Abstract
The effective and environmentally sustainable control of mosquitoes is a challenge of essential importance. This is due to the fact that some invasive mosquitoes, with special reference to the Aedes genus, are particularly difficult to control, due to their high ecological plasticity. Moreover, the indiscriminate overuse of synthetic insecticides resulted in undesirable effects on human health and non-target organisms, as well as resistance development in targeted vectors. Here, the leaf essential oil (EO) extracted from a scarcely studied plant of ethno-medicinal interest, Blumea eriantha (Asteraceae), was tested on the larvae of six mosquitoes, including Zika virus vectors. The B. eriantha EO was analyzed by GC and GC-MS. The B. eriantha EO showed high toxicity against 3rd instar larvae of six important mosquito species: Anopheles stephensi (LC50=41.61 μg/ml), Aedes aegypti (LC50=44.82 μg/ml), Culex quinquefasciatus (LC50 =48.92 μg/ml), Anopheles subpictus (LC50=51.21 μg/ml), Ae. albopictus (LC50=56.33 μg/ml) and Culex tritaeniorhynchus (LC50=61.33 μg/ml). The major components found in B. eriantha EO were (4E,6Z)-allo-ocimene (12.8%), carvotanacetone (10.6%), and dodecyl acetate (8.9%). Interestingly, two of the main EO components, (4E,6Z)-allo-ocimene and carvotanacetone, achieved LC50 lower than 10 μg/ml on all tested mosquito species. The acute toxicity of B. eriantha EO and its major constituents on four aquatic predators of mosquito larval instars was limited, with LC50 ranging from 519 to 11.431 μg/ml. Overall, the larvicidal activity of (4E,6Z)-allo-ocimene and carvotanacetone far exceed most of the LC50 calculated in current literature on mosquito botanical larvicides, allowing us to propose both of them as potentially alternatives for developing eco-friendly mosquito control tools.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquitoes constitute a major public health problem as vectors of serious human and animal diseases, such as malaria, filariasis, Japanese encephalitis, dengue fever, chikungunya, yellow fever, and – more recently – Zika virus. These diseases cause high mortality and morbidity among people living in tropical and sub-tropical zones (Benelli 2015a; Mehlhorn 2015; Benelli et al. 2016; Yakob and Walker 2016). Nowadays, the effective and environmentally sustainable control of mosquitoes is a challenge of essential importance. This is due to the fact that some mosquitoes, with special reference to the Aedes genus, are particularly difficult to control, due to their high ecological plasticity. A good example is the Asian tiger mosquito, Aedes albopictus, which is now ranked among the one hundred most invasive organisms worldwide (Benedict et al. 2007; Becker 2008; Becker et al. 2013; Benelli and Mehlhorn 2016). In addition, the indiscriminate overuse of synthetic insecticides resulted in undesirable effects on human health and non-target organisms, as well as resistance development in targeted vectors, with consequent loss of efficacy (see Hemingway and Ranson 2000, as well as Naqqash et al. 2016 for dedicated reviews). This current scenario is worsened by the lack of access to modern and costly mosquito control tools by rural populations of developing countries, which are the most afflicted by mosquito-borne diseases (Benelli 2015a).
Therefore, the use of plant derivatives with multiple mechanisms of action and eco-friendly features has been proposed as alternative tools against Culicidae, ticks, and other vectors (Benelli 2015b; Pavela and Benelli 2016a, b). In latest years, plant essential oils (EOs), plant extracts, plant extraction byproducts (e.g. neem cake), as well as botanical-fabricated capped mosquitocidal nanoparticles have been evaluated as mosquito control agents (Amer and Mehlhorn 2006a, b, c, d; Dinesh et al. 2015; Subramaniam et al. 2015, 2016; Murugan et al. 2016a, b; Panneerselvam et al. 2016; Benelli 2016a, b, 2017; Benelli and Govindarajan 2017).
In particular, a wide number of EOs extracted from aromatic plants have been tested as mosquitocides, ovideterrents and/or repellents, against different mosquito species (Benelli 2015a; Pavela 2015; Pavela and Govindarajan 2016; et al. 2016a, b, c). Good examples include Origanum majorana (El-Akhal et al. 2014), Ocimum gratissimum (Pratheeba et al. 2015), Lavandula stoechas (El Ouali et al. 2016), Corymbia citriodora (Santi and Simone 2014), Myristica fragrans (Carolina and Maman 2016), Crataeva magna (Veni et al. 2016), Laurus nobilis (Verdian-Rizi 2009), Apium graveolens (Kumar et al. 2014), Clausena anisata (Jayaraman et al. 2015), Melissa officinalis (Baranitharan et al. 2016) and Coleus aromaticus (Govindarajan et al. 2013b; Baranitharan et al. 2017). Results underlined that the plant EOs may be an alternative source of mosquito larval control agents, since are rich in bioactive compounds that show multiple mechanisms of action, are biodegradable into non-toxic products, and potentially suitable for use in IPM programs (Pavela and Benelli 2016a, b).
However, most of the studies focused on routine testing of EOs without digging deep in their chemical composition (i.e. no GC-MS, HPLC-MS, HPTLC and NMR analyses have been performed) and evaluating the bioactivity of selected pure constituents, formulated alone or in synergistic blends (Benelli et al. 2017a, b, c). Most importantly, as recently reviewed by Pavela (2015), the majority of tested EOs achieved LC50 higher than 50 ppm on mosquitoes, highlighting the value of systematic screening endemic flora of tropical and sub-tropical countries searching for effective mosquitocidal and antiplasmodial products (Benelli and Mehlhorn 2016).
