Abstract
Insecticidal toxicities of bioactive constituent from Diospyros kaki roots and its structural derivatives were evaluated against the larvae of Aedes aegypti, Culex pipiens pallens, and Ochlerotatus togoi. Bioactive constituent of D. kaki roots was isolated by some chromatographic methods and identified as 5-hydroxy-2-methyl-1,4-naphthoquinone. Based on the LC50 values of 5-hydroxy-2-methyl-1,4-naphthoquinone structural derivatives, 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (0.89, 0.80, and 1.04 μg/mL) had the most potent insecticidal activity, followed by 2,3-dibromo-1,4-naphthoquinone (1.32, 1.28, and 1.94 μg/mL), 5,8-dihydroxy-1,4-naphthoquinone (3.57, 3.34, and 5.04 μg/mL), 2,3-dichloro-1,4-naphthoquinone (4.76, 3.89, and 5.33 μg/mL), 5-hydroxy-2-methyl-1,4-naphthoquinone (5.66, 5.43, and 6.09 μg/mL), and 2,2′-bi(3-hydroxy-1,4-naphthoquinone) (33.93, 32.82, and 36.17 μg/mL) against Ae. aegypti, Cx. pipiens pallens, and Oc. togoi, respectively. In this regard, 5-hydroxy-2-methyl-1,4-naphthoquinone and its structural derivatives could be suitable as insecticidal agents to control the larvae of the three mosquito species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Mosquito is the most important intermediator with respect to human disease and global distribution (James 1992). They function as vectors for various diseases, which are dengue fever, encephalitis, and filariasis (Rajeswary and Govindarajan 2013). These diseases are accompanied by economic losses, particularly in tropical and subtropical countries (James 1992). Synthetic mosquito larvicides, such as growth regulators (methoprene) and organophosphates (chlorpyrifos and temephos), have been used for several decades to control the larvae of mosquitoes (Yang et al. 2002). Despite their effectiveness, continuous use of synthetic larvicides has caused undesirable effects, such as reducing biological control by natural enemies, development of larvicide resistance, and toxicity to non-target and beneficial organisms (Park and Shin 2005). The problems have been needed for the new development of safer insecticides for the larvae of mosquitoes (Yang et al. 2002).
Plant substances may be an alternative source of insecticides and larvicides because they constitute the rich materials of biological compounds (Lee 2002; Lee et al. 2013; Yang et al. 2013). In particular, a variety of plant species with chemical defense mechanisms against herbivores could provide a new source of control agents against a wide spectrum of insect vectors (Kim et al. 2002; Yang et al. 2002). Various studies have focused on essential oils, phytochemicals, and plant extracts, such as mosquito control agents (Tabanca et al. 2013). Therefore, it is established that natural products derived from plants have the potential as alternatives to conventional repellents, insecticides, and larvicides (Ali et al. 2013; Tabanca et al. 2013). Diospyros kaki (Ebenaceae) is native to Korea, China, and Japan and 90 % of its production occurs in eastern Asia (Sunity and Himanshu 2011). D. kaki has been continuously used as traditional medicine because of its active compounds, such as carotenoids, epicatechin, gallocatechin, and polyphenols (George and Redpath 2008; Sunity and Himanshu 2011). D. kaki roots contain abundant phenolic compounds, including anthocyanidin, chlorogenic acid, quinones, syringic acid, and tannin (Sattar et al. 1992; Suzuki et al. 2005). Phenolic compounds possess biological effects, such as anticarcinogenic, antimutagenic, insecticidal, and radioprotective activities (Bachrach and Wang 2002; Lee and Lee 2008; Pu et al. 2013). Although various studies have reported on the compounds derived from D. kaki roots, the insecticidal activity of an active compound from D. kaki roots against mosquito larvae has not been reported previously. In this regard, the objective of the present study is to isolate the bioactive compound from D. kaki roots and evaluate the insecticidal toxicities of the bioactive compound and its structural derivatives against the three types of the mosquito species.
Materials and methods
Chemicals
2.2′-Bi(3-hydroxy-1,4-naphthoquinone) and pirimiphos-methyl were purchased from Fluka (Switzerland). 2,3-Dibromo-1,4-naphthoquinone, 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone, 2,3-dichloro-1,4-naphthoquinone, and 5,8-dihydroxy-1,4-naphthoquinone were provided by Sigma-Aldrich (USA).
