Abstract
Modification of the adsorbent surfaces has been considered a fascinating strategy that enhances biomass-based adsorption properties for efficient removal of organic pollutants. This is based on the attempt to replace the cost-ineffectiveness of the commercial activated carbon. The present study discusses different modification strategies and a review on modified biomass materials for the sorption of organic contaminants. Unlike previous literatures in the field, wider range of these pollutants are discussed in this study under different categories including pesticides (such as insecticides, herbicides, fungicides), pharmaceutical (e.g. analgesic and antipyretic drugs, antibiotic drugs, non-steroidal anti-inflammatory drugs and antimalaria drugs), and dyes (e.g. azo, xanthene, miscellaneous diagnostic, tri-aryl methane, and phenol-derived polymeric dyes). It was observed that the acid-activated Posidonia oceanica and HNO3-modified rice husk displayed the highest and lowest adsorption capacities of 2681.9 and 0.35 mg/g for removing Rhodamine B dye and methyl parathion pesticide, respectively. The mechanistic aspects of organic pollutants adsorption, their corresponding regeneration studies, and environmental challenges with chemical modifications are also discussed. The use of computational (optimization) models for modified biomass-based adsorbents to remove organic pollutants is devoid in previous reviews but discussed in the present study. To foster more advancement in this field, the concluding part presents various challenges and knowledge gaps for furthering research towards more realistic industrial implementations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
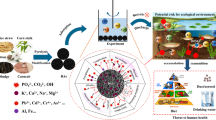
The advances in the industrial revolution and urbanization have resulted in day-to-day increase in manufacturing of chemical substances like pesticides, herbicides, pharmaceutical and personal care products, dyes, etc. with their respective ever rising and continuous demands for different applications (Philippot et al. 2015; Ahmad et al. 2019; Ibrahim et al. 2021; Adegoke et al. 2022c, a). Discharge of effluents from these manufacturing and applications industries has led to the discharge of hazardous compounds into the ecosystems (De Gisi et al. 2016; Bulgariu et al. 2019; Agboola and Bello 2020; Kassegne et al. 2020; Li et al. 2020; Ahmad et al. 2021c; Bello et al. 2021). The accumulation and movement of same into water bodies via erosion, drought, etc. have become a global threat into both human and aquatic habitat (Fig. 1) (Adegoke and Bello 2015; Ahmad et al. 2016; De Gisi et al. 2016; Singh et al. 2017; Thambiraj et al. 2018; Bulgariu et al. 2019; Adebusuyi et al. 2022). Pollutants pose adverse effects on the ecosystem, thereby making the sustainable environment sustenance/maintenance practically impossible (Bello et al. 2015b; Bellec et al. 2015; Wang et al. 2016; Bello et al. 2017a; Adegoke et al. 2017, 2019; Jain and Gogate 2017b; Yaseen and Scholz 2018). Water is important to life and it is a major component of humanity, and other aquatic systems; therefore, water pollution resulting from climate change, industrialization, urbanization, and population is projected to result in possible potable water scarcity in the nearest future (Afolabi et al. 2020a; Ahmad et al. 2021c, b).
However, the rise in applications and conversion of biomass-based wastes into products with added value such as adsorbents and catalysts for different applications has been witnessed in recent years. Based on this, numerous adsorbents have been produced from biomass-based origin as alternative for the cost-ineffective commercial activated carbon which are not sustainable (Bello et al. 2015a, 2019a; Pathania et al. 2017). Also, this approach remains a fascinating research hotspot since it possesses the benefit of enhancing environmental management system by preventing biomass wastes’ accumulation in the environment. This is because the accumulation of these wastes causes serious air, water, and soil pollutions. They become worse in rainy season, generate terrible odour and stink at the fall of rain. They are difficult to manage by environmentalists and usually end-up in streams, rivers and oceans thereby endangering the aquatic habitat. Burning these wastes even during dry season causes air pollution and the death of important microbes/biological systems (Bello et al. 2017d, 2020c, 2021; Ojedokun and Bello 2017a; Bello et al. 2019a, b, c; Yu et al. 2019; Afolabi et al. 2020b; Ahmad et al. 2021b, 2022). These abnormalities have inspired researchers worldwide to convert the biomass wastes into important products for water/wastewater treatment.
It has however been observed that raw adsorbents derived from biomass sources have poor adsorption capacity; therefore, various enhancing strategies have been employed to facilitate the sorption ability adsorbents for effective removal of contaminants (Khodaie et al. 2013; Fernandez et al. 2014; Utomo et al. 2015; Singh et al. 2017). One major fascinating strategy is surface modification of the adsorbent thereby resulting in both physical and chemical changes with structural enhancement needed for effective adsorption properties such as higher active sites, higher surface areas, enhanced particle and pores sizes, improved porosities, and pore volumes (Üner et al. 2017; Khasri et al. 2018; Sham and Notley 2018; Bello et al. 2019b, 2020a; Ahmad et al. 2020). For example, some recent studies conducted using modified biomass-based materials such as Dragon peel (Ahmad et al. 2021b), pod of Mangifera indica (Bello et al. 2021), lemon grass (Ahmad et al. 2019, 2021a, 2022; Putri et al. 2020), pomegranate fruit peel (Ahmad et al. 2020); leaves of Gmelina aborea (Bello et al. 2020a), husks of coconut (Bello et al. 2019a), Parkia biglobosa (Bello et al. 2019b), bean husk (Bello et al. 2017d), Moringa oleifera leaf (Bello et al. 2017b, c), guava leaf (Ojedokun and Bello 2017b), berry leaves (Ahmaruzzaman et al. 2015), Glossogyne tenuifolia leaves (Yang and Hong 2018), Ficus racemosa (Jain and Gogate 2017c, a), coconut leaves (Cazetta et al. 2011; Rashid et al. 2018), C. camphora leaves (Tang et al. 2017), leaves of plane trees (Gong et al. 2013), senescent plant leaves (Gunasekar and Ponnusami 2013), durian leaves (Hussin et al. 2015), and bamboo (Ghosh and Bandyopadhyay 2017), Prunus dulcis (Jain and Gogate 2017b) have been reported. They have reported how effective the strategy was for enhancing sorption properties of adsorbents for different organic pollutants removal. Thus, fabricating novel functionalized adsorbents for effective removal of different organic pollutants remain a global research hotspot.
Conversely, it should be noted that some studies have reported the utilization of numerous sorbents derived from clay, sand, nanoparticles, and other synthetic adsorbents. However, no study has presented the comprehensive information on the modified biomass-based adsorbent for organic pollutant removal focusing entirely on pesticides, e.g. organophosphate, oximino carbamate, atrazine and thioatrazine, etc.; insecticides, e.g. neoncotinoids, n-phenylurea, sulphometuron-methyl, etc.; fungicides, e.g. azoxystrobin; pharmaceutical pollutants, e.g. paracetamol (acetaminophen), ciprofloxacin, doxycycline, ibuprofen, lumefantrine, dicloxacillin, phenacetin, levofloxacin, hydrochlorothiazide, etc.; and dyes, e.g. Congo red, tartrazine, rhodamine B, methylene, phenol-derived polymeric dyes, indigo carmine, methyl red, acid green 3, azo dye, reactofix red 3BFN, direct blue 53, reactive red 4 dye, etc. discussed in this review. In addition, the mechanistic aspects of organic pollutants’ adsorption and corresponding regeneration studies are also discussed. Unlike existing reviews in the field, the present study presents the review of various computational (optimization) models for the adsorption of organic pollutants using modified biomass-based adsorbents, covering response surface methodology and artificial intelligence. The concluding part of the work presents various challenges, and knowledge gaps for furthering research in the field towards more realistic industrial implementations.
Routes of exposure to organic pollutants and their ecotoxicological effects
Toxicity means ability of a substance to cause harmful effects in living organisms depending on its dose, route of entry, frequency and duration of exposure, environmental factors, chemical combinations, and inter- and intraspecies variations. There are three major routes by which organic pollutants can enter into the body system of living organisms: inhalation, absorption (skin or eye), and ingestion (Vafeiadi et al. 2014). Inhalation is the most common route of entry of organic pollutants into living organisms. The inhaled and deposited organic pollutants are bioaccumulated in fatty tissues of living organisms and may cause various health effects. Absorption of organic pollutants through the skin (dermal) is also a route of entry into the body of living organisms. Once organic pollutants are absorbed in large quantities by the skin, they may produce systemic damage to internal organs. The eyes can also absorb organic pollutants and transport them to other parts of the body thereby causing harm to the organism. Ingestion of organic pollutants is also very common through the consumption of foods that are contaminated with organic pollutants (Kassegne et al. 2020). These organic pollutants are absorbed by the lining of the gastrointestinal tract and then transported to internal organs where they cause harmful effects. Several organic pollutants have endocrine disrupting properties (Schug et al. 2011), and many are considered to be carcinogenic in living organisms. Organic pollutants usually have different potencies, and this is further complicated by the fact that no environmental exposure to organic pollutants is by a single chemical but by a mixture of organic pollutants. Several environmental conditions can affect the toxicity of organic pollutants such as climatic condition, temperature, salinity, nutritional status, adaptation to climatic change, and pH.