Blumea is a genus of shrubs and small trees, comprising about 80 species distributed in tropical and subtropical Asia, Africa, and Oceania (Liang et al. 2011). This genus includes some key medicinal plants largely used in traditional medicine. For example, Blumea membranacea EO led to blood pressure reduction (Mehta et al. 1986). Besides the ethno-pharmacological potential of Blumea species, the EOs from B. mollis (Senthilkumar et al. 2008), Blumea perrottetiana (Owolabi et al. 2010) and B. densiflora (Zhu and Tian 2011) have been reported for their insecticidal activity, while B. membranacea EO shows antifungal activity (Mehta et al. 1986).
Blumea eriantha is an annual aromatic herb, which grows abundantly along roadsides and degraded forestlands. Common names are “Nimurdi” in Marathi and “Kukronda” in Hindi. B. eriantha is distributed in Bihar, Karnataka, Madhya Pradesh, Maharashtra, Orissa, Uttar Pradesh and Southern India (Singh et al. 2011). The juice extracted from this herb has been reported as a carminative, while the warm leaf infusion is used as sudorific, and the cold infusion is considered as a diuretic and herbal emmenagogue. The EO extracted from B. eriantha is traditionally recognized for its antibacterial and antifungal uses in folk medicine (Khare 2007). A recent study focused on the antimicrobial efficacy of B. eriantha EO (Pednekar et al. 2012).
However, the toxicity of B. eriantha EO against insect vectors of medical and veterinary relevance is unknown. In the present research, we investigated the environmentally sustainable use of B. eriantha EO and its main chemical components for the development of new products to combat the spread of the mosquito-borne diseases, with special reference to dengue and Zika virus. We tested the B. eriantha EO larvicidal activity on six key mosquito vectors, i.e. Anopheles stephensi, An. subpictus, Ae. aegypti, Ae. albopictus, Culex quinquefasciatus, and Cx. tritaeniorhynchus. Furthermore, B. eriantha EO was analyzed using gas chromatography-mass spectrometry (GC-MS). The major components of B. eriantha EO, i.e. (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate, were also tested against the six mosquito vectors. Lastly, to shed light on non-target effects of the B. eriantha EO as well as (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate, we evaluated them in acute toxicity tests on four non-target predators of mosquito larvae.
Materials and methods
Extraction, GC and GC-MS of the B. eriantha essential oil
Fresh leaves of B. eriantha were collected in the Munnar mountains, India (10°05’21”N 77°03’35”E, 1700 m a.s.l.) in May 2016. Blumea eriantha EO was hydro-distilled using 3 kg of fresh leaves, then analyzed by GC and GC-MS as described by Govindarajan and Benelli (2016a, b). Compound identification was carried out comparing retention indices and mass spectra with those available in NIST 98.1, Mass Finder 3.1 and Adams (2007).
Larvicidal activity of (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate
The B. eriantha EO as well as (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate, were tested against 3rd instar larvae of An. stephensi, Ae. aegypti, Cx. quinquefasciatus, An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus following the protocol by WHO (2005), slightly modified by Govindarajan and Benelli (2016c). For each tested product, 5 replicates of each dose were prepared. 10 3rd larvae were transferred into each beaker. Mortality was assessed after 24 h of exposure.
Toxicity on non-target predators
The effect of B. eriantha EO as well as (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate, on non-target aquatic predators was assessed following the method by Sivagnaname and Kalyanasundaram (2004) modified Govindarajan et al. (2016d, e). The toxicity of the B. eriantha EO, (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate was tested against adults of the non-target biological control agents of mosquito young instars, including adult backswimmers and water bugs i.e. Anisops bouvieri and Diplonychus indicus , and larvivorous fish Gambusia affinis and Poecilia reticulata. The non-target species were reared as reported by Govindarajan and Benelli (2016d). The B. eriantha EO, (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate were evaluated at doses higher than 50xLC50 calculated on the six mosquito species. 10 replicates were performed for each dose. 4 control replicates were also done (where no B. eriantha EO, (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate were added to the water). Mortality of each non-target predator was assessed after 48 h of exposure (Govindarajan and Benelli 2016b; Benelli et al. 2017c).
Data analysis
Mortality data were analyzed by probit analysis (Benelli 2017). LC50 and LC90 were calculated following Finney (1971). Concerning non-target predators, the Predator Safety Factor (PSF) was calculated as described by Deo et al. (1988). Data were analyzed by SPSS version 16.0.
Results
Yield and composition of B. eriantha essential oil
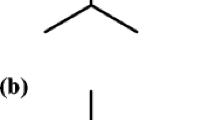
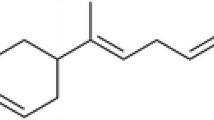
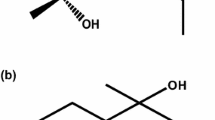
The yield of B. eriantha leaf EO was 2.5 ml/kg of leaf fresh weight. Table 1 showed a total of 34 chemical constituents, representing 94.2% of the B. eriantha leaf EO. The major constituents of B. eriantha EO were (4E,6Z)-allo-ocimene (12.8%), carvotanacetone (10.6%) and dodecyl acetate (8.9%). The chemical structures of (4E,6Z)-allo-ocimene, carvotanacetone and dodecyl acetate are reported in Figure 1. The amount of remaining 31 molecules ranged from 0.7 % to 4.2 % (Table 1).
Larvicidal activity of (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate
The B. eriantha EO showed acute toxicity against third instar larvae of An. stephensi, Ae. aegypti, Cx. quinquefasciatus, An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus, with LC50 of 41.61, 44.82, 48.92, 51.21, 56.33 and 61.33 μg/ml, respectively (Table 2).