Sample preparation
Diospyros kaki roots were supplied from the Jeonju market in Korea during spring 2012 and dried at 30 °C. After washing, the roots (2 kg) were homogenized with a grinder and then extracted with methanol in an incubator at 25 °C for 48 h. The methanol extract (628 g, yield 31.4 %) was filtered and consecutively divided with hexane, chloroform, ethyl acetate, butanol, and distilled water. Five fractions were concentrated at 45 °C using a vacuum evaporator and the distilled water fraction was freeze-dried.
Isolation and identification
Chromatographic methods were used to isolate the insecticidal compound of the chloroform fraction partitioned from the methanol extract of D. kaki roots (Lee and Lee 2008). The chloroform fraction (9 g) was loaded on an open column, containing a silica gel (70–230 mesh, 500 × 701 mm; Merck, USA) and sequentially eluted with a mixture of organic solvents consisting of hexane:chloroform (3:7, 1:9, and 0:10, v:v). All fractions were analyzed using thin layer chromatography and the fractions with similar thin layer chromatography patterns were combined. As a result, six fractions (DK1–DK6) were produced. The active DK1 fraction (4.4 g) was again chromatographed using a silica gel column and a mixed organic solvent consisting of hexane:chloroform (3:7, v:v), and six fractions (DK11–DK16) were obtained. The active DK11 fraction (1.2 g) was isolated using prep HPLC (JAI Co., Ltd., Japan), following the analytical conditions: JAI GS column (GS310 500 + GS310 500 mm, 20 × 500 mm, Japan); mixed organic solvents consisting of hexane:chloroform:isopropanol (30:70:2, v:v:v); flow rate of 5 mL/min; and UV detection at an absorbance of 268 nm. As a result, five fractions (DK111–DK115) were obtained. The active DK114 fraction (940 mg) was chromatographed on a JAI W column (W 253 500 mm, 20 × 500 mm) under the same conditions. Finally, the active DK1144 fraction (380 mg) was isolated. The structure of the DK1144 fraction was measured by spectroscopic methods. 1H and 13C distortionless enhancement by polarization transfer (DEPT) nuclear magnetic resonance spectra were documented with the JNM-ECA 600 spectrometer (JEOL Ltd., Japan) using CDCl3 at 600 and 150 MHz, respectively. Tetramethylsilane served as the chemical shifts, and internal standards are given in delta (δ, parts per million). A spectrum of absorbance was obtained using an ultraviolet spectrometer (model: DR 4000, HACH, Korea).
Mosquitoes and bioassay
The mosquito species were Ae. aegypti, Cx. pipiens pallens, and Oc. togoi supplied from Seoul National University in 2012. Each larva was reared in a plastic tray (200 × 350 × 70 mm) and was provided with a sterile diet consisting of chick chow powder: yeast (4:1, v:v). The mosquito adults were supplied with a blood from the mouse and 10 % sucrose solution. The mosquito larvae and adults were maintained on a light:dark photoperiod (16:8 h) at 28 °C and 70 % relative humidity. The insecticidal activities of active component and its structural derivatives against the larvae of the three mosquito species were conducted using the standard method modified by WHO (2009). Twenty-fourth-instar larvae were placed in distilled water (24.5 mL), containing an each sample (0.5 mL), was added in a 30 mL cup. For comparison, pirimiphos-methyl, which is one of the commercial insecticides, was used as the positive control. The negative control was prepared from distilled water (24.5 mL) and dimethyl sulfoxide solution (0.5 mL), which were added with mosquito larvae. All treatments were replicated five times and were incubated for 24 h at 28 °C. Percent mortalities were investigated and transformed to arcsine square root values for variance analysis with SPSS statistical software (version 18.0, SPSS Inc., USA). Treatment means were compared with Scheffe’s test (p < 0.05). Means values of untransformed data are reported. LC50 values in all treatments were calculated by probit analysis.