Organic pollutants are chemical substances which possess enormous ability to adversely affect the endocrine system of living organisms, can be either synthetic or natural (Arthur et al. 2019). They are usually leached from agricultural lands and dump sites into water bodies via erosion (Kabir et al. 2015). These pollutants are also transported to soils around during infiltration and water percolation, which then seep into groundwater via water movement in due course. This means that environmental pollutants can enter into human body system via contaminated food, air, and water.
They tend to alter normal endocrine and coordination functions through their action on hormonal glands (Falconer et al. 2006; Jung et al. 2015; Wee and Aris 2017). The level of coordination of hormonal system is very high with a very high specificity such that slight alteration in their concentration and timing result in massive distortions in their physiological properties leading to adverse health effects (Bai et al. 2016; Tapia-Orozco et al. 2016; Tursi et al. 2018). This therefore has enormous adverse human health effects because they cause the impairment of soil and water bodies. This suggests that their effects are troublesome since the alteration in genetic programming during their development in the early stages pose serious and significant effects which may result in transgenerational inheritance diseases (Schug et al. 2011; Skinner 2011; Raja et al. 2022). Previous study has demonstrated an illustrative model suggesting that all endocrine systems are targeted by these organic pollutants and these organs including the hypothalamic pituitary adrenal axis, pancreas, thyroid gland, and reproductive organs are vulnerable to attack by these organic pollutants since they are known to influence brain functions and hormone-dependent metabolic systems (Raja et al. 2022). Based on these exposure routes to organic pollutants and the corresponding ecological effects in the ecosystem, there is a serious need to develop efficient methods and readily available materials for decontaminating these noxious pollutants and ensuring sustainable and safer environment.
Fabrication processes of activated carbon for removal of organic pollutants
The quest for the use of activated carbon for adsorption of pollutants have been attributed to their desirable properties such as having microporous and homogenous structure in addition to their relatively high surface area as well as abrasion and very low cost of production in comparison to commercial activated carbon. However, characteristics of an activated carbon are reliant on chemical nature of the precursor and the fabrication process, which comprises two stages of carbonization and activation. The carbonization stage is a pyrolysis process that leads to thermal decomposition of the biomass-based material at temperature below 800 °C in the presence of minimal or no oxygen. This leads to the breaking down of lignocellulose structure of the biomass-based material and production of charcoal (Adegoke et al. 2022b).
The activation stage is required subsequent to the carbonization stage in order to copiously develop the pore structure that enhances activated carbon’s surface area, which expedite the capture and retention of substances. The activation stage is performed using either chemical or physical treatment method which leads to the generation of activated carbon (Adegoke et al. 2022b). These various treatment methods during their preparation are responsible for the alteration of shape and size of leaves used as adsorbent (Adegoke and Bello 2015; Yahya et al. 2015). Treatment process by physical means takes place mainly through activation and carbonization. The precursors must first be carbonized before activation using carbon (IV) oxide (CO2) or steam. Whereas, in chemical treatments; precursor materials are first of all impregnated by “activating agent” prior to heating in an inert atmosphere (Yahya et al. 2015). Dissolution of cellulosic constituents present in leaves are achieved by activating agents thereby enhancing the formation of crosslinks (Adegoke and Bello 2015; Yahya et al. 2015). In comparison, advantages of chemical activation over physical activation process are numerous and these include high production yield, ability to generate well formed microporous sites, high surface area, low temperatures, involvement of only one step (González-García et al. 2013; Adegoke and Bello 2015; Yahya et al. 2015), and mineral matter contents reduction that is higher (Bello et al. 2017b). Nevertheless, some shortcomings of chemical method of activation such as necessities to wash the activated/carbonized adsorbents to get rid of impurities that are associated with activation agents and corrosive nature of activating reagents still exist (Adegoke and Bello 2015; Yahya et al. 2015).
Preparation techniques for adsorbents through functionalization/modification
Among the several modification agents available, H3PO4 and ZnCl2 are most widely utilized for activation of lignocellulosic adsorbents (González-García 2018). By comparison, many authors have shown H2SO3, H3PO4, citric acid, and NaOH (Hussin et al. 2015; Makeswari et al. 2016; Ghosh and Bandyopadhyay 2017; Jain and Gogate 2017a, 2017c; Tang et al. 2017; Khan et al. 2018) preference to ZnCl2 for modification of leaf because they are eco-friendly in nature. They have the ability to produce mesopores with high volume and diameter, and high surface areas (Adegoke and Bello 2015; Bello et al. 2017b; Ojedokun and Bello 2017b). Also, activated carbon produced are usually suitable for used in food and pharmaceutical companies (Yahya et al. 2015). Therefore, the use of H3PO4 as modification agent offers advantages such as (i) easy recovery of carbon product during processing, (ii) high amount of activated carbon is produced, and (iii) does not pose any toxic effect (Adegoke and Bello 2015; Yahya et al. 2015; Bello et al. 2017b; Ojedokun and Bello 2017b).
The mechanism of reaction for each activating reagent is different, for example, ZnCl2 enhances extraction of H2O molecules from lignocellulosic materials’ structures while H3PO4 chemically associates within lignocellulosic materials (Yahya et al. 2015). Surface chemistry modification of leaves is an emerging, efficient, and promising method for applications of carbon in many fields. Modification of leaves involves oxidation and subsequent grafting onto leaf surfaces new molecule(s) such as cyclodextrin and functional group(s) such as –NH2 and -COOH. Various modification methods of achieving oxidation are by acid, alkaline, ozone, microwave, and plasma. Commonly used modification methods are briefly discussed as follows:
Functionalization/modification by acidic methods
Acidic modification methods are majorly employed for oxidizing the porous carbon surfaces in order to increase the acidic properties thereby removing the excess mineral substances and also enhance the hydrophilic ability of the adsorbent surfaces (Table 1) (Shen et al. 2010). In recent years, nitric and sulphuric acids have been the most used acids though many others were used for modifying leaf adsorbents (Jawad et al. 2016; Jain and Gogate 2017b; Khan et al. 2018). These authors have established that utilization of acid functional group(s) existed on the surface of carbon upon modification for efficient dye removal from wastewater and aqueous solutions due to their oxygen-functional group containing-proton-donors.
The effect of acidic modification of bamboo leaves using citric acid was investigated for methylene blue adsorption in aqueous solution (Ghosh and Bandyopadhyay 2017). They tested seven isotherm models and Temkin isotherm gave the best description for the adsorption process while pseudo-second-order kinetic model best described the adsorption. Highest percentage removal achieved was 99.97%, with adsorption capacity of 725 mg/g. Bello et al. reported H3PO4-modified Moringa oleifera leaf for adsorptive removal of reactive blue (RB) dye (Bello et al. 2017c). They observed that the adsorption process of RB red dye was best described by Langmuir and pseudo-second-order models with intraparticle diffusion observed as adsorption process. They also observed that the adsorption means energy evaluated from Dubinin–Radushkevich isotherms confirmed the involvements of physical adsorption having a capacity of 934.4 mg/g. Many other authors also reported remarkable adsorption capacities with acid treatment (Oyelude 2015; Ghosh and Bandyopadhyay 2017; Ojedokun and Bello 2017b; Yang and Hong 2018; Mahmoudi et al. 2020). They discussed the different dye-uptake capacities attained from modified leaf samples in relation to surface chemical property of each adsorbent. They observed that anionic dyes on the prepared adsorbent samples by thermal treatment under hydrogen flow at elevated temperatures were favoured on considering their dispersive and/or electrostatic interaction.
Critical observation from literature established that acidic modification of leaf activated carbons (ACs) enhanced the adsorption of dyes onto the leaves-modified counterpart owing to a significant surface chemistry change. This is because acidic modifications have affinity to remove OH group(s), thereby producing the O2-containing functional group(s), as the amounts of these functional groups have a close relationship with the ability of leaf ACs to remove dye compounds. Yang et al. (2016) used acid-treated vermicompost-derived adsorbent for the removal of congo red and methylene blue dyes from aqueous solution and observed similar findings. Xing et al. (2007) prepared active solid from carbon materials via sulphonation and they reported an enhanced material due to high steam-to-carbon ratio attributed to the acid treatment. This was further confirmed with FTIR results that indicated sulphonated groups at 1032 cm−1 (Xing et al. 2007) and ether groups at 1151 cm−1 (Lai et al. 2010) in similar experiments. N2 isotherm curves indicated microporosity due to acid treatment (Güzel et al. 2017).
Functionalization/modification by alkaline (basic) methods
Alkaline (basic) modifications of leaf ACs produce positive-charge surfaces that help adsorb the significant amounts of negatively charged species. The porous carbons having the basic surface characters/properties can be achieved by treating the leaf materials in inert hydrogen or ammonia atmosphere at higher temperature such as 400–900 °C (treatment of leaves with NH3) or at 400–600 °C (treatment with amide, aromatic amines or protonated amides) leading to the creation of basic formations with sufficient nitrogen functionalities (Faria et al. 2004). It is noteworthy here that adsorbents with nitrogen-containing functional groups are endowed with basic properties having the ability to trigger the enhancement of porous carbons and acid molecular interaction, e.g. hydrogen bonding, dipole–dipole interaction and/or covalent bonding. Interestingly, it is highly expected that the hydroxyl ions (OH−) have a strong affinity to react with leaf ACs surface functional groups under a basic condition thus making this modification treatments advantageous to enhance the adsorption of dyes. Basic modifications on leaves can be achieved through nitric acid treatment, partial gasification of oxygen, urea impregnations followed by pyrolysis. Generally, one can easily conclude that OH− ions are expected to react with the leaf surface functional group under alkaline modification thereby leading to the abundance creation of positive charges on leaf surfaces of AC which is favourable to enhance the negative-charged species from H2O.
Zheng et al. (2013) modified activated carbon using NaOH and reported that there was increase in pore volume concentration and surface area of the adsorbent and thus improved adsorption of organic pollutants. However, using similar chemical, Sadaf and Bhatti (2016) reported that organic pollutants, such as anionic dyes, might not be effectively removed by alkaline-modified adsorbent because of the perceived surface deprotonation functionality due to –OH and –COOH that could cause an electrostatic repulsion of these dyes.
Hayati and Mahmoodi (2012) examined utilization of NaOH in surface modification of activated carbon and subsequent use of the prepared modified activated carbon in the removal of dyes. Their results showed that NaOH was effective for the surface modification. Compared to ordinary activated carbon surface, their results indicated higher maximum adsorption capacity of 9.17 and 11.77 mg/g onto the surface-modified activated carbon for Acid Red 14 (AR14) and Acid Blue-92 (AB92), respectively.
Functionalization/modification by impregnation methods
The phenomenon called impregnation is defined as fine, even or uniform distribution of chemical reagents and particles in the pores of leaf ACs. ACs from leaves can be impregnated with metal compounds from silver, copper, aluminium, iron, etc. due to their adsorption capacities that is significantly high. It should be noted that effect of impregnation ratio must be considered. This is the weight ratio of the activation agents to that of the precursor. This ratio is the most important factor in the activation process by chemical method because, with the increasing ratio, the leaf adsorbent surface area is expected to increase. Bello et al. impregnated Moringa oleifera leaf with NaOH and H2SO4 and used different characterization techniques to establish the effects of modification by impregnation. The study concluded that impregnated Moringa oleifera leaf could be employed for dyes recovery in lieu of commercial activated carbon (Bello et al. 2017b).
Magnetic modification
Aryee et al. (2020) prepared magnetic-modified biomass for dye removal using co-precipitation and yielded a crystalline mesoporous material that possess superparamagnetic characteristics. The results obtained show improved sorption capacity of 32.5 mg/g by the modified biomass towards methylene blue. Safarik and Safarikova (2010) observed a monolayer adsorption in decontamination of different dye types using a magnetic-modified adsorbent via Langmuir model. Chen et al. (2011) carried out biomass pyrolysis with FeCl3 and FeCl2 to derive a magnetic material that could be applied in the separation of solid–liquid mixtures. They prepared the magnetic biochar via chemical co-precipitation of iron hydroxides on orange peel powder and subsequent pyrolysis. Orange peel was used as material and magnetite as magnetic medium. They observed that the novel magnetic biochar derived through their modification exhibited an enhanced adsorption capacity (several times) for phosphate and organic contaminants than untreated biomass. Hence, they indicated that magnetic-modified adsorbents could be an extraordinary sorbent for contaminants removal. The synthesis of magnetic-modified biomass has been carried out by scientists using different kinds of methods with various biomass and magnetic medium.
Functionalization/modification by microwave methods
Modification of leaf ACs via microwave radiation has been given wide popularity nowadays owing to its heating capacity at molecular levels which results in quick thermal and homogenous reactions (Khan et al. 2018). Unlike conventional heating methods, microwave heating/irradiations offer significant advantages: (i) microwave energy neatly heats the leaf materials from inside out, (ii) provision of rapid heating by microwave energy, (iii) no need of heating convection through fluids, (iv) process of heating can be controlled easily, (v) direct contacts between the microwave heating sources and the heated leaf materials cannot occur, (vi) capable of been operated at higher temperatures, (vii) it saves energy and time, (viii) increasing chemical reactivities, (ix) cost-effective (x) systems processing are also moderately portable, compact and easy to maintain (Khan et al. 2018). For instance Khan et al. (2018) used spent black tea leaves prepared using microwave-assisted method as effective low-costs and green adsorbent for adsorption of dye (Khan et al. 2018). Their results revealed that Langmuir model best described the adsorption with adsorption capacity of 242.72 mg/g at 25 °C.
Performance of different modified biomass-derived adsorbents for removing organic pollutants
There has been series of scientific reports on application of modified biomass-derived adsorbent for removing organic and emerging contaminants such as dyes, pesticides, and pharmaceuticals from water/wastewater as discussed below. The corresponding adsorptive removal process (Fig. 2) and performances at different operating conditions, kinetics, isotherms and their mechanisms are summarized in Table 2.
Modified biomass-derived adsorbents for pesticides removal
Insecticides
The biomass-derived adsorbent from spent tea leaves has been reportedly used in removing organophosphate pesticides such as dimethoate and chlorpyrifos in a concentration array of 20 to 100 mg/L. The spent leaves derived biomass adsorbent displayed a mean percentage removal of 42.11 and 72.98% for dimethoate and chlorpyrifos pesticides, respectively (Beheary et al. 2018). Furthermore, some studies about the feasibility of modified rice bran, rice husk, Moringa oleifera pod, and sugar can bagasse for removing organophosphate pesticides from aqueous solution at varied operating adsorptive conditions have been reported. This involves modification of these biomasses with HNO3 and the utilization of the resulting adsorbents for removing methyl parathion. These adsorbents demonstrated adsorption capacity of 28 m2/g (rice bran), 17 m2/g (rice husk), 27 m2/g (Moringa oleifera pod) and 25 m2/g (sugar cane bagasse) for methyl parathion removal (Akhtar et al. 2007; Ahmad et al. 2010). The recounted adsorption percentage removal and optimum adsorption capacity at optimum experimental conditions for removing methyl parathion were 96.31% and 0.196 mg/g, respectively. Langmuir model gave the best description of the adsorption isotherm with respective maximum adsorption capacities of 0.39 mg/g (rice bran), 0.35 mg/g (rice husk), 0.39 mg/g (Moringa oleifera pod) and 0.36 mg/g (sugar cane bagasse) (Akhtar et al. 2007; Ahmad et al. 2010). Application of the same adsorbents for the adsorption of methyl parathion from wastewater samples gave percentage removal of 99, 97, 98 and 99% for rice bran, rice husk, Moringa oleifera pod and sugar cane bagasse, respectively. The studies on thermodynamics displayed an exothermic, spontaneous, and reaction that was feasible with a low entropy. On the other hand, the adsorption energy deduced from Dubinin–Radushkevich model ranging from 10.1 to 11.6 kJ/mol, which indicates a chemical adsorption mechanism (Akhtar et al. 2007).
More recently, adsorption of chloropyrifos, dimethoate, and malathion were studied by Jocić and co-workers on viscose fibre-derived activated carbon. In the study, dimethoate gave the least adsorption efficiency followed by malathion, while chlorpyrifos gave the most efficient adsorption. The material properties were observed to correlate with the uptake of these organophosphorus pesticides. It was concluded that activated carbon fibre was optimally efficient for the adsorptive removal of chlorpyrifos, with 240 mg/g as experimentally observed adsorption capacitances (Jocić et al. 2022).
The modification of Ricinodendron heudelotii shells (akpi shells) with ortho-phosphoric acid to prepare microporous activated carbon with enormous surface area of 11,179 m2/g has been reported. The use of ortho-phosphoric acid to modify the akpi shell denotes a positive influence in adsorption properties for effective adsorptive removal of neoncotinoids such as imidacloprid from an aqueous solution (Urbain et al. 2017). A maximum imidacloprid adsorption removal was reported to be 90% while the adsorption capacity of 43.48 mg/g was observed. Langmuir isotherm model fitted well with R2 value of 0.990 and pseudo-second-order model with R2 of 0.998 with a chemisorption mechanism approach (Urbain et al. 2017).
The biomass-derived from spent tea leaves has been reportedly used in removing imidacloprid in a concentration array of 20 to 100 mg/L, demonstrating a mean percentage removal of 93.01% (Beheary et al. 2018). More recently, corn stalk and ZIF-67 were used to prepare a novel magnetic porous carbon which was prepared via an in-suit process followed by pyrolysis at high-temperature and acid picking, and used for adsorptive removal of thiamethoxam and imidacloprid in aqueous solution (Yang et al. 2022). The prepared ZIF-67/CS@C exhibited efficient absorption capacities for thiamethoxam and imidacloprid in water with maximum adsorption capacities of 133 and 189 mg/g, respectively. The efficiencies of removal for thiamethoxam and imidacloprid reached peak values of 99.13 and 99.65%, respectively. Further probe into the mechanism of adsorption revealed that pore filling, H-bond, and π-π electron donor–acceptor interaction might be main driving forces for the adsorption by ZIF-67/CS@C, and the reasonable pore size distribution and high external surface area was attributed to why the ZIF-67/CS@C showed better adsorption performance.