Furthermore, the three major pure compounds extracted from the B. eriantha EO, (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate, were tested individually against six mosquito vector larval populations. We observed that (4E,6Z)-allo-ocimene, carvotanacetone and dodecyl acetate were extremely toxic to the six mosquito species. (4E,6Z)-allo-ocimene LC50 values estimated on An. stephensi, Ae. aegypti, Cx. quinquefasciatus, An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus, were 4.06, 4.52, 4.92, 6.14, 6.70 and 7.26 μg/ml, respectively (Table 3). Carvotanacetone LC50 values were 6.20, 6.77, 7.38, 8.43, 9.21 and 10.02 μg/ml, respectively (Table 4). Dodecyl acetate LC50 values were 10.22, 11.18, 12.16, 12.31, 13.45 and 14.68 μg/ml, respectively (Table 5). No mortality was recorded in controls.
Toxicity on non-target predators
The acute toxicity of B. eriantha EO was tested on the four non-target predators A. bouvieri, D. indicus, P. reticulata and G. affinis. Results are reported in Table 6 . B. eriantha EO LC50 values were 4139.79, 6285.59, 10251.51 and 11431.04 μg/ml, respectively.
Furthermore, the three major pure compounds extracted from the B. eriantha EO, (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate, were tested individually against four important non-target predators of mosquito larvae. We observed that (4E,6Z)-allo-ocimene, carvotanacetone and dodecyl acetate were scarcely toxic to the non-target predators. (4E,6Z)-allo-ocimene LC50 estimated on A. bouvieri, D. indicus, P. reticulata and G. affinis were 519.97, 845.65, 1656.78 and 1854.25 μg/ml, respectively (Table 7). Carvotanacetone LC50 were 631.59, 1051.39, 1863.86 and 2075.07 μg/ml, respectively (Table 8). Dodecyl acetate LC50 were 823.94, 1483.11, 2065.56 and 2369.78 μg/ml, respectively (Table 9). No mortality was recorded in control treatments.
The estimated PSF indicated that the B. eriantha EO and its main constituents showed little toxicity on A. bouvieri, D. indicus, P. reticulata and G. affinis (Table 10).
Lastly, focal observations conducted daily until 10 days from the exposure to B. eriantha EO and its main constituents, formulated at the LC50 and LC90 calculated on the targeted six mosquito vectors, indicated that the survival and swimming activity of the non-target predators were not damaged.
Discussion
Wide chemical diversity of Blumea essential oils
Results from GC and GC-MS analyses showed that 34 compounds were identified in the B. eriantha EO, with (4E,6Z)-allo-ocimene, carvotanacetone and dodecyl acetate as main components. This highlighted a quite different chemical composition, if compared to other EOs extracted from close-related Blumea species. Currently, the EOs of several species of the genus Blumea have been examined. Good examples are B. balsamifera (Sakee et al. 2011), Blumea lacera (Khair et al. 2014), B. balsamifera (Norikura et al. 2008), and B. lacera (Jahan et al. 2014). At variance with our results on B. eriantha, it is worthy to note that B. perrottetiana aerial part EO was mostly composed by 2,5-dimethoxy-p-cymene (30.0 %), 1,8-cineole (11.0 %) and sabinene (8.1 %) (Owolabi et al. 2010). Blumea balsamifera leaf EO was mostly composed by borneol (33.22 %), caryophyllene (8.24 %) and ledol (7.12 %) (Bhuiyan et al. 2009). Blumea mollis leaf EO was mainly composed by linalool (19.43 %) and γ-elemene (12.19 %) (Senthilkumar et al. 2008). Blumea brevipes EO main chemicals were terpinen-4-ol (27.6 %) and germacrene-D (15.4 %) (Mwangi et al. 1994), while B. lanceolaria EO mainly contained methyl thymol (Dung et al. 1991). Lastly, B. lacera leaf EO was composed by thymoquinol di-mether, β-caryophyllene, α-humulene, and (E)-β-farnesene (Laakso et al. 1989). Main compounds in B. densifora EO were borneol (11.43%), germacrene D (8.66%) and β-caryophyllene (6.68%) (Zhu and Tian 2011). Based on the presence of (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate in our GC-MS analysis of B. eriantha EO, we selected these molecules for further toxicity screenings on mosquito vectors.
Blumea essential oils really work against mosquitoes!
The use of plant EOs in vector control may represent a cheap alternative method to minimize the side effects of chemical pesticides on human health and the environment (Benelli 2015b; Govindarajan et al. 2013a, b, 2016a, b, c; Pavela 2015). Although a number of compounds of botanical origin have been currently reported (Wang et al. 2006; Cheng et al. 2009; Pavela and Benelli 2016b), the discovery of more effective plant products is of paramount importance to improve insecticide formulation and develop environmentally acceptable insecticides (Alkofahi et al. 1989).
In our experiments, the EO extracted from the leaves of B. eriantha showed high toxicity against 3rd instar larvae of Anopheles, Aedes and Culex species, including Zika virus vectors, with LC50 ranging from 41.61 to 61.33 μg/ml. Our results fit the criteria of EO larvicidal efficacy outlined by Pavela (2015). Earlier, it has been reported that two other EOs from the Blumea genus are highly toxic to anopheline vectors. Indeed, the B. densiflora EO tested on Anopheles anthropophagus larvae showed a LC50 of 10.55 ppm after 24 h, and 22.32 ppm after 12 h (Zhu and Tian 2011), and Senthilkumar et al. (2008) showed the larvicidal effectiveness of B. mollis EO on Cx. quinquefasciatus, with LC50 of 52.2 ppm.
