contacting is essential for oviposition deterrence of Rhodojaponin-III in Spodoptera litura. Arch Insect Biochem Physiol 86:122–136
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Acknowledgements
The work was funded by a minor research project provided by the University Grants Commission [F. No. PSW-025/13-14], New Delhi, Government of India. The author is highly grateful to the USIC department for SEM study, Chemical Ecology Laboratory for GC-FID and the Department of Chemistry for FT-IR, The University of Burdwan, West Bengal, India. He is also thankful to the IICB, Kolkata, West Bengal, India, for GC–MS analysis.
Author information
Authors and Affiliations
Contributions
NR designed the whole study including sample collection, chemical analysis and data analysis and prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roy, N. Jute Leaf Physicochemical cue-mediated Behavioral Responses of Diacrisia casignetum Kollar. Agric Res 8, 287–296 (2019). https://doi.org/10.1007/s40003-018-0362-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-018-0362-2