The experimental study on the utilization of H3PO4 to modify silkworm faeces in preparing environmentally friendly activated carbon has reportedly yielded an adsorbent with desirable properties. These properties include respective mean pore diameter and specific surface area of 0.2035 and 75.219 cm2/g for the adsorption removal of oxamyl, which is regarded as an oximino carbamate pesticide (Mohammad and Ahmed 2017). Furthermore, it was suggested that the equilibrium removal of oxamyl on modified silkworm faeces in the adsorption process was attained at contact time of 120 min. Freundlich model with R2 of 0.9975 gives the best interpretation for the adsorption isotherm when compared with Langmuir and Temkin models with respective R2 of 0.9217 and 0.9163.
Salman and Hameed prepared banana stalk activated carbon for sorption of carbofuran insecticide from aqueous media at varied contact time, temperature, initial concentration, and pH. From the adsorption study, equilibrium sorption of carbofuran was observed to decrease slightly from 65.33 to 63.54 mg/g when the initial pH of the aqueous media was varied from 2 to 12. This was attributed to presence of excess H+ ions which was considered to accelerate the adsorption of carbofuran with the anion OH− in the aqueous solution (Salman and Hameed 2010). El-Geundi and co-workers observed that activated carbon prepared from cotton stalks was an efficient adsorbent for the adsorption of methomyl which is an insecticide from aqueous media. In the study, phosphoric acid-activation surface area of the activated carbon was relatively high (1600 m2/g), equilibrium adsorption was achieved in 2.5 h and the sorption capacity of the activated carbon increased with increasing methomyl concentration and contact time; however, it decreased with increasing temperature as well as activation energy of sorption was reported as − 2.35 kJ/mol suggesting that the sorption process was an exothermic reaction (El-Geundi et al. 2013).
Herbicides
Mandala and co-workers prepared biochars using eucalyptus bark, bamboo chips, corn cob, rice husk and rice straw for adsorption of atrazine from aqueous solution. They went further to prepare acid-treated rice straw and among these unreacted biochars, it was rice straw that showed the maximum adsorption for atrazine (70.7%) (Mandal et al. 2017). It was also noted that phosphoric acid treatment of rice straw improved its sorption. Besides, adsorption of atrazine on rice straw was best explained using the pseudo-second-order model. It was noted that the sorption decreases with increasing concentration of the atrazine in solution.
Recently, Phan et al. (2022) prepared various hydrochar using microwave-assisted hydrothermal carbonization under various conditions such as temperature ranging from 150 to 200 °C, residence time (20–60 min), and liquid to solid ratio from 5:1 to 15:1 mL/g as alternative adsorbent for atrazine removal. They activated the surface of hydrochar using different concentrations of H2O2 and KOH. Their results showed that as-prepared hydrochar at higher temperatures, longer residence times, and lower liquid to solid ratios showed significantly higher adsorption capacities for atrazine. From the results, KOH-activated hydrochar was reported to have highest adsorption capacity (4.06 mg/g) and even higher than biochar with higher surface areas. Other similar studies include hydrochar prepared by hydrothermal treatment of Prunus serrulata bark which is a novel and effective absorbent to adsorb atrazine in river waters with the maximum sorption capacity of 63.35 mg/g (Netto et al. 2022), while that of atrazine by HCl-modified corncob bio-waste sorbents at different pyrolysis temperatures and residence times revealed maximum adsorption in the range of 11.31–19.58 mg/g (Binh et al. 2022).
Recently, adsorption mechanism of rice straw biochar (RSB) to phenylurea herbicides (Monuron, Diuron, and Linuron) was studied via batch sorption experiments with three factors influencing the mechanism which are as follows: RSB dosage, ionic strength (IS), and pH, using orthogonal test (Dan et al. 2021). At pH value at 3, RSB dosage of 60 mg, and IS of 0.1 M, which was the optimal conditions for sorption, the maximum rate of removal attained for Diuron, Monuron, and Linuron were 25%, 41.9%, and 56.8%, respectively. Cara and co-workers investigated adsorption of sulphonylurea (chlorsulphuron) from an aqueous solutions using alkaline-treated wheat and corn (straw) mixed with soil and reported 337 mg/g maximum adsorption capacity for treated wheat and 318 mg/g for treated corn (Cara et al. 2017). Also, results obtained showed that maximum adsorption capacity in alkaline-treated straw was higher than in the soil (166 mg/g). Factors like the quality of raw material, hydrogen bonding interaction between chlorsulphuron and polar groups on the surface of the straw, π-π interactions between electron donor and acceptor, and hydrophobic moieties from the straw surface affect the adsorption capacity. Therefore, they concluded that the alkaline-treated straw has biosorption properties suitable for sulphonylurea removal. In another recent study, sulphuric acid was used to treat wheat husks (Fagopyrum esculentum) for its modification and applied as adsorbent for the removal of 2,4-dichlorophenoxyacetic acid (2,4-D) pesticide from aqueous solutions (Franco et al. 2021). Their findings showed that the maximum adsorption capacity attained was 161.1 mg/g at 298 K and electrostatic interactions was involved in adsorption mechanism.
Another novel study on the use of oxygen-defective graphdiyne as an material for removing the pesticides from water under various conditions showed remarkable sorption properties for seven sulphonylureas (Zhu et al. 2022). The maximum sorption capacity of oxygen-defective graphdiyne was 795.51 mg/g for iodosulphuron-methyl sodium, which was 130 folds higher than that of modified- graphene oxides and biochar with 6 and 1.5 mg/g respectively. The Langmuir isotherm model and pseudo-second-order kinetic model showed more suitability in describing this adsorption process, revealing that sulphonylureas onto oxygen-defective graphdiyne was monolayer coverage.
Fungicides
Adsorbent gotten from cow bone exhibited characteristic surface pore volume of 0.225 cm3/g as well as surface area of 200 m2/g. They have been apparently utilized as adsorbent at various doses from 0.01 to 1 g in removing azoxystrobin fungicide that belongs to the class of β-methoxyacrylates pesticidal compounds (Mendes et al. 2017). It was stated that 0.01 g dose of cow bone char displayed azoxystrobin fungicide adsorptive removal from contaminated water. However, an increase in adsorptive azoxystrobin fungicide removal was proposed to increase with a consistent increase in the adsorbent doses of the cow bone char from 0.01 to 1 g. Furthermore, the mechanism of adsorption was proposed to occur by chemical interaction among functional groups on pesticides and the cow bone char which resulted in π–π interaction from the heterocyclic ring π electron donor (Mendes et al. 2017).
Modified biomass-derived adsorbents for pharmaceutical pollutants removal
Analgesic and antipyretic drugs
The utilization of NaOH has been presented to modify rice husk for removing paracetamol at varied operating parameters of pH (2.0–3.5), dose of adsorbent (0.025–0.45 g), initial concentration (50–110 mg/L) and contact time (0–150 min) in aqueous solution (George Nche et al. 2017). NaOH-modified rice husk exhibited 20.964 mg/g adsorption capacity for paracetamol as deduced from Langmuir isotherm with R2 of 0.951. The kinetics of adsorption was best fitted into pseudo-second-order and intraparticle diffusion model with both having R2 of 0.976, and the adsorption process was suggested to proceed in a competitive mechanism between physisorption and chemisorption (George Nche et al. 2017).
Chemically modified Moringa oleifera seed pods have been used in sorption of paracetamol from aqueous solution and the effects of operating experimental factors including initial concentration of paracetamol (10–50 mg/L), dose of adsorbent (0.1 g), contact time (0–300 min), pH (2–11) along with temperature (303–323 K) were considered (Ogunmodede et al. 2021). They observed that chemically modified Moringa oleifera showed a sorption capacity of 20.284 mg/g and Langmuir isotherm gave the best description of the sorption with R2 = 0.9947 (Ogunmodede et al. 2021). The sorption kinetics was best fitted with the pseudo-second order with R2 = 0.9998, while sorption removal of paracetamol was observed to proceed through the chemisorption process. Furthermore, thermodynamic study revealed that the removal of paracetamol using modified Moringa oleifera resulted in spontaneous, feasible, and endothermic chemical process. The cost analysis revealed that the chemically modified Moringa oleifera was about 10 times less expensive when compared to the commercial activated carbon premised on their respective cost price of 40.17 USD per kg and 398.79 USD per kg (Ogunmodede et al. 2021).
Modification of sugarcane bagasse using H2SO4, NaOH and urea as economical adsorbent for removing paracetamol from aqueous solution at various adsorption experimental operating conditions of initial drug concentration (2–10 mg/L), adsorbent dose (0.02–0.1 g) and contact time (15–120 min) has been studied (Khan et al. 2012). It was reported that the adsorptive removal of paracetamol increased proportionally with increasing initial paracetamol drug concentration from 2 to 8 mg/L, beyond which there was no noticeable significant increase in the adsorptive removal of paracetamol. Likewise, at optimal concentration of 8 ppm and varied adsorbent dose, a linear response in the relationship amid quantity of paracetamol drug adsorbed and dose of the modified sugarcane bagasse was observed. This connotes that adsorption capacity of the adsorbent increased with increase in the adsorbent dose (Khan et al. 2012). In addition, Langmuir model best described adsorption of paracetamol drug on modified sugarcane bagasse with adsorption capacities ranging from 1.604 to 3.096 mg/g (Khan et al. 2012).