Concerning the larvicidal activity of other EOs and extracts from the Asteraceae family, Macêdo et al. (1997) evaluated the toxicity of Tagetes minuta extract on Ae. fluviatilis (1.0 mg/l). The ethyl acetate leaf extract of Eclipta prostrata achieved a LC50 of 119.89 ppm on Cx. tritaeniorhynchus (Elango et al., 2009), while Achilea millefolium methanolic stem extract led to LC50 of 120.0 ppm on Cx. quinquefasciatus (Pavela 2008), Tanacetum vulgare methanolic flower extract (LC50 = 178.0 ppm) and methanolic stem extract of Otanthus maritimus (LC50 = 195.0 ppm) were also toxic to Cx. quinquefasciatus (Pavela et al. 2009).
Larvicidal activity of (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate
Interestingly, two of the main B. eriantha EO components, (4E,6Z)-allo-ocimene and carvotanacetone, achieved LC50 lower than 10 μg/ml on all tested mosquito species. As mentioned above, a wide number of plant EOs have been tested against mosquitoes. Indeed, screening the abundance of research products using “essential oil mosquito” as keywords on Scopus database (accessed: January 2017) we found more than 900 studies on this topic.
Unfortunately, only a very limited number of them considered testing single molecules identified in the EO (Pavela 2015; Benelli et al. 2017a). Some recent and noteworthy exceptions with larvicidal LC50 lower than 50 ppm are reviewed here. For example, the leaf EO from Clausena anisata contained β-pinene, sabinene, germacrene-D, estragole and linalool, which achieved LC50 of 23.17, 19.67, 16.95, 11.01 and 35.17 ppm on An. stephensi, LC50 of 27.69, 21.20, 18.76, 12.70 and 38.64 ppm on Ae. aegypti, and LC50 of 32.23, 25.01, 21.28, 14.01 and 42.28 ppm on Cx. quinquefasciatus (Govindarajan 2010). The LC50 of germacrene D-4-ol from Zanthoxylum monophyllum EO ranged from 6.12 to 7.26 μg/mL, while the LC50 for α-cadinol ranged from 10.27 to 12.28 μg/mL (Pavela and Govindarajan 2016). ar-curcumene and epi-β-bisabolol from Hedychium larsenii EO were toxic to An. stephensi (LC50 = 10.45 and 14.68 μg/ml), Ae. aegypti (LC50 = 11.24 and 15.83 μg/ml) and Cx. quinquefasciatus (LC50 = 12.24 and 17.27 μg/ml) (AlShebly et al. 2017). Artemisia absinthium EO-isolated (E)-β-farnesene, (Z)-en-yndicycloether, and (Z)-β-ocimene were toxic to An. stephensi (LC50 = 8.13, 16.24 and 25.84 μg/ml), An. subpictus (LC50 = 10.18, 20.99, and 30.86 μg/ml), Ae. aegypti (LC50 = 8.83,17.66, and 28.35 μg/ml), Ae. albopictus (LC50 = 11.38, 23.47, and 33.72 μg/ml), Cx. quinquefasciatus (LC50 = 9.66, 19.76, and 31.52 μg/ml), and Cx. tritaeniorhynchus (LC50 = 12.51, 25.88, and 37.13 μg/ml) (Govindarajan and Benelli 2016a). Lavandulyl acetate and bicyclogermacrene from Heracleum sprengelianum EO were toxic to An. subpictus (LC50 = 4.17 and 10.3 μg/ml), Ae. albopictus (LC50 = 4.60 and 11.1 μg/ml) and Cx. tritaeniorhynchus (LC50 = 5.11 and 12.5 μg/ml) (Govindarajan and Benelli 2016b). from Syzygium zeylanicum EO was a source of α-humulene and β-elemene, which were toxic to An. subpictus (LC50 = 6.19 and 10.26 μg/ml), Ae. albopictus (LC50 = 6.86 and 11.15 μg/ml), and Cx. tritaeniorhynchus (LC50= 7.39 and 12.05 μg/ml) (Govindarajan and Benelli 2016c). Eugenol, α-pinene and β-caryophyllene were identified in the Plectranthus barbatus EO, and they showed toxicity to An. subpictus (LC50 = 25.45, 32.09 and 41.66 μg/ml, respectively), Ae. albopictus (LC50 = 28.14, 34.09 and 44.77 μg/ml) and Cx. tritaeniorhynchus (LC50 = 30.80, 36.75 and 48.17 μg/ml) (Govindarajan et al. 2016a). Carvacrol and terpinen-4-ol isolated from the Origanum vulgare EO showed toxicity to An. stephensi (LC50 = 21.15 and 43.27 μg/ml), An. subpictus (LC50 = 24.06 and 47.73 μg/ml), Cx. quinquefasciatus (LC50 = 26.08 and 52.19 μg/ml) and Cx. tritaeniorhynchus (LC50 = 27.95 and 54.87 μg/ml) (Govindarajan et al. 2016d). δ-cadinene, calarene and δ-4-carene from the Kadsura heteroclita EO acted as larvicides on An. stephensi (LC50 = 8.23, 12.34 and 16.37 μg/ml), Ae. aegypti (LC50 = 9.03, 13.33 and 17.91 μg/ml) and Cx. quinquefasciatus (LC50 = 9.86, 14.49 and 19.50 μg/ml) (Govindarajan et al. 2016e).
Testing selected chemicals from plant essential oils is really important, since in a number of instances the single molecules are more effective if compared to the raw oil. In addition, testing single products for which the mechanism (s) of action is well known may help to shed light on the precise alterations induced on insect biochemical pathways (Pavela and Benelli 2016b). Lastly, the most effective molecules could be formulated in dedicated blends to shed light on possible synergistic and antagonistic toxicity effects (Benelli et al. 2017b, d). Further research on potential synergic as well as antagonistic effects occurring among the B. eriantha-borne molecules tested in blend is ongoing.
Toxicity of selected Blumea-borne molecules on non-target aquatic predators
The acute toxicity of B. eriantha EO, as well as (4E,6Z)-allo-ocimene, carvotanacetone, and dodecyl acetate on four aquatic predators was limited, with a LC50 range of 519-11.431 μg/ml (Tables 6, 7, 8, and 9). Plant EOs have been recognized as important sources of biopesticides, with limited toxic effects on human health and non-target organisms (Pavela and Benelli 2016b). For instance, recent research showed very limited acute toxicity of Pinus kesiya EO on A. bouvieri, D. indicus and G. affinis, with LC50 ranging from 4135 to 8390 mg/ml. Also in the above-cited research, in agreement with the present results, G. affinis has been found less susceptible to EO-based treatments, if compared to A. bouvieri, D. indicus and P. reticulata (Govindarajan et al. 2016c). Besides size differences, enzymatic assays to shed light on the reasons at the basis of this differential susceptibility are required. Furthermore, Govindarajan et al. (2016b) reported that the Zingiber nimmonii EO was safer towards D. indicus and G. affinis, with LC50 of 3241.53 and 9250.12 μg/ml, respectively. Pavela and Govindarajan (2016) showed that Z. monophyllum EO and its main constituents germacrene D-4-ol and a-cadinol tested on G. affinis had LC50 of 4234, 414 and 635 μg/ml, respectively. H. sprengelianum EO and its two major compounds lavandulyl acetate and bicyclogermacrene tested on A. bouvieri, D. indicus and G. affinis led to LC50 ranging from 414 to 4219 μg/ml. The S. zeylanicum EO (LC50 = 20,374 μg/ml), β-elemene (LC50 = 2073 μg/ml), and α-humulene (LC50 = 1024 μg/ml) from Syzygium zeylanicum are scarcely toxic towards G. affinis (Govindarajan and Benelli 2016c). Taken together, the data reported above underline the eco-friendly features of EO-borne molecules used as mosquito larvicides, allowing us to claim their potential employ as larvicides in urban and rural areas, with special reference to developing countries where mosquito-borne diseases are endemic and people should synergize different control tools in the fight against mosquitoes (see also Benelli 2015a).
Conclusions
Overall, the present research showed the toxicity of B. eriantha EO on six important mosquito vectors. Besides the effective larvicidal potential of the B. eriantha EO, which led to LC50 values lower than 50 ppm for most of the tested mosquitoes (Pavela 2015), is extremely noteworthy the toxicity of two main EO components, (4E,6Z)-allo-ocimene and carvotanacetone, which achieved LC50 lower than 10 μg/ml on all tested mosquito species, including two aedine vectors of Zika virus. Therefore, the extremely high larvicidal activity of (4E,6Z)-allo-ocimene and carvotanacetone far exceed most of the LC50 calculated in current literature on botanical mosquito larvicides, coupled with their eco-friendly features on non-target aquatic predators of mosquito larval instars, allowing us to propose both of them as potentially alternatives for developing eco-friendly mosquito control tools.
References
Adams RP (2007) 4th Ed. Carol Stream, Illinois: Allured Publishing Corporation; Identification of essential oil components by gas chromatography/mass spectroscopy
Alkofahi A, Rupprecht JK, Anderson JE, Mclaughlin JL, Mikolajczak KL, Scott BA (1989) Search for new pesticides from higher plants. In: Arnason JT, Philogene BJR, Morand P (eds) Insecticides of Plant Origin. American Chemical Society, Washington, DC, pp 25–43
AlShebly MM, AlQahtani FS, Govindarajan M, Gopinath K, Vijayan P, Benelli G (2017) Toxicity of ar-curcumene and epi-β-bisabolol from Hedychium larsenii (Zingiberaceae) malaria, chikungunya and St. Louis encephalitis mosquito vectors. Ecotoxicol Environ Saf 137:149–157
Amer A, Mehlhorn H (2006a) Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol Res 99:478–490
Amer A, Mehlhorn H (2006b) The sensilla of Aedes and Anopheles mosquitoes and their importance in repellency. Parasitol Res 99:491–499
Amer A, Mehlhorn H (2006c) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99:466–472
Amer A, Mehlhorn H (2006d) Persistency of larvicidal effects of plant oil extracts under different storage conditions. Parasitol Res 99:473–477
Baranitharan M, Dhansekaran S, Murugan K, Kovendan K, Gokulakrishnan J (2016) Chemical composition and laboratory investigation of Melissa officinalis essential oil against human malarial vector mosquito, Anopheles stephensi L. (Diptera: Culicidae). J Coast Life Med 4(12):969–973
Baranitharan M, Dhanasekaran S, Kovendan K, Murugan K, Gokulakrishnan J, Benelli G (2017) Coleus aromaticus leaf extract fractions: a source of novel ovicides, larvicides and repellents against Anopheles, Aedes and Culex mosquito vectors? Process Saf Environ Prot 106:23–33
Becker N (2008) Influence of climate change on mosquito development and mosquito-borne diseases in Europe. Parasitol Res 103(Suppl 1):19–28
Becker N, Geier M, Balczun C, Bradersen U, Huber K, Kiel E, Krueger A, Luehken R, Orendt C, Plenge-Boenig A, Rose A, Schaub GA, Tannich E (2013) Repeated introduction of Aedes albopictus into Germany, July to. October 2012. Parasitol Res 112:1787–1790
Benedict MQ, Levine RS, Hawley WA, Lounibos LP (2007) Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic Dis 7:76–85
Benelli G (2015a) Research in mosquito control: current challenges for a brighter future. Parasitol Res 114:2801–2805
Benelli G (2015b) Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol Res 114:3201–3212
Benelli G (2016a) Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res 115:23–34
Benelli G (2016b) Green synthesized nanoparticles in the fight against mosquito-borne diseases and cancer – a brief review. Enzym Microb Technol 95:58–68
Benelli G (2017) Commentary: Data analysis in bionanoscience – issues to watch for. J Clust Sci. doi:10.1007/s10876-016-1143-3
Benelli G, Govindarajan M (2017) Green-synthesized mosquito oviposition attractants and ovicides: towards a nanoparticle-based "lure and kill" approach? J Clust Sci. doi:10.1007/s10876-016-1088-6
Benelli G, Mehlhorn H (2016) Declining malaria, rising dengue and Zika virus: insights for mosquito vector control. Parasitol Res 115:1747–1754
Benelli G, Lo Iacono A, Canale A, Mehlhorn H (2016) Mosquito vectors and the spread of cancer: an overlooked connection? Parasitol Res 115:2131–2137
Benelli G, Pavela R, Maggi F, Petrelli R, Nicoletti M (2017a) Commentary: Making green pesticides greener? The potential of plant products for nanosynthesis and pest control. J Clust Sci. doi:10.1007/s10876-016-1131-7
Benelli G, Pavela R, Iannarelli R, Petrelli R, Cappellacci L, Cianfaglione K, Afshar FH, Nicoletti M, Canale A, Maggi F (2017b) Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind Crop Prod 96:186–195
Benelli G, Rajeswary M, Govindarajan M (2017c) Towards green oviposition deterrents? Effectiveness of Syzygium lanceolatum (Myrtaceae) essential oil against six mosquito vectors and impact on four aquatic biological control agents. Environ Sci Poll Res. doi:10.1007/s11356-016-8146-3
Benelli G, Pavela R, Canale A, Cianfaglione K, Ciaschetti G, Conti F, Nicoletti M, Senthil-Nathan S, Mehlhorn H, Maggi F (2017d) Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: synergistic and antagonistic effects. Parasitol Int. doi:10.1016/j.parint.2017.01.012
Bhuiyan MNI, Chowdhury JU, Begum J (2009) Chemical components in volatile oil from Blumea balsamifera(L.) DC. Bangladesh J Botany 38:107–109
Carolina A, Maman M (2016) Larvicidal activity of essential oils from the leaves and fruits of nutmeg (Myristica fragrans Houtt) against Aedes aegypti (Diptera: Culicidae) Turkish. J Agric Food Sci Technol 4(7):552–556
Cheng SS, Chua MT, Chang EH, Huang CG, Chen WJ, Chang ST (2009) Variations in insecticidal activity and chemical composition of leaf essential oils from Cryptomeria japonica at different ages. Biores Technol 100:465–470
Deo PG, Hasan SB, Majumdar SK (1988) Toxicity and suitability of some insecticides for household use. Int Pest Control 30:118–129
Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, Nicoletti M, Jiang W, Benelli G, Chandramohan B, Suresh U (2015) Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol Res 114:1519–1529
Dung NX, Loi DT, Hung DT, Leclercq PA (1991) Chemical composition of the oil of Blumea lanceolaria (Roxb.) Druce from Vietnam. J Essent Oil Res 3:285–286
El Ouali LA, El-Akhal F, Maniar S, Ez Zoubi Y, Taghzouti K (2016) Chemical Constituents and larvicidal activity of Essential Oil of Lavandula Stoechas (Lamiaceae) from Morocco against the malaria vector Anopheles Labranchiae (Diptera: Culicidae). J Pharmacogn Phytochem Res 8(3):505–511
El-Akhal F, El Ouali LA, Ez Zoubi Y, Greche H, Guemmouh R (2014) Chemical composition and larvicidal activity of essential oil of Origanum majorana (Lamiaceae) cultivated in Morocco against Culex pipiens (Diptera: Culicidae). Asian Pac J Trop Biomed 4(9):746–750
Elango G, Rahuman AA, Bagavan A, Kamaraj C, Zahir AA, Venkatesan C (2009) Laboratory study on larvicidal activity of indigenous plant extracts against Anopheles subpictus and Culex tritaeniorhynchus. Parasitol Res 104:1381–1388
Finney DJ (1971) Probit analysis. Cambridge University Press, London, pp 68–72
Govindarajan M (2010) Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) against three mosquito species. Asian Pac J Trop Med 3:874–877
Govindarajan M, Benelli G (2016a) Artemisia absinthium-borne compounds as novel larvicides: effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol Res 115(12):4649–4661
Govindarajan M, Benelli G (2016b) Eco-friendly larvicides from Indian plants: effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotox Environ Saf 133:395–402
Govindarajan M, Benelli G (2016c) α-humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol Res 115:2771–2778
Govindarajan M, Benelli G (2016d) Facile biosynthesis of silver nanoparticles using Barleria cristata: mosquitocidal potential and biotoxicity on three non-target aquatic organisms. Parasitol Res 115:925–935
Govindarajan M, Sivakumar R, Rajeswari M, Yogalakshmi K (2013a) Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.) against Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (Diptera: Culicidae). Exp Parasitol 34:7–11
Govindarajan M, Sivakumar R, Rajeswary M, Veerakumar K (2013b) Mosquito larvicidal activity of thymol from essential oil of Coleus aromaticus Benth. against Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (Diptera: Culicidae). Parasitol Res 112:3713–3721
Govindarajan M, Rajeswary M, Hoti SL, Bhattacharyya A, Benelli G (2016a) Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol Res 115:807–815
Govindarajan M, Rajeswary M, Arivoli S, Samuel T, Benelli G (2016b) Larvicidal and repellent potential of Zingiber nimmonii (J. Graham) Dalzell (Zingiberaceae) essential oil: an eco-friendly tool against malaria, dengue and lymphatic filariasis mosquito vectors? Parasitol Res 115:1807–1816
Govindarajan M, Rajeswary M, Benelli G (2016c) Chemical composition, toxicity and effects on non-target organisms of Pinus kesiya essential oil: an eco-friendly larvicide against mosquito vectors. Ecotox Environ Saf 129:85–90
Govindarajan M, Shine K, Naiyf S, Alharbi, Benelli G (2016d) Acute toxicity and repellent activity of the Origanum scabrum Boiss. & Heldr. (Lamiaceae) essential oil against four mosquito vectors of public health importance and its biosafety on non-target aquatic organisms. Environ Sci Pollut Res 23:23228–23238
Govindarajan M, Rajeswary M, Benelli G (2016e) δ-Cadinene, calarene and δ-4-carene from Kadsura heteroclita essential oil as novel larvicides against malaria, dengue and filariasis mosquitoes. Comb Chem High Throughput Screen 19:565–571
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391
Jahan K, Sukalyan Kumar K, Abdul Bake MD (2014) Evaluation of antimicrobial and cytotoxic activities of the methanolic and petroleum ether extract of Blumea lacera Burm.f in Bangladesh. J Pharmacogn Phytochem 2(6):104–108
Jayaraman M, Senthilkumar A, Adaikala Raj G, Venkatesalu V (2015) Isolation of mosquito larvicidal molecule from the leaves of Clausena anisata. J Exp Sci 6:12–16
Khair A, Ibrahim M, Ahsan Q, Homa Z, Kuddus MR, Rashid RB, Rashid MA (2014) Pharmacological activities of Blumea lacera (Burm. f) DC: a medicinal plant of Bangladesh. Br J Pharm Res 4:1677–1687
Khare CP (2007) Indian medicinal plants—an illustrated dictionary. Springer Science Business Media, LLC. (eds.) 233 Spring Street, New York, NY 10013, USA
Kumar S, Mishra M, Wahab N, Warikoo R (2014) Larvicidal, repellent, and irritant potential of the seed-derived essential oil of Apium graveolens against dengue vector, Aedes aegypti L. (Diptera: Culicidae). Front Public Health 18:147
Laakso I, Seppanen-Laakso T, Hiltunen R, Ekundayo O (1989) Composition of the essential oil of Blumea lacera DC. (Asteraceae) leaves from Nigeria. Flav Frag J 4:73–75
Liang Z, Ying-Juan T, Li Y, Jian-Gou J (2011) Chemical composition and antimicrobial activities of essential oil of Blumea megacephala. EXCLI J 10:62–68
Macêdo ME, Rotraut Consoli AGB, Telma Grandi SM, Anjos AMG, Oliveira AB, Mendes NM, Queiróz RO, Zani CL (1997) Screening of Asteraceae (Compositae) plant extracts for larvicidal activity against Aedes fluviatilis (Diptera: Culicidae). Mem Inst Oswaldo Cruz, Rio de Janeiro 92(4):565–570
Mehlhorn H (ed) (2015) Encyclopedia of parasitology, 4th edn. Springer, New York
Mehta SC, Vardhan H, Saxena SP (1986) Some pharmacological actions of the essential oil of Blumea membranacea. Indian J Physiol Pharmacol 30:149–154
Murugan K, Panneerselvam C, Samidoss CM, Madhiyazhagan P, Suresh U, Roni M, Chandramohan B, Subramaniam J, Dinesh D, Rajaganesh R, Paulpandi M, Wei H, Aziz AT, Saleh Alsalhi M, Devanesan S, Nicoletti M, Pavela R, Canale A, Benelli G (2016a) In vivo and in vitro effectiveness of Azadirachta indica-synthesized silver nanocrystals against Plasmodium berghei and plasmodium falciparum, and their potential against malaria mosquitoes. Res Vet Sci 106:14–22
Murugan K, Aruna P, Panneerselvam C, Madhiyazhagan P, Paulpandi M, Subramaniam J, Rajaganesh R, Wei H, Alsalhi MS, Devanesan S, Nicoletti M, Syuhei B, Canale A, Benelli G (2016b) Fighting arboviral diseases: low toxicity on mammalian cells, dengue growth inhibition (in vitro) and mosquitocidal activity of Centroceras clavulatum-synthesized silver nanoparticles. Parasitol Res 115:651–662
Mwangi JW, Achola KJ, Lwande W, Hassanali A, Laurent R (1994) Constituents of the essential oil of Blumea brevipes (Oliv. and Hiern) Willd. Flav Frag J 9:233–235
Naqqash MN, Gökçe A, Bakhsh A, Salim M (2016) Insecticide resistance and its molecular basis in urban insect pests. Parasitol Res 115:1363–1373
Norikura T, Kojima-Yuasa A, Shimizu M, Huang X, Xu S, Kametani S, Rho S, Kennedy DO, Matsui-Yuasa I (2008). Mechanism of growth inhibitory effect of Blumea balsamifera extract in Hepatocellular Carcinoma. Biosci Biotechnol Biochem 72:1183–1189
Owolabi MS, Lajideh L, Villanueva HE, Setzer WN (2010) Essential oil composition and insecticidal activity of Blumea perrottetiana growing in southwestern Nigeria. Nat Prod Comm 3:1135–1138
Panneerselvam C, Murugan K, Roni M, Aziz AT, Suresh U, Rajaganesh R, Madhiyazhagan P, Subramaniam J, Dinesh D, Nicoletti M, Higuchi A, Alarfaj AA, Munusamy MA, Kumar S, Desneux N, Benelli G (2016) Fern-synthesized nanoparticles in the fight against malaria: LC/MS analysis of Pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitol Res 115:997–1013
Pavela R (2008) Larvicidal activities of some Euro-Asiatic plants against Culex quinquefasciatus Say (Diptera: Culicidae). J Biopest 1:81–85
Pavela R (2015) Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind Crop Prod 76:174–187
Pavela R, Benelli G (2016a) Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors—a review. Exp Parasitol 167:103–108
Pavela R, Benelli G (2016b) Essential oils as eco-friendly biopesticides? Challenges and constraints. Tr Plant Sci 21(12):1000–1007
Pavela R, Govindarajan M (2016) The essential oil from Zanthoxylum monophyllum a potential mosquito larvicide with low toxicity to the non-target fish Gambusia affinis. J Pest Sci. doi:10.1007/s10340-016-0763-6
Pavela R, Vrchotova N, Triska J (2009) Mosquitocidal activities of thyme oils (Thymus vulgaris L.) against Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 105:1365–1370
Pednekar PP, Vakil BV, Sane RT, Datar AG (2012) Antimicrobial activity of essential oil of Blumea eriantha DC against skin pathogens. Int J Pharm Pharm Sci 4:296–299
Pratheeba T, Prabhavathi O, Yuvarajan R, Murugan N, Natarajan D (2015) Identification of mosquitocidal compounds from the leaf extracts of Ocimum gratissimum (lamiaceae) against dengue and chikungunya vector Aedes aegypti (L.). Int J Entomol Res 3:67–79
Sakee U, Maneerat S, Cushnie TP, De-Eknamkul W (2011) Antimicrobial activity of Blumea balsamifera (Lin.) DC. extracts and essential oil. Nat Prod Res 25:1849–1856
Santi E, Simone (2014) Evaluating the toxicity of oil of lemon eucalyptus, Corymbia citriodora (Hook.), against larvae of the Asian tiger mosquito and non-target fish and larval amphibians. Ann Biologia 36:97–105
Senthilkumar A, Kannathasan K, Venkatesalu V (2008) Chemical constituents and Larvicidal property of the essential oil of Blumea mollis (D. Don) Merr. against Culex quinquefasciatus. Parasitol Res 103:959–962
Singh UP, Singh AK, Sarathy RP (2011) Effect of methanolic extracts of Blumea eriantha DC dc leaves on protein metabolism and marker enzymes in streptozotocin induced hyperglycemic animals. Int J of Pharm Sci 4(1):235–238
Sivagnaname N, Kalyanasundaram M (2004) Laboratory evaluation of methanolic extract of Atlantia monophylla (Family: Rutaceae) against immature stages of mosquitoes and non-target organisms. Mem Inst Oswaldo Cruz 99:115–118
Subramaniam J, Murugan K, Panneerselvam C, Kovendan K, Madhiyazhagan P, Kumar PM, Dinesh D, Chandramohan B, Suresh U, Nicoletti M, Higuchi A, Hwang JS, Kumar S, Alarfaj AA, Munusamy MA, Messing RH, Benelli G (2015) Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: towards an integrative approach? Environ Sci Pollut Res Int 22:20067–20083
Subramaniam J, Murugan K, Panneerselvam C, Kovendan K, Madhiyazhagan P, Dinesh D, Mahesh Kumar P, Chandramohan B, Suresh U, Rajaganesh R, Saleh Alsalhi M, Devanesan S, Nicoletti M, Canale A, Benelli G (2016) Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles: high antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatus predators. Environ Sci Poll Res 23:7543–7558
Veni T, Pushpanathan T, Mohanraj J (2016) Ovicidal and larvicidal efficacy of Crataeva magna (lour.) dc. (Family: Capparidaceae) against the Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Int J Pure Appl Zool 4:149–154
Verdian-Rizi M (2009) Chemical composition and Larvicidal activity of the essential oil of Laurus nobilis L. from Iran. Iran J Pharm Sci 5:47–50
Wang SY, Lai WC, Chu FH, Lin CT, Shen SY, Chang ST (2006) Essential oil from the leaves of Cryptomeria japonica acts as a silverfish (Lepisma saccharina) repellent and insecticide. J Wood Sci 52:522–526
World Health Organization (2005) Guidelines for laboratory and field testing of mosquito larvicides. Communicable disease control, prevention and eradication, WHO pesticide evaluation scheme. WHO, Geneva, WHO/CDS/WHOPES/GCDPP/1.3.
Yakob L, Walker T (2016) Zika virus outbreak in the Americas: the need for novel mosquito control methods. Lancet Glob Health S2214-109X(16)00048–6
Zhu L, Tian Y (2011) Chemical composition and larvicidal activity of Blumea densiflora essential oils against Anopheles anthropophagus: a malarial vector mosquito. Parasitol Res 109:1417–1422
Acknowledgements
We are grateful to H. Mehlhorn and the anonymous reviewers for their useful suggestions on an earlier version of our study. The authors extend their sincere appreciations to the Deanship of Scientific Research at King Saud University for funding this Prolific Research Group (PRG-1437-36). The authors would like to thank Professor and Head, Department of Zoology, Annamalai University, for the laboratory facilities provided.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Benelli, G., Govindarajan, M., Rajeswary, M. et al. Larvicidal activity of Blumea eriantha essential oil and its components against six mosquito species, including Zika virus vectors: the promising potential of (4E,6Z)-allo-ocimene, carvotanacetone and dodecyl acetate. Parasitol Res 116, 1175–1188 (2017). https://doi.org/10.1007/s00436-017-5395-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5395-0