Results and discussion
The insecticidal activity of the methanol extract from D. kaki roots against the larvae of the three mosquito species is shown in Table 1. The D. kaki extract exhibited 100 % mortality against Cx. pipiens pallens, Ae. aegypti, and Oc. togoi at 100 μg/mL. Based on the 50 and 25 μg/mL concentrations, the methanol extract of D. kaki roots had potent larvicidal activity against Cx. pipiens pallens (85.7 and 40.3 % mortality), followed by Ae. aegypti (72.7 and 28.6 % mortality) and Oc. togoi (60.5 and 9.8 % mortality). A previous study reported that plants are particularly suitable for crop protection because of the presence of defense chemicals with more than one mode of action (Jiang et al. 2002). Furthermore, the methanol extract (600 g) of D. kaki roots was partitioned using the polarity of several solvent systems to obtain the five fractions of hexane (22 g, yield 3.7 %), chloroform (67 g, yield 11.2 %), ethyl acetate (188 g, yield 31.3 %), butanol (109 g, yield 18.2 %), and distilled water (83 g, yield 13.8 %). The chloroform fraction possessed excellent larvicidal toxicities (86.5, 100, and 69.5 % mortality at 50 μg/mL) against Cx. pipiens pallens, Ae. aegypti, and Oc. togoi, respectively. Therefore, the chloroform fraction partitioned from the D. kaki extract was isolated using some chromatographic methods to identify bioactive compound.
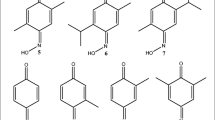
Silica gel column and prep HPLC were conducted to isolate the insecticidal compound of the chloroform fraction. The DK1144 fraction was isolated as the bioactive compound in D. kaki roots and the DK1144 structure was identified by some spectroscopic methods, such as 1H, 13C, and DEPT NMR spectra. The DK1144 fraction was characterized as 5-hydroxy-2-methyl-1,4-naphthoquinone (C11H8O3) (Fig. 1A); EI-MS (70 eV) m/z M+ 188 (100), 173 (17), 160 (19), 131 (18), 120 (11), 92 (9), 63 (5); 1H NMR (CDCl3, 600 MHz) δ 2.086–2.090 (d, 3H, J = 1.6 Hz), 6.693–6.697 (d, 1H, J = 1.6 Hz), 7.124–7.148 (m, 1H, J = 9.6 Hz), 7.470–7.488 (d, 1H, J = 7.2 Hz), 7.508–7.523 (d, 1H, J = 6.0 Hz); 13C NMR (CDCl3, 150 MHz) δ 16.54 (CH), 113.98 (C), 118.16 (CH), 123.02 (CH), 130.89 (C), 134.28 (CH), 134.94 (CH), 148.44 (C), 159.96 (C), 183.54 (C), 189.03 (C). The analytical result of 5-hydroxy-2-methyl-1,4-naphthoquinone was consistent with the NMR data of previously reported study (Lee and Lee 2008).
Structures of 5-hydroxy-2-methyl-1,4-naphthoquinone derivatives: (A) 5-hydroxy-2-methyl-1,4-naphthoquinone, (B) 2,3-dibromo-1,4-naphthoquinone, (C) 2,3-dichloro-1,4-naphthoquinone, (D) 5,8-dihydroxy-1,4-naphthoquinone, (E) 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone, (F) 2,2′-bi(3-hydroxy-1,4-naphthoquinone)
The insecticidal toxicity of 5-hydroxy-2-methyl-1,4-naphthoquinone was evaluated against the three mosquito larvae (Table 2). The LC50 values of 5-hydroxy-2-methyl-1,4-naphthoquinone (A) were 5.66, 5.43, and 6.09 μg/mL against Ae. aegypti, Cx. pipiens pallens, and Oc. togoi, respectively. The larvicidal toxicity of 5-hydroxy-2-methyl-1,4-naphthoquinone (A) has not been reported, and these findings indicate that the sensitivity of 5-hydroxy-2-methyl-1,4-naphthoquinone (A) against Cx. pipiens pallens was the highest among the three mosquito species. According to previous study, the resistance of natural substances derived from plants against Ae. aegypti and Oc. togoi was higher than that of a biomaterial against Cx. pipiens pallens because of the influence of phytochemicals (pellitorine, guineensine, and retrofractamide A) and biochemical factors (detoxifying enzyme, acetylcholinesterase, and penetration of sodium channel) (Yang et al. 2013; Kim et al. 2008).
The structural toxicity relationships of 5-hydroxy-2-methyl-1,4-naphthoquinone (A) and its structural derivatives, including 2,2′-bi(3-hydroxy-1,4-naphthoquinone) (F), 2,3-dibromo-1,4-naphthoquinone (B), 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (E), 2,3-dichloro-1,4-naphthoquinone (C), and 5,8-dihydroxy-1,4-naphthoquinone (D) were determined (Table 2; Fig. 1). Based on the LC50 values of-5-hydroxy-2-methyl-1,4-naphthoquinone derivatives against Ae. aegypti, it was found that 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (E) (0.89 μg/mL) had the most potent larvicidal toxicity, followed by 2,3-dibromo-1,4-naphthoquinone (B) (1.32 μg/mL), 5,8-dihydroxy-1,4-naphthoquinone (D) (3.57 μg/mL), 2,3-dichloro-1,4-naphthoquinone (C) (4.76 μg/mL), and 2,2′-bi(3-hydroxy-1,4-naphthoquinone) (F) (33.93 μg/mL); 2,3-Dichloro-5,8-dihydroxy-1,4-naphthoquinone (E) (0.80 μg/mL) possessed the strongest larvicidal toxicity against Cx. pipiens pallens, followed by 2,3-dibromo-1,4-naphthoquinone (B) (1.28 μg/mL), 5,8-dihydroxy-1,4-naphthoquinone (D) (3.34 μg/mL), 2,3-dichloro-1,4-naphthoquinone (C) (3.89 μg/mL), and 2,2′-bi(3-hydroxy-1,4-naphthoquinone) (F) (32.82 μg/mL). In the case of Oc. togoi, 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (E) (1.04 μg/mL) showed excellent larvicidal toxicity, followed by 2,3-dibromo-1,4-naphthoquinone (B) (1.94 μg/mL), 5,8-dihydroxy-1,4-naphthoquinone (D) (5.04 μg/mL), 2,3-dichloro-1,4-naphthoquinone (C) (5.33 μg/mL), and -2,2′-bi(3-hydroxy-1,4-naphthoquinone) (F) (36.17 μg/mL). The larvicidal toxicities of the 5-hydroxy-2-methyl-1,4-naphthoquinone structural derivatives were less than those of pirimiphos-methyl (0.18, 0.13, and 0.24 μg/mL) against Ae. aegypti, Cx. pipiens pallens, and Oc. togoi. Nevertheless, 5-hydroxy-2-methyl-1,4-naphthoquinone (A) and its structural derivatives could be suitable as natural insecticidal agents to control mosquito larvae.
2,3-Dibromo-1,4-naphthoquinone (B) possessed potent insecticidal toxicity against the three mosquito larvae when 1,4-naphthoquinone contained Br, Cl, and OH functional groups, followed by 5,8-dihydroxy-1,4-naphthoquinone (D) and 2,3-dichloro-1,4-naphthoquinone (C). However, when combined with an OH functional group on 2,3-dichloro-1,4-naphthoquinone (C), the larvicidal toxicities of 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (E) were approximately 5.35, 4.86, and 5.13 times more effective than those of 2,3-dichloro-1,4-naphthoquinone (C) against Ae. aegypti, Cx. pipiens pallens, and Oc. togoi, respectively. In contrast, when a CH3 functional group was on 5-hydroxy-1,4-naphthoquinone, the insecticidal toxicities of 5-hydroxy-2-methyl-1,4-naphthoquinone (A) were less than those of 5,8-dihydroxy-1,4-naphthoquinone (D) against the three mosquito larvae. These findings indicate that the presence of some functional groups on 1,4-naphthoquinone influenced insecticidal toxicity against the Cx. pipiens pallens, Ae. aegypti, and Oc. togoi larvae. In particular, when the Cl and OH functional groups were placed on 1,4-naphthoquinone at the same time, insecticidal toxicity against the three mosquito larvae was the highest among compounds. In previous study, Ribeiro et al. (2009) have reported the insecticidal toxicities of 1,4-naphthoquinone structural derivatives against the Ae. aegypti larvae. Based on the LC50 values against Ae. aegypti, it was found that 3-bromo-5-hydroxy-1,4-naphthoquinone (0.873 μg/mL) possessed the potent larvicidal toxicity, followed by 5-hydroxy-1,4-naphthoquinone (3.587 μg/mL), 3-bromo-5-methoxy-1,4-naphthoquinone (5.752 μg/mL), and 3-bromo-5-acetoxy-1,4-naphthoquinone (7.287 μg/mL). These findings indicated that 1,4-naphthoquinone structural derivatives, containing Br and OH functional groups on 1,4-naphthoquinone, had the potent insecticidal effects against the mosquito larvae. From these results, the present study suggest that 1,4-naphthoquinone structural derivatives are insecticidal agents against the mosquito larvae.
References
Ali A, Murphy CC, Demirci B, Wedge DW, Sampson BJ, Khan IA, Baser KHC, Tabanca N (2013) Insecticidal and biting activity of rose-scented geranium (Pelargonium species) individual compounds against Stephanitis pyrioides and Aedes aegypti. Pest Manage Sci 69:1385–1392
Bachrach U, Wang YC (2002) Cancer therapy and prevention by green tea: role of ornithine decarboxylase. Amino Acids 22:1–13
George AP, Redpath S (2008) Health and medicinal benefits of persimmon fruit: a review. Adv Hortic Sci 22:244–249
James AA (1992) Mosquito molecular genetics: the hands that feed bite back. Science 257:37–38
Jiang ZL, Zhang X, Feng JR (2002) A critical review of the essential oils and its application in plant protection. J Shaanxi Agric Sci 1:32–36
Kim DH, Kim SI, Chan KS, Ahn YJ (2002) Repellent activity of constituents identified in Foeniculum vulgare fruits against Aedes aegypti (Diptera: Culicidae). J Agric Food Chem 50:6993–6996
Kim NJ, Byun SG, Cho JE, Chung K, Ahn YJ (2008) Larvicidal activity of Kaempferia galanga rhizome phenylpropanoids towards three mosquito species. Pest Manag Sci 64:857–862
Lee HS (2002) Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J Agric Food Chem 50:1400–1403
Lee CH, Lee HS (2008) Acaricidal activity and function of mite indicator using plumbagin and its derivatives isolated from Diospyros kaki Thunb. roots (Ebenaceae). J Microbiol Biotechnol 18:314–321
Lee NH, Lee SM, Song DH, Yang JY, Lee HS (2013) Antimicrobial effect of emodin isolated from Cassia tora Linn. seeds against food-borne bacteria. J Appl Biol Chem 56:187–189
Park IK, Shin SC (2005) Fumigant activity of plant essential oils and components from garlic (Allium sativum) and clove bud (Eugenia caryophyllata) oils against the Japanese termite (Reticulitermes speratus). J Agric Food Chem 53:4388–4392
Pu F, Ren XL, Zhang XP (2013) Phenolic compounds and antioxidant activity in fruits of six Diospyros kaki genotypes. Eur Food Res Technol 237:923–932
Rajeswary M, Govindarajan M (2013) Mosquito larvicidal and phytochemical properties of Ageratina adenophora (Asteraceae) against three important mosquitoes. J Vector Borne Dis 50:141–143
Ribeiro KAL, de Carvalho CM, Molina MT, Lima EP, Lopez-Montero E, Reys JRM, de Oliveira MBF, Pinto AV, Santana AEG, Goulart MOF (2009) Activities of naphthoquinones against Aedes aegypti (Linnaeus, 1762) (Diptera: Culicide), vector of dengue and Biomphalaria glabrata (Say, 1818), intermediate host of Schistosoma mansoni. Acta Trop 111:44–50
Sattar A, Bibi N, Chaudry MA (1992) Phenolic compounds in persimmon during maturation and on tree ripening. Mol Nutr Food Res 36:466–472
Sunity S, Himanshu J (2011) Diospyros kaki (Ebenaceae): a review. Asian J Res Pharm Sci 1:55–58
Suzuki T, Someya S, Hu F, Tanokura M (2005) Comparative study of catechin compositions in five Japanese persimmons (Diospyros kaki). Food Chem 93:149–152
Tabanca N, Bernier UR, Ali A, Wang A, Demirci B, Blythe EK, Khan SI, Baser KHC, Khan IK (2013) Bioassay-guided investigation of two Monarda essential oil for repellent activity against yellow fever mosquito Aedes aegypti. J Agric Food Chem 61:8573–8580
World Health Organization (2009) Report of the WHO informal consultation on the evaluation on the testing of insecticides. World Health Organization, Geneva
Yang YC, Lee SG, Lee HK, Kim MK, Lee SH, Lee HS (2002) A piperidine amide extracted from Piper longum L. fruit shows activity against Aedes aegypti mosquito larvae. J Agric Food Chem 5:3765–3767
Yang JY, Cho KS, Chung NH, Kim CH, Suh JW, Lee HS (2013) Constituents of volatile compounds derivative from Melaleuca alternifolia leaf oil and acaricidal toxicities against house dust mites. J Korean Soc Appl Biol Chem 56:91–94
Acknowledgments
This research was carried out with the support of Cooperative Research Program for Agriculture Science & Technology Development (Project title: Development of integrated pest management techniques using natural products in the grain storage, Project No: PJ01004502), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, MG., Lee, HS. Insecticidal toxicities of naphthoquinone and its structural derivatives. Appl Biol Chem 59, 3–8 (2016). https://doi.org/10.1007/s13765-015-0115-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-015-0115-x