NH4Cl-modified activated carbon derived from pomegranate fruit peels has been reportedly used to remove paracetamol from contaminated water at varied experimental parameters of pH (2–9), temperature (10–40 °C), and initial concentration (100–500 mg/L) (Mashayekh-Salehi and Moussavi 2016). Langmuir isotherm with R2 = 0.999 gave the best description with a sorption capacity of 233 mg/g and removal percentage of 99.4%. The adsorption kinetics was well interpreted using pseudo-second-order kinetic model with a R2 of 0.9981. Thermodynamic study showed that the adsorption process was feasible, exothermic, and spontaneous with a low degree of entropy (Mashayekh-Salehi and Moussavi 2016).
Activated carbon produced from thermally treated and microwave-activated tea waste has been utilized in removing paracetamol at varied operating conditions such as temperature (283–323 K), contact time (0–120 min), dose of adsorbent (1–2 g), initial paracetamol concentration (20–100 mg/L) and pH (3–8) (Dutta et al. 2015). At optimum conditions of pH (3), initial adsorbent concentration (100 mg/L), dose of adsorbent (1 g) and temperature (303 K), the maximum adsorption capacity of 195.95 mg/g and percentage removal of 99.42% were obtained (Dutta et al. 2015). Furthermore, adsorption isotherm and kinetics for removing paracetamol were excellently described with by Langmuir and pseudo-second-order kinetic models with respective R2 of 0.993 and 0.999. Thermodynamic study showed that at a temperature range of 283 to 323 K, the adsorptive removal of paracetamol was endothermic and non-spontaneous with a high degree of entropy. In addition, the desorption studies revealed a 97.97% recovery of adsorbent in a basic medium (Dutta et al. 2015).
H3PO4 has been used to modify Ayous sawdust and Cucurbitaceae peelings for removing phenacetin from simulated pharmaceutical wastewater at operating experimental parameters of 0.5 g of adsorbent and pH 2 (Ngakou et al. 2019). The obtained results showed the Langmuir maximum adsorption capacities of 15.43 and 5.33 mg/g for the Cucurbitaceae peelings and Ayous sawdust, respectively, with R2 of 0.931 and 0.990 receptively. The adsorption isotherm was best described by Langmuir isotherm for the modified Cucurbitaceae peelings while Freundlich model best described the experimental data when ayous sawdust was used (Ngakou et al. 2019). In addition, both adsorbents were best described by pseudo-second-order kinetic model with respective R2 of 0.852 and 0.943. Adsorption mechanism showed displayed a π–π interactions of the phenacetin on modified ayous sawdust and Cucurbitaceae peelings (Ngakou et al. 2019).
Antibiotic drugs
Biomass-derived adsorbent prepared from rice straw has been used to remove ciprofloxacin and doxycycline at different operating conditions. The maximum adsorption capacity of 432.90 and 131.58 mg/g for doxycycline and ciprofloxacin, respectively, was obtained. Freundlich isotherm gave the best description of the adsorption with R2 of 0.994 and 0.984, respectively. In addition, the adsorption kinetics was best fitted into the pseudo-second-order model with R2 of 0.997 and 0.998, respectively, for doxycycline and ciprofloxacin, while the adsorption process was suggested to proceed through hydrogen bonding and π–π interaction mechanism (Zeng et al. 2018).
Similarly, aqueous solution of KOH-modified pomegranate fruit peel has employed in removing ciprofloxacin at various adsorption conditions that include pH (2–12), contact time (15–150 min), dose of adsorbent (0.025–0.2 g) and initial concentration (50–300 mg/L). The experimental findings showed 86.4% removal efficiency and maximum sorption capacity of 2.353 mg/g. The Freundlich isotherm adsorption model gave the best description for the removal ciprofloxacin using KOH-modified pomegranate fruit peels with R2 of 0.991 (Elhag Elhussien 2017).
Furthermore, biomass-derived char prepared from tea leaves at a temperature of 450 °C has been reportedly utilized to remove ciprofloxacin from aqueous solution at varied adsorption conditions of initial concentration (150–500 mg/L), pH (4–10), temperature (30–60 °C) at different time interval for 24 h (Li et al. 2018b). The sorption model that gave the best fit was Langmuir isotherm model and it showed a maximum adsorption capacity of 238.10 mg/g at optimum adsorption conditions, while the sorption kinetics was fitted into the pseudo-second-order kinetic model. The adsorption process was feasible and controlled by hydrogen bonding, π–π and electrostatic interaction mechanisms (Li et al. 2018b).
Thermal activation of Chinese herbal medicine (Astragalus mongholicus) waste at a temperature of 800 °C to prepare biomass-derived adsorbent for remediation of ciprofloxacin contaminated water has been reported (Shang et al. 2016). Under the optimum condition of pH at 7.0, the sorption capacity of 43.668 mg/g was obtained from the Langmuir model with R2 of 0.999. Furthermore, pseudo-second-order model with R2 of 0.999 gave the best interpretation of the adsorption kinetics. The mechanistic studies on the adsorption removal of ciprofloxacin onto the thermally activated Astragalus mongholicus residue was observed to be controlled by hydrogen bond, hydrophobic, electrostatic and π–π interactions (Shang et al. 2016).
Batch adsorption studies at various operating conditions that comprises of initial concentration (0.5–70 mg/L), adsorption contact time (5–60 min), pH (2.5–9.5) and 0.1 g adsorbent dose has been investigated on removing ciprofloxacin from aqueous solution by using bamboo charcoal prepared by burning bamboo wood at a temperature of 800 °C in a kiln (Wang et al. 2017). At optimum pH of 5.5, bamboo charcoal exhibited maximum adsorption capacity of 36.02 mg/g for removing ciprofloxacin from an aqueous solution. Langmuir isotherm best described the adsorption with R2 of 0.97. The adsorption kinetics for removing ciprofloxacin using bamboo charcoal was best interpreted using pseudo-second-order kinetic model. Furthermore, adsorption mechanism involving the ion exchange and hydrogen bonding was controlled the adsorptive removal of ciprofloxacin on bamboo charcoal. In addition, the presence of metal ion was also observed to influence the adsorptive removal of ciprofloxacin from aqueous solution (Wang et al. 2017).
The modification of Indian almond leaf has been reported for removing dicloxacillin from pharmaceutical aqueous solution. It was suggested that Langmuir isotherm model provided the best description of the adsorption of dicloxacillin on the modified Indian almond leaf with a high R2 of 0.9650. A maximum adsorption capacity of 71.04 mg/g with a percentage removal of 86.93% was attained at optimum experimental adsorption parameters of pH (6.0), adsorbent dose (0.1 g), initial concentration of dicloxacillin (20 mg/L), temperature (283.15 K) and contact time (24 h) (Sunsandee et al. 2020). Additionally, the adsorption kinetics was suggested to be favoured by pseudo-second-order kinetic model with a R2 of 0.9983 when compared to the pseudo-first-order kinetic model. Thermodynamic studies revealed that the adsorption process was feasible, spontaneous and exothermic with a poor degree of entropy proceeding with a mechanism of intermolecular attraction force such as van der Waal force and hydrogen bonding (Sunsandee et al. 2020).
The modification of sugarcane bagasse with H2SO4, NaOH and urea has been reported as an economical adsorbent for removing levofloxacin from aqueous solution at various adsorptive experimental operating conditions (Khan et al. 2012). They reported that adsorptive removal of the drugs increased proportionally as the initial drug concentration increased from 2 to 8 ppm, beyond which there was no positive change in the adsorption rate of levofloxacin. Similarly, when 8 mg/L was used as the optimum drug concentration at varied adsorbent dose, a linear response relationship between the quantity of levofloxacin adsorbed and the dose of the modified sugarcane bagasse was noticed. This suggests that the adsorption capacity of the adsorption process increases with an increase in the adsorbent dose (Khan et al. 2012). The adsorption of the levofloxacin from aqueous solution attained equilibrium time within 180 min. It was opined that increase in levofloxacin adsorption with contact time was as a consequence of the availability of active site on the adsorbents. While a decrease in the amount of levofloxacin removed beyond the optimal contact time resulted from the rate of exhaustion of the active sites on the activated carbon. The Langmuir model was described to deliver the best fit for understanding the adsorption of the levofloxacin on the modified sugarcane bagasse with adsorption capacities in the range of 0.847–2.0465 mg/g for levofloxacin antibiotic drug (Khan et al. 2012).
Non-steroidal anti-inflammatory drugs
Bean (Phaselous vulgaris) husks which are usually disposed as debris that forms heaps are of environmental concern has been regarded to be edible to livestock especially in its fresh form but not edible to humans. These wastes are commonly available and are said to be economically viable materials for preparing the adsorbents. In recent times, there has been a quest for the production of activated carbon from biomass and this was the basis for preparing activated carbon from bean husk as an alternative for expensive commercial activated carbon (Bello et al. 2019c). The application of bean husk modified with ortho-phosphoric acid has been documented as an auspicious adsorbent for removing ibuprofen from water has been reported. It was estimated that at a pH of 4.75 and temperature of 50 °C, a maximum adsorption capacity of 50.0 mg/g was recorded using functionalized bean husk for removing ibuprofen from aqueous system. Langmuir adsorption isotherm gave best interpretation, while pseudo-second-order explained the adsorption kinetics model, while thermodynamic studies showed that the process proceeded through an endothermic and spontaneous route. Moreover, a desorption study using HCl, H2O and NaOH revealed that the ortho-phosphoric acid functionalized bean husk exhibited a higher degree of regeneration and re-usability for removing ibuprofen from wastewater (Bello et al. 2019c).
The employment of ortho-phosphoric acid to modify orange peels and application in removing ibuprofen under different adsorption parameters showed a Langmuir maximum adsorption capacity of 49.30 mg/g at a temperature of 50 °C. Also, thermodynamics confirmed that adsorption process was feasible, spontaneous, and exothermic in chemical nature. Furthermore, kinetic studies revealed that adsorption process occurred via pseudo-second order with R2 of 0.999. The process was suggested to occur by physisorption mechanism (Bello et al. 2020b).
Antimalaria drugs
Banana has been considered as the second largest quantity of produced fruits from the total world’s production of fruits. Banana peels have been recognized to be a food waste that was classified as non-recyclable with a 1:2 ratio of banana product to waste. However, there is much more to do with the non-recyclable banana peels, and this includes composting, extraction of valuable organic compounds from banana peel in addition to preparation of activated carbons for several applications such as in wastewater treatment. For instance, the utilization of ortho-phosphoric acid-modified banana stalk for the removal of pharmaceutical contaminants such as lumefantrine drug from aqueous solution at varied operating parameters that include pH (3–11), contact time (0–200 min), initial lumefantrine concentration (20–100 mg/L), temperature (303–323 K) and 0.1 g adsorbent dose has been investigated (Agboola et al. 2021). The experimental results revealed that at optimum pH of 6, contact time of 120 min and temperature of 303 K, the maximum Langmuir adsorption capacity of 102.1 mg/g with R2 of 0.9998 was achieved. Furthermore, pseudo-second-order kinetic model with R2 ranging from 0.9845 to 0.9997 provided the best interpretation for the adsorptive removal of lumefantrine on chemically modified banana stalk. Additionally, thermodynamic studies showed that the process was spontaneous, feasible, and endothermic proceeding through intraparticle diffusion and boundary layer effect mechanisms (Agboola et al. 2021).
Modified biomass-derived adsorbents for dyes removal
Azo dyes
The quest for a cheap source of activated carbon as substitute to expensive commercial activated carbon in the treatment and remediation of aquatic ecosystem from environmental pollution with dyes has resulted in the employment of plant biomass as starting material for the synthesis of economically friendly activated carbon. For example, modified coconut mesocarp has been utilized for removing organic pollutants such as azo-anionic dyes of Congo red and tartrazine from simulated wastewater. Effects of various experimental factors including initial concentration (40–100 mg/L), adsorbent dose (5–12 mg/L), etc. were investigated (Cocos 2021). It has been reported that modified cationic cellulose derived from coconut mesocarp yielded adsorption capacities of 19.61 mg/g and 19.99 mg/g for removing tartrazine and Congo red from simulated wastewater. Freundlich and Dubinin–Radushkevich models were, respectively, employed to identify adsorption isotherm process of tartrazine and Congo red. In addition, pseudo-second-order model gave a worthy depiction for the adsorption kinetics in tartrazin and Congo red with R2 of 0.999 for the adsorptive removal from wastewater (Cocos 2021).
The modification of lemon grass to produce viable adsorbent for removing methyl red has been investigated. The experimental outcome revealed that modification of lemon grass with ortho-phosphoric acid yielded activated carbon with desirable properties such as 836.4 m2/g surface area, 598.06 m2/g mesopore surface area, with a total pore volume and average pore diameter of 0.472 cm3 and 3.62 nm, respectively (Ahmad et al. 2019). Effects of various operating parameters such as 25–500 mg/L initial concentration, 0–24 h contact time, 30–60 °C temperature and 2–12 solution pH revealed that the removal of methyl red using lemon grass was spontaneous and endothermic. It proceeded via physisorption process with 63.87% percentage adsorptive removal and 76.923 mg/g maximum monolayer adsorption capacity as described using Langmuir isotherm with R2 of 0.983, while kinetic studies confirmed the favourability of pseudo-first-order kinetic model (Ahmad et al. 2019).
Removal of methylene blue has been successfully experimented using H2SO4-modified coconut shell. It was estimated that optimum adsorption capacity of 50.6 mg/g was achieved when H2SO4-modified coconut shell was utilized as tremendous adsorbent for removing methylene blue from wastewater. The adsorption kinetics and isotherm were best described using pseudo-second-order and Freundlich models, respectively. The mechanism of adsorption process occurred via hydrogen bonding, π–π, and electrostatic interactions (Jawad et al. 2020).
Acid-activated Posidonia oceanica has been reportedly utilized for the remediation of methylene blue from spiked brackish wastewater from a lake in Egypt called Manzala (Elmorsi et al. 2019). From the batch adsorption findings, it was suggested that at optimum experimental operating conditions, there was an adsorption percentage removal in the range of 91.5–99.9%. Furthermore, when compared with the Freundlich and Langmuir isotherm models, the experimental findings suggested that the Dubinin–Radushkevich model gave the best description for the adsorption isotherm with a R2 of 0.992 (Elmorsi et al. 2019).
Acid/thermal-modified rice husk has also been used for removing methylene blue with a percentage removal of 96.7% and maximum Langmuir adsorption capacity of 103.11 mg/g with R2 of 0.9962 (Moeinian and Mehdinia 2019). Similarly, the use of modified lemon grass has been employed in removing methylene blue from aqueous environment (Ahmad et al. 2021a). It was reported that maximum percentage removal of 64.34% methylene blue was recorded at optimum pH 12 and maximum sorption capacity of 342.9 mg/g. Koble-Corrigan model isotherm best described the adsorption with a R2 of 0.999. The adsorption process was endothermic and its adsorption kinetics was best described by pseudo-first-order kinetic model proceeding via physisorption mechanism (Ahmad et al. 2021a).
KOH-modified pomegranate fruit peels have been used in removing methylene blue dye from simulated wastewater under the influence of operational conditions, including temperature (303–313 K), initial methylene blue dye concentration (25–300 mg/L) and contact time (0–24 h) (Ahmad et al. 2021c). The percentage removal of 83.4% removal and a maximum Langmuir sorption capacity of 235.58 mg/g with a R2 of 0.977 were obtained. Freundlich isotherm model gave the best interpretation of the isotherm adsorption model with a R2 of 0.998 (Ahmad et al. 2021c). Compared to pseudo-second order, the sorption kinetics was well interpreted using the pseudo-first order at a temperature range of 303–312 K. The sorption process was observed to be feasible, exothermic, and spontaneous proceeding through a physisorption mechanism of adsorption (Ahmad et al. 2021c).
The utilization of polyvinyl alcohol (PVA) coated activated carbon derived from the stems of Crotolaria burhia and Opuntia dellinii as adsorbents have been reported in removing azo dyes that include methylene red and methylene blue from aqueous solution (Gehlot et al. 2009, 2011). It was noted that the PVA coated activated carbon displayed 89.1% removal efficiency and a maximum Langmuir adsorption capacity of 19.23 mg/g with a R2 of 0.9975. The adsorptive removal of azo dyes using PVA coated activated carbons derived from the stems of Crotolaria burhia and Opuntia dellinii was excellently described by Freundlich isotherm model with a R2 of 0.994 (Gehlot et al. 2009, 2011).
The modification of wheat husk with 30% hydrogen peroxide for 24 h, which was preceded by washing with distilled water then oven drying at 60 °C, has been investigated in removing Reactofix Red 3BFN from aqueous solution. The investigated batch adsorption conditions are adsorbent dose (4.0–24.0 g), pH (2–10), initial dye concentration (1.0 × 10−5–6.0 × 10−5 mg/L) and temperature (30–50 °C) (Jain et al. 2006a). The results obtained from the experimental findings showed that at optimum pH of 2.0, the modified wheat husk displayed 90% removal of Reactofix Red 3BFN. At an optimum temperature of 30 °C, there was a maximum adsorption capacity of 80.37 mg/g with a R2 of 0.962. Furthermore, thermodynamic studies showed that at temperature range of 30–50 °C, the batch adsorptive removal of Reactofix Red 3BFN from an aqueous solution using H2O2-modified wheat husk followed a spontaneous, feasible, and endothermic chemical path (Jain et al. 2006a).
Xanthene dyes
The utilization of ortho-phosphoric acid functionalized coconut husk in sequestering Rhodamine B dye has been demonstrated to achieve a maximum Langmuir adsorption capacity of 1666.67 mg/g with R2 of 0.99. Furthermore, premised on the thermodynamic and kinetic studies, the adsorption process was said to be chemically endothermic and spontaneous proceeding via a pseudo-second-order adsorption kinetic model (Bello et al. 2019a).
Similarly, the adsorptive removal of Rhodamine B dye using functionalized locust bean pod has been investigated at varied experimental conditions of Rhodamine B dye concentrations (200–1000 mg/L), dose (0.1 g), temperature (303, 313, 323 K), and agitation speed of 120 rpm. The adsorption mechanism of using ortho-phosphoric acid-modified locust ban pod for removing Rhodamine B dye proceeded via physisorption mechanism with endothermic, spontaneous, and feasible reaction path. This yielded maximum monolayer adsorptive removal capacity of 1111.1 mg/g as deduced from Langmuir isotherm that best interpreted the adsorption. The adsorption kinetics was best described with pseudo-second-order kinetic model (Bello et al. 2019b).
In addition, the use of functionalized Gmelina aborea leaf for removing Rhodamine B dye from water at experimental conditions of pH, temperature, adsorbent dose, initial dye concentration and contact time has been reported to yield maximum adsorption capacity of 1000 mg/g (Bello et al. 2020a). Additionally, adsorption kinetics was best described with pseudo-second-order kinetic model and thermodynamics study revealed that sequestering of Rhodamine B dye using modified Gmelina aborea leaf was characterized by an endothermic, spontaneous, and feasible chemical process that proceed via a physisorption mechanism. Moreover, from the recycling and regeneration studies using HCl, H2O and NaOH revealed that 0.5 M HCl for desorbing Rhodamine B dye from modified Gmelina aborea leaf yielded a regeneration efficacy that increased from 8.26 to 92.74% (Bello et al. 2020a).
The employment of modified mango pod has been investigated in removing Rhodamine B dye from aqueous media at varied operating conditions which include pH (2–9), adsorption contact time (0–120 min), dose of adsorbent (0.1 g), temperature (303–323 K) and initial Rhodamine B dye concentration (200–1000 mg/L) (Bello et al. 2021). Adsorption kinetics and isotherm were best described via pseudo-second-order kinetic model and Freundlich isotherm model, with R2 of 0.99 (Bello et al. 2021). Maximum adsorptive removal capacity of 500 mg/g was obtained and thermodynamic studies showed that the adsorptive removal process was feasible, endothermic, and spontaneous proceeding under the influence of both intraparticle diffusion and boundary layer mechanisms. In addition, the cost analysis revealed that the utilization of modified mango pod to prepared adsorbent with a cost price of 34.20 USD/kg in removing Rhodamine B dye from aqueous media was less expensive compared to commercial activated carbon with a cost price of 259.5 USD/kg, thereby translating to about 8 times less expensive compared than the commercial activated carbon (Bello et al. 2021).
Miscellaneous diagnostic dyes
The modification of rice husk by treatment with hydrogen peroxide preceding the acetic acid treatment to prepare a vital adsorbent has been investigated in removing indigo carmine dye from aqueous solution at various conditions of pH (2–12), initial dye concentration (1.0 × 10−4 –9.0 × 10−4 mg/L), temperature (40–60 °C), dose of adsorbent (0.5–3.5 g) and contact time (10–70 min) (Jain et al. 2006b). Adsorptive removal of indigo carmine dye increased with increase in adsorbent dosage in addition to contact time, nonetheless, decreases with increasing initial dye concentration, solution pH and temperature. At an optimum temperature of 40 °C, a maximum adsorption capacity of 9.275 mg/g was recorded as obtained from Langmuir model. Pseudo-first-order model gave the best description for the adsorption kinetics at room temperature, fixed pH and adsorbent dose, while the thermodynamic studies showed that adsorptive removal of indigo carmine dye onto the modified rice husk was exothermic and spontaneous (Jain et al. 2006b).
Tri-aryl methane dyes
The modification of Azolla filiculoides with 0.1 M HCl has been studied as a feasible adsorbent for removing Acid Green 3 dye. The experimental findings demonstrated that the optimum conditions were contact time (90 min), pH (3), dose of adsorbent (4 g/L) and initial concentration (10 mg/L) (Balarak et al. 2016). The utilization of HCl-modified Azolla filiculoides exhibited 99.1% removal for Acid green 3 dye at optimum adsorption parameters. Moreover, the Langmuir adsorption isotherm model gave the best description of the adsorption process with maximum adsorption capacity of 37.5 mg/g and R2 of 0.999. Adsorption kinetic proceeded via pseudo-second-order kinetic model which displayed a R2 of 0.999 (Balarak et al. 2016).
Phenol-derived polymeric dyes
The employment of thermally and chemically modified rice husk for the removal of phenol (a precursor for phenol-derived polymeric dyes) has been reported. The thermally treated rice husk exhibited higher surface of 24–201 m2/g over the calcium hydroxide-treated rice husk with surface area of 3.19 m2/g, and also displayed a higher adsorptive removal capacity of 36–64%, compared to 28% displayed by the calcium hydroxide-treated rice husk (Daffalla et al. 2020). The emergence of research on the use of modified biomass wastes has led to numerous studies in the recent years for their employment in wastewater remediation containing different organic pollutants as presented in Table 2.
Mechanistic aspects of organic pollutants adsorption
During adsorption process, the interactions between the surfaces of organic pollutants (adsorbates) and adsorbent proceeds until dynamic equilibrium is attained. There are three major processes that occur during the adsorption of an adsorbent by an adsorbate and they are as follows: physical adsorption, precipitation and complexation, and pore filling (Fagbohungbe et al. 2017). Physical adsorption is the stage at which adsorbates settle on the surface of adsorbent but any adsorption does not take place; hence, it is called the clear zone. On the other hand, the precipitation and complexation stage is caused by the deposition of the adsorbates on the surface coverage area of the adsorbent. This stage involves the mass transfer of adsorbates into the adsorbent with the adsorption progressing by chemical bonding. Therefore, it is called as the mass transfer zone. Pore filling stage is premised on the condensation and retention of adsorbate into pores of the adsorbent until equilibrium is attained, therefore it is referred the exhausted zone (Fagbohungbe et al. 2017; Ambaye et al. 2021). From these three stages can be observed the three critical zones which are as follows: clear zone, mass transfer zone, and the final stage which is referred to as exhausted/saturated zone, where equilibrium is attained (Ambaye et al. 2021). Inverse proportionality is observed between saturated zone and clear zone. Meanwhile, mass transfer zone remains unaltered, apart from the increase in adsorbate’s concentration. This process continues until the adsorbent is saturated, and this point is called breakthrough point (Moreno-Castilla 2004). A removal mechanism for adsorption of organic pollutants onto biomass is presented in Fig. 3. Hydroxyl, carboxyl, carbonyl, and amine, which are organic functional groups, favour the adsorption of organic molecules on the surface of the biomass. This is an example of a donor/acceptor electron type of adsorption mechanism based on the unbalanced electron distribution between the functional groups of the biomass and the organic compound. Notably is the highly reduced bonding between the adsorbent and organic compounds with active chloro- and nitro- substituent groups. Therefore, the binding energy between the biomass and the organic compound is increased (Mu’azu et al. 2017; Ambaye et al. 2021) due to the substituent group being a strong electron acceptor (Atkinson et al. 2010).
Different adsorption mechanisms for organic contaminants. Partition/adsorption are indicated with circles on adsorbent’s particles. I—electrostatic interaction between the modified biomass adsorbent and organic contaminant, II—electrostatic attraction between the modified biomass adsorbent and organic contaminant that are polar, and III—electrostatic attraction between the modified biomass adsorbent and non-polar organic contaminant (Ahmad et al. 2014a)
The mechanisms of adsorption of organic pollutants have been classified as hydrophobic interaction, pore filling, electrostatic interaction, partitioning, and electron donor–acceptor (EDA) interaction. The partitioning mechanism involves the diffusion of the adsorbates into the pores of the non-carbonized portion of the adsorbent due to their easy interaction between the organic adsorbates. Therefore, the adsorptive removal of the organic pollutants is reliant on the properties of the non-carbonized portion of the adsorbent, and the partitioning mechanism is highly effective when the adsorbent possesses high content of volatile matter in the presence of high concentration of organic pollutants (Ambaye et al. 2021). The electrostatic interaction mechanism is the most significant mechanism that takes place in the adsorption of ionizable organic compounds to adsorbents that exhibit positively charged surfaces through electrostatic interaction. However, the ionic strength as well as the solution’s pH determines the efficiency of the electrostatic interaction mechanism in attracting and repelling organic pollutants. This is attributed to the increase in repulsive electrostatic interaction between adsorbate and adsorbent when they both possess same charges (Ambaye et al. 2021).
The pore filling mechanism occurs by the passage of the organic pollutants through the micropores and mesopores of the adsorbent but the mechanism is dependent on the polarity of the organic pollutants as well as the nature and type of the adsorbent. However, the efficiency of the pore filling mechanism depends on the occurrence of petty content of volatile matter in the presence of low concentration of organic pollutants. The electron donor and acceptor interaction mechanism frequently occurs in the adsorptive interaction of aromatic compounds on adsorbents that exhibit a graphene-like structure, which is only attained at a temperature above 1100 °C (Ambaye et al. 2021). Nevertheless, the pyrolysis temperature employed in the fabrication of the adsorbent determines the development of enriched or deficient π–electron adsorbent. That is, system of adsorbent’s π aromatic compound acts as electron acceptor and donor when the adsorbents are obtained at temperature above and below 500 °C. The hydrophobic interaction is habitually employed in adsorptive removal of neutral and hydrophobic organic compounds through the portioning as well as hydrophobic interaction processes, but requires less amount of energy in comparison with the partitioning mechanistic process. This is subsequent to the decline in the quantity of polar functional groups on the surface of the adsorbent as an outcome of pyrolysis temperature (Ambaye et al. 2021).
Regeneration studies and environmental challenges with chemical modifications
The regeneration of adsorbent used is dependent on the source of the adsorbent, regeneration method, and adsorbent dosage. During a regeneration study, the recovery of the adsorbates can be achieved through the principles of desorption and decomposition (Jia et al. 2013; Wang et al. 2015; Ambaye et al. 2021; Bello et al. 2021). The regeneration studies on the use of eucalyptus leaves to prepare magnetic biomass for decontamination of organic pollutants revealed that the FTIR spectra band shift did not change until six continuous cycles (Wang et al. 2015). They attributed this observation to the increment in the ash content of the prepared biomass as well as the effect of surface functional groups of the biomass that did not change significantly. The pore sizes and surface area of the prepared and regenerated magnetic biomass are noticed to be similar, thus allowing high separation and adsorptive rates. The process of adsorption is made more economical by the regeneration method. It was further observed that the regeneration study using a basic chemical such as NaOH is more efficient. Another method of chemical regeneration is altering the pH of adsorbents to desorb substances that are non-reactive, like dyes and aniline (Fagbohungbe et al. 2017). However, major challenges associated with regeneration studies using chemical methods are the high cost of some required reagents and the potential to aggravate environmental pollution. Based on this, the disposal of digestate is therefore very important in the management of biomass.
Recently, thermal regeneration method has been recognized as the best technique for the recovery of biomass since it permits the development of tiny pore sizes contrasted with the first pores of the initial biomass, mainly when it is completed at high temperatures. The researchers noticed that temperature increase improved regeneration efficiency. For instance, in a study on removal of organic compounds such as pyrene and benzopyrene using biomass adsorbent derived from Enteromorpha prolifera and the regeneration studies carried out at varied temperatures of 80 °C, 150 °C, and 200 °C, an increased in temperature improved the regeneration efficiency (Qiao et al. 2018). The adsorption efficiency of pyrene was observed to increase by 35.0%, 45.0%, and 48.0%, while that of benzopyrene increased by 31.0%, 41.0%, and 40.0%, respectively (Qiao et al. 2018). Furthermore, it was also observed that dissolved organic carbon could be reduced by biomass regeneration using the thermal method.
Regeneration of biomass had also been carried out using irradiation by microwave method. Results obtained show that the biomass regeneration was very rapid. Additionally, it allowed easy control of the operating temperature. Inducement of polar molecules in the biomass was observed when it was irradiated by microwave, thus leading to the formation of dipole/polarization. However, this method is still at the experimental stage and laboratory scale because its application on an industrial/large scale is currently absent (Jia et al. 2013; Ahmad et al. 2014b, 2021b; Khasri et al. 2018; Li et al. 2018a; Ambaye et al. 2021; Yusop et al. 2022). Recently, the operating pressure was adjusted using supercritical fluid in order to investigate biomass regeneration (Hu 2018; Dai et al. 2019). It was observed by the authors that no change occurred in the physicochemical properties of the biomass when used to adsorb high molecular weight volatile organic compounds. This method shows advantages such as minimal loss of biomass, a short operating cycle, and low operating temperatures. However, because of the complexity of the method, the costly materials, equipment, and high-pressure resistance, the method is still at the experimental stage and needs to be scaled up. In addition, of all the methods used for the regeneration and management of biomass in the previous studies, the thermal regeneration process seems to be most efficient due to minimal operation cost and maximal economic applicability. However, carbon loss is very high when compared with other methods. Further research is required which should focus on the economic value of biomass used for adsorption.
Realizing that the use of chemical modification has become very popular for improving the adsorption uptake of organic pollutants, several studies conducted for adsorption of various organic pollutants confirmed that this approach is feasible for translating the applicability of modified biomass adsorbents into industrial application. This is based on the efficient adsorbent properties such as availability of the biomass material at low/zero cost, enhanced porosities, and larger surface area. However, evaluating the effects that these modified adsorbents pose to ensure the global environmental safety is highly important. After a successful adsorption process, the life cycle experiment through desorption studies is necessary to ensure the number of cycles that these adsorbents are able to complete before ripping off of their maximum adsorption capacities. For waste disposal, different strategies such as catalytic technology, biological treatment, ion exchange and advanced bio-physical methods have been developed to eradicate the different imbalances that the spent (used) adsorbent pose to the immediate environment (Xu et al. 2017; Ogunlalu et al. 2021). The adsorbents that are not capable of been reused should be burnt off at a very higher temperatures such as 1100 °C to 1200 °C and the resultant gases should be collected using a specialized equipment (Zhou et al. 2008; Ogunlalu et al. 2021).
Computational (optimization) models for modified biomass adsorbents for removal of organic pollutants
Response surface methodology
The response surface methodology (RSM) has two most commonly used designs which are central composite design (CCD) and Box–Behnken design (BBD). The systematic utilization of RSM for the adsorptive removal of malachite green, methylene blue, azo and anthraquinone dyes from effluents has been reported (Song et al. 2018; Chowdhury et al. 2019; Ahmad et al. 2020). In addition, adsorptive removal of acid dye using derived activated carbon for antibiotic (ciprofloxacin) removal from wastewater using rice husk as well as other research findings on the employment of RSM for the adsorptive removal of organic contaminants from aqueous system has established the effective application of RSM as an efficient computational model in wastewater treatment (Amini et al. 2008; Niad et al. 2014; Samuel et al. 2015; Zhou et al. 2019).
RSM has been confirmed to be suitable in studying the removal of methyl orange using modified sugar beet bagasse and results obtained show concordance between experimental and predicted values. The optimum adsorption process conditions generated using RSM for the aqueous removal of methyl orange were 2.51, 100 mg/L, and 0.37 g/L for pH, initial methyl orange concentration, and adsorbent dose, respectively, with predicted adsorption capacity and adsorptive removal efficiency of 221.5 mg/g and 51.8%, respectively (Ghorbani and Kamari 2017). RSM using two-factor interaction models and quadratic models have been applied in optimizing the preparation of functionalized pomegranate fruit peels. Its application in removing Remazol brilliant blue R dye resulted in a 31.2% yield of activated carbon derived from functionalized pomegranate fruit peels and 81.35% removal of Remazol brilliant blue R dye. The model suitability was performed by means of analysis of variance. It was suggested from the percentage error between the actual and predicted result calculated to be 1.92% that there was a good correlation between experimental and predicted results (Ahmad et al. 2020).
In a similar study, RSM was used to model the adsorption of methylene blue (MB) from aqueous system using pomegranate fruit peel activated carbon (PFPAC) which was prepared by KOH impregnation and CO2 gasification methods. Results obtained reveal that the optimum sorption conditions for the removal of MB dye are 375 W, 4.5 min, and 0.9, for radiation power, activation time, and impregnation ratio, respectively. These optimum adsorption conditions resulted in 83.4% removal of MB dye and PFPAC yield of 30.8%, respectively (Ahmad et al. 2021c). Similarly, use of eggshell in the adsorptive removal of Malachite green (MG) dye was studied using CCD to investigate effects of operating conditions on the adsorptive removal of the dye. There was significant agreement between predicted values obtained with experimental results at R2 value of 0.9388. The optimum adsorptive removal was 90.66% at operating parameters of 1.25 g adsorbent dosage, pH 6, and contact time of 40 min. Results from this study further revealed that the eggshell could be a good adsorbent for MG removal from aqueous solution (Hoo et al. 2022). The second-order polynomial equation of CCD has been reportedly utilized in the optimization of adsorption parameters for the adsorptive removal of methylene blue, brilliant green and Congo red with their variable levels. The predicted optimum adsorption condition was 30 °C, 9.8, 2.5 g/L and 99% for temperature, pH, adsorbent dose, and percentage adsorptive removal of dye using CCD. The qualitative fit of the model was defensible by the R2 of 0.9966, which exceeded the R2 of 0.88 suggested as best correlation from previous studies (Fegousse et al. 2019). Grape leaves prepared activated carbon was used for the removal of MB from aqueous solution. Data modelling and design of the experiment were carried out using RSM. The results obtained show that the optimum percentage removal of MB was 97.4% at adsorption conditions of adsorbent dose 12.5 g/L, MB concentration of 100 mg/L, pH 11, and contact time of 90 min. Furthermore, obtained results indicate that adsorbent dosage and initial dye concentration play major role in increasing the efficiency of MB adsorption (Alireza et al. 2022).
Green pea which is a low-cost adsorbent was investigated for the adsorptive removal of Reactive Blue 19 dye using Box–Behnken design to deduce the effect of independent and interaction influences of process variables such as temperature, pH, and adsorbent dosage. The RSM experimental results revealed that maximum removal was 99.42% at optimum conditions of temperature (35 °C), pH 2, and adsorbent dosage (1.5 g/100 mL). Furthermore, the kinetic study showed that Freundlich isotherm fitted best for the removal of the dye and it followed pseudo-second-order model (Demirhan 2020).
The adsorptive removal of Direct Violet 51 dye from aqueous solution by biosorption using sugarcane bagasse biomass was conducted by different physical and chemical treatments (Sadaf et al. 2015). Box–Behnken experimental design was used to ascertain the effect of three independent variables (biosorbent dose, pH, and initial dye concentration). Optimum removal of Direct Violet 51 dye was 63.0 mg/g and was attained at 0.05 g biosorbent dose and pH 2. Desorption study on the loaded biosorbent using 1 M NaOH solution removed 61.58% dye (Sadaf et al. 2015). Additionally, the use of plackett–Burman design and RSM has been employed in the statