Abstract
Purpose
Staphylococcus aureus is an important cause of infections in hospitalized neonates. Preterm or low birthweight infants are especially at risk to develop a S. aureus infection due to the immaturity of the immune system, length of hospital stay and invasive procedures. Exfoliative toxin (ET)-producing S. aureus is often responsible for neonatal infections, causing clinical manifestations such as staphylococcal scalded skin syndrome, characterized by both localized blisters or generalized exfoliation of the skin.
Methods
We describe an outbreak due to an S. aureus strain producing ETA occurring in a local hospital in Northern Italy. Molecular typing of the isolates included spa typing and multilocus sequence typing. DNA microarray hybridization was also performed on one representative strain.
Results
In the period from July 2013 to February 2014, 12 neonates presented with skin infections, mainly bullae or pustules. Cultures of skin swabs yielded methicillin-susceptible S. aureus (MSSA). By molecular typing, an epidemic strain (t1393/ST5) was identified in nine neonates; microarray analysis and PCR revealed that it contained the ETA encoding gene. Screening of staff, mothers and healthy neonates and environmental cultures did not reveal the presence of the epidemic strain. However, the father of an infected neonate was found to be a carrier of MSSA t1393 five months after the outbreak started.
Conclusion
Implementation of hygiene procedures and sanitization of the ward twice terminated the outbreak. Timely surveillance of infections, supported by molecular typing, is fundamental to prevent similar episodes among neonates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus represents one of the major human pathogens both in hospitals and in community. This pathogen can cause a wide variety of diseases ranging from serious invasive infections (e.g., sepsis, meningitis, pneumonia, endocarditis, osteomyelitis) to skin infections with different degrees of severity [1, 2]. Serious infections can be associated with the production of toxins such as toxic shock syndrome toxin-1 (TSST-1), staphylococcal enterotoxins, Panton–Valentine leukocidin (PVL), γ-hemolysin and exfoliative toxins (ET)s which are considered among the most important virulence factors of S. aureus [3]. Several studies have highlighted the importance of toxigenic S. aureus in causing outbreaks in neonatal units in various geographical areas. An outbreak of skin and soft tissue infections (SSTIs) involving seven neonates in Scotland in 2004 was caused by a methicillin-resistant S. aureus (MRSA) that harbored both enterotoxin D and exfoliative toxin A [4]. In 2010, an outbreak of SSTIs caused by a community-associated (CA)-MRSA clone USA300 that contained PVL occurred in a hospital newborn nursery in Italy [5]. In Germany, an outbreak occurring in a neonatal intensive care unit in 2011 was caused by TSST-1 and enterotoxin A-producing methicillin-susceptible S. aureus (MSSA) [6]. In the first weeks of life, from 40 to 50% of neonates are colonized by S. aureus and nasal carriage represents one of the most important risk factors for S. aureus infections [7, 8]. Moreover, hospitalized neonates, especially preterm and low birthweight infants, are at higher risk to develop S. aureus infections due to an immature immune system, the invasive procedures they undergo, and a long hospital stay [9, 10]. In newborns, S. aureus infections mostly involve the skin with erythema and papulae or pustules in the diaper area [11], but more serious skin conditions can develop such as staphylococcal scarlet fever rash and staphylococcal scalded skin syndrome (SSSS) [12,13,14]. SSSS is caused by ET-producing S. aureus and includes two forms: the generalized exfoliative syndrome (GES), also known as Ritter’s disease, that involves the whole body surface presenting as a severe and life-threatening illness and the local form or bullous impetigo (BI), characterized by erythematous blisters with surrounding redness, especially in the diaper area [14, 15]. Exfoliative toxins associated with human skin infections are ETA, ETB and ETD [13]. The production of ETA and ETB is most commonly associated with BI and GES, respectively [13, 14], while ETD is associated with milder manifestations such as furuncles or cutaneous abscesses [12, 16].
SSSS affects mainly neonates and children under 5 years of age [12, 15, 17], but predisposed adults such as immunocompromised subjects or patients with kidney diseases can also be affected [18]. The severity of the infection depends largely on the toxigenic profile of the microorganism and the immune status of the neonates [13, 14]. In particular, GES affects mostly preterm or low birthweight neonates with immature immune response as well as immature renal clearance system [14, 19]. The mortality rate for SSSS is low in infants (< 5%), but can increase to about 60% in adults [12, 13, 20]. SSSS can be treated successfully with a timely and appropriate therapy [14, 21].
In this study, we describe an outbreak of skin infections due to ETA-producing S. aureus occurring in an 8-month period among neonates born in a hospital in Lombardy, North Italy.
Methods
Description of outbreak and setting
The outbreak occurred in a 120-bed hospital located in the city of Codogno in Lombardy, North Italy. The maternity ward of the hospital is composed of nine rooms for mother–infant rooming in (total number of beds = 14) and one common room for neonatal care or medical procedures. The medical and non-medical staff includes 65 persons. Healthy neonates are generally discharged 3–4 days after birth and re-evaluated at a control visit in the outpatient clinic after 7–10 days.
In July 2013, two neonates, one at discharge and the other at a control visit, presented with localized bullous lesions on the trunk and/or the belly; in both cases, cultures of skin swabs grew S. aureus. More cases of skin infections emerged in the following months, and a total of 12 neonates with S. aureus skin infection were observed from July 2013 to February 2014 (Table 1) clustering in July–September 2013 and in December 2013–February 2014 (Fig. 1). At birth, the affected neonates were healthy, with normal weight and without virus-like symptoms or underlining diseases. Skin infection developed from few days to 2 weeks after birth, consisting in bullae or pustules involving a limited skin area, more frequently the trunk or groins. Only one neonate (case 3) had in more sites bullous lesions with exfoliation.
To detect the source of the outbreak, the local committee for health-care-associated infections (CIO) started an investigation in September 2013. Environmental swabs were obtained from the surface and the equipment of the delivery room, the common room and the staying rooms in the maternity ward. In particular, samples were obtained from cribs, changing tables and other tables for the care of neonates, scales, sinks, faucets, infusion pumps, stethoscopes and other medical equipment. In October, nasal screening for S. aureus was carried out in the staff, in healthy neonates and mothers at discharge. In addition, parents and siblings of infected neonates were screened for S. aureus at control visits.
Microbiological methods
Isolates identification and antimicrobial susceptibility were determined by using an automatic system (Vitek 2; bioMérieux, Marcy l’Etoile, France). PCR assays to confirm S. aureus species and the methicillin-resistance status of the isolates were performed [22]. The presence of PVL, TSST-1, ETA, ETB and ETD as well as the determination of accessory gene regulator (agr) groups was also investigated by PCR using published primers [23, 24].
Molecular typing of the isolates was performed by spa typing [25]. The sequences were analysed using the Ridom Staph Type software (http://www.ridom.de/spaserver). In selected isolates, multilocus sequence typing (MLST) was carried out according to the recommended method [26] and the allelic profiles were compared with those deposited at the MLST database (http://saureus.mlst.net). DNA microarray hybridization was performed on one representative strain by using a previously described DNA microarray platform (Alere Technologies, GmbH, Jena, Germany) [27].
Results
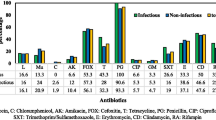
All S. aureus isolates from infected neonates were confirmed as susceptible to methicillin (MSSA) and to all the antibiotics tested with the exception of three isolates (cases 5, 11 and 12) that showed resistance to penicillin (Table 1).
Molecular typing showed that 9 out of 12 isolates belonged to the same spa type (t1393), while the other three isolates were associated with three different spa types (t078, t091, t4565) (Table 1). Therefore, MSSA t1393 was considered the epidemic strain. All t1393 (n = 9) were isolated in the period July 1, 2013–January 7, 2014, while strains (n = 3) with spa types other than t1393 were identified during the outbreak period (strain t4565) and toward the end of the outbreak (strains t078, t091) (Fig. 1).
The t1393 isolates were susceptible to all the antibiotics tested including penicillin, while strains associated with different spa types were resistant to penicillin (Table 1). Therefore, susceptibility to penicillin was an additional criterion to discriminate outbreak strains from the others. By MLST, t1393 isolates were found to belong to ST5, clonal complex (CC) 5. The three different spa types t078, t091 and t4565 belonged to ST26, ST7 and ST22, respectively. Microarray analysis performed on MSSA t1393 from case 2 revealed the presence of eta gene. Additional characteristics of the epidemic strain, obtained by microarray, corresponded to the typical features of CC5, including the presence of the immune-evasion cluster, of the enterotoxin gene cluster egc and sea-N315, of leukocidines and adhesive matrix molecules [28]. The only resistance gene detected by microarray was fosB, coding for fosfomycin resistance [29]. A remnant of the recombinase ccrC was detected, a sign of a possible previous presence of the staphylococcal chromosome cassette (SCC) mec. These same features were confirmed in all nine isolates belonging to t1393 by PCR assays. In addition, the epidemic strain was shown to belong to the agr group II, typically found in isolates belonging to CC5. For one non-epidemic strain (case 11), the PCR assay revealed the presence of the eta and etb genes (Table 1).
Neonates with skin infections were treated with systemic antibiotics (2 neonates with linezolid, 9 with amoxicillin–clavulanic acid and 1 with amoxicillin) and with intranasal mupirocin.
The cultural screening revealed that 35% (13 out of 37) of the family members of infected neonates, 30% (16 out of 54) of the staff, 32% (17 out of 53) of the healthy neonates and 33% (14 out of 42) of the mothers were S. aureus carriers. No S. aureus was detected from samples obtained from environment and medical equipment.
None of the isolates obtained from the screening was spa t1393 with the exception of the isolate from the father of case 2 who was found colonized by the epidemic strain on the occasion of a control visit for the baby in December, five months after the beginning of the outbreak.
Staff members and the family members of infected neonates who were S. aureus carriers received intranasal mupirocin. Control measures to terminate the outbreak were enforced in September, including implementation of hygienic procedures such as hand washing and use of hydroalcoholic gel, for which dispensers were made available in each room. All surfaces and instruments in the delivery room and the maternity ward were sanitized with a chlorine-based disinfectant. However, a second cluster of cases started in December, indicating that the source of the outbreak had not been eliminated. After a second deep cleaning of the ward, no new cases related to the outbreak strain occurred after January 7, 2014.
Discussion
This outbreak was caused by an ETA-producing MSSA associated with an uncommon spa type (t1393), belonging to CC5, that affected neonates during an 8-month period. Neonates showed skin manifestations, consisting in BI and BI with exfoliation in one case, from few days to 2 weeks after birth. They likely acquired MSSA in hospital although the source of the epidemic was not determined. The father of one of the first infected neonates (case 2) was found to be colonized by the outbreak strain five months after the beginning of the outbreak. Whether he was a carrier from the beginning or acquired the strain subsequently from the infected child could not be established. The application of appropriate control measures implemented twice, in September and in December, led to the termination of the outbreak.
To our knowledge, this is the first report of an outbreak of skin infection caused by ETA-producing S. aureus, associated with an uncommon spa type, not previously reported in Italy, and belonging to CC5. CC5 was widely spread in European and Italian hospitals at the beginning of the last decade mostly among MRSA [22, 30], while, among MSSA, CC5 was less common with a prevalence of about 5% [30]. Examining the sequence of the spa repeats, spa type 1393 is related to t067 (CC5), one of the most prevalent spa types in Spain in the period from 2006 to 2011, mostly among MRSA [30, 31]. One peculiar feature of the outbreak strain was susceptibility to penicillin that in Italy is only present in approximately 14% of MSSA (unpublished data).
MSSA represents one of the major causes of skin infections in pediatrics [9]; however, reports involving MSSA are rare since more importance is usually given to infections caused by MRSA. Skin infections can occur with different clinical presentations and severity depending on the production of bacterial toxins and host susceptibility [32].
ETA and ETB, responsible for SSSS, are produced by only 5% of S. aureus strains with a prevalence that varies in different countries [12, 17]. ETA is more common in Europe, USA and Africa and is expressed by more than 80% of ET-producing strains, while ETB-producing strains are more prevalent in Japan [12]; ETD is less common than ETA and ETB and data are limited [16]. Several outbreaks involving ETA or ETB-producing S. aureus have been described.
An outbreak of SSSS due to ETA-producing S. aureus involving 13 neonates occurred in 2002 in France [21]. In 2011, an outbreak of BI caused by an ETB-producing strain occurred in a neonatal ward in the Netherlands and an environmental contamination was considered the source of infection [33]. In 2013, in England, eight healthy neonates developed SSSS due to ETA- and ETB-producing strains and a health-care worker was identified as the source of infection [34].
In the outbreak we described, reinforcement of appropriate control measures including handwashing and sanitization of the ward had to be implemented in two different periods, but finally led to the end of the outbreak. No new cases due to the epidemic strain were detected since. Timely surveillance of infections, supported by molecular typing, and continuous implementation of hygienic measures are fundamental to prevent similar episodes among neonates.
Conclusions
In this outbreak, spa typing, a rapid and relatively inexpensive typing method was of utmost importance to identify the epidemic strain and to understand the extent of the outbreak. Therefore, it is important to consider typing not only for MRSA that are particularly threatening in neonatal units, but also for MSSA, especially when the isolates carry a toxin such as ETA associated with potential fatal manifestations. In addition, since a high percentage of neonates and other individuals can be MSSA carriers, typing is essential to distinguish outbreak from non-outbreak strains to apply control measures.
References
Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–61.
Monaco M, Pimentel de Araujo F, Cruciani M, Coccia EM, Pantosti A. Worldwide epidemiology and antibiotic resistance of Staphylococcus aureus. Curr Top Microbiol Immunol. 2016. doi:http://dx.doi.org/10.1007/82_2016_3.
Kong C, Neoh HM, Nathan S. Targeting Staphylococcus aureus toxins: A potential form of anti-virulence therapy. Toxins (Basel). 2016;8:72. doi:http://dx.doi.org/10.3390/toxins8030072.
Gould IM, Girvan EK, Browning RA, MacKenzie FM, Edwards GF. Report of a hospital neonatal unit outbreak of community-associated methicillin-resistant Staphylococcus aureus. Epidemiol Infect. 2009;137:1242–8.
Sanchini A, Spitoni MG, Monaco M, Raglio A, Grigis A, Petro W, et al. Outbreak of skin and soft tissue infections in a hospital newborn nursery in Italy due to community-acquired meticillin-resistant Staphylococcus aureus USA300 clone. J Hosp Infect. 2013;83:36–40.
Layer F, Sanchini A, Strommenger B, Cuny C, Breier AC, Proquitte H, et al. Molecular typing of toxic shock syndrome toxin-1-and Enterotoxin A-producing methicillin-sensitive Staphylococcus aureus isolates from an outbreak in a neonatal intensive care unit. Int J Med Microbiol. 2015;305:790–8.
Peacock SJ, Justice A, Griffiths D, de Silva GD, Kantzanou MN, Crook D, et al. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J Clin Microbiol. 2003;41:5718–25.
Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–62.
Gaynes RP, Edwards JR, Jarvis WR, Culver DH, Tolson JS, Martone WJ. Nosocomial infections among neonates in high-risk nurseries in the United States. National Nosocomial Infections Surveillance System. Pediatrics. 1996;98:357–61.
Healy CM, Palazzi DL, Edwards MS, Campbell JR, Baker CJ. Features of invasive staphylococcal disease in neonates. Pediatrics. 2004;114:953–61.
Tuzun Y, Wolf R, Baglam S, Engin B. Diaper (napkin) dermatitis: a fold (intertriginous) dermatosis. Clin Dermatol. 2015;33:477–82.
Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301–7.
Ladhani S. Understanding the mechanism of action of the exfoliative toxins of Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:181–9.
Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224–42.
Kapoor V, Travadi J, Braye S. Staphylococcal scalded skin syndrome in an extremely premature neonate: a case report with a brief review of literature. J Paediatr Child Health. 2008;44:374–6.
Bukowski M, Wladyka B, Dubin G. Exfoliative toxins of Staphylococcus aureus. Toxins (Basel). 2010;2:1148–65.
Lamand V, Dauwalder O, Tristan A, Casalegno JS, Meugnier H, Bes M, et al. Epidemiological data of staphylococcal scalded skin syndrome in France from 1997 to 2007 and microbiological characteristics of Staphylococcus aureus associated strains. Clin Microbiol Infect. 2012;18:E514–21.
Nhan TX, Leclercq R, Cattoir V. Prevalence of toxin genes in consecutive clinical isolates of Staphylococcus aureus and clinical impact. Eur J Clin Microbiol Infect Dis. 2011;30:719–25.
Yamasaki O, Yamaguchi T, Sugai M, Chapuis-Cellier C, Arnaud F, Vandenesch F, et al. Clinical manifestations of staphylococcal scalded-skin syndrome depend on serotypes of exfoliative toxins. J Clin Microbiol. 2005;43:1890–3.
Neylon O, O’Connell NH, Slevin B, Powell J, Monahan R, Boyle L, et al. Neonatal staphylococcal scalded skin syndrome: clinical and outbreak containment review. Eur J Pediatr. 2010;169:1503–9.
El Helali N, Carbonne A, Naas T, Kerneis S, Fresco O, Giovangrandi Y, et al. Nosocomial outbreak of staphylococcal scalded skin syndrome in neonates: epidemiological investigation and control. J Hosp Infect. 2005;61:130–8.
Monaco M, Sanchini A, Grundmann H, Pantosti A. Vancomycin-heteroresistant phenotype in invasive methicillin-resistant Staphylococcus aureus isolates belonging to spa type 041. Eur J Clin Microbiol Infect Dis. 2010;29:771–7.
Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002;70:631–41.
Tinelli M, Monaco M, Vimercati M, Ceraminiello A, Pantosti A. Methicillin-susceptible Staphylococcus aureus in skin and soft tissue infections, Northern Italy. Emerg Infect Dis. 2009;15:250–7.
Harmsen D, Claus H, Witte W, Rothganger J, Turnwald D, Vogel U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41:5442–8.
Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci USA. 2002;99:7687–92.
Monecke S, Slickers P, Ehricht R. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol Med Microbiol. 2008;53:237–51.
Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE. 2011;6:e17936.
Roberts AA, Sharma SV, Strankman AW, Duran SR, Rawat M, Hamilton CJ. Mechanistic studies of FosB: a divalent-metal-dependent bacillithiol-S-transferase that mediates fosfomycin resistance in Staphylococcus aureus. Biochem J. 2013;451:69–79.
Grundmann H, Aanensen DM, van den Wijngaard CC, Spratt BG, Harmsen D, Friedrich AW. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 2010;7:e1000215.
Perez-Vazquez M, Vindel A, Marcos C, Oteo J, Cuevas O, Trincado P, et al. Spread of invasive Spanish Staphylococcus aureus spa-type t067 associated with a high prevalence of the aminoglycoside-modifying enzyme gene ant(4′)-Ia and the efflux pump genes msrA/msrB. J Antimicrob Chemother. 2009;63:21–31.
Changchien CH, Chen SW, Chen YY, Chu C. Antibiotic susceptibility and genomic variations in Staphylococcus aureus associated with Skin and Soft Tissue Infection (SSTI) disease groups. BMC Infect Dis. 2016;16:276.
Koningstein M, Groen L, Geraats-Peters K, Lutgens S, Rietveld A, et al. The use of typing methods and infection prevention measures to control a bullous impetigo outbreak on a neonatal ward. Antimicrob Resist Infect Control. 2012;1:37.
Paranthaman K, Bentley A, Milne LM, Kearns A, Loader S, Thomas A, et al. Nosocomial outbreak of staphyloccocal scalded skin syndrome in neonates in England, December 2012–March 2013. Euro Surveill. 2014;19:1–7.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Local Surveillance and Infection Control Committee (CIO) approved the study on September 13, 2013. Informed consent was obtained from parents of infected and non-infected neonates and from medical and non-medical staff. Clinical data and samples (bacterial isolates) were anonymized before being used for the study. Approval by the ethics committee was not required as the study was regarded as part of routine surveillance measures for infection control.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pimentel de Araujo, F., Tinelli, M., Battisti, A. et al. An outbreak of skin infections in neonates due to a Staphylococcus aureus strain producing the exfoliative toxin A. Infection 46, 49–54 (2018). https://doi.org/10.1007/s15010-017-1084-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-017-1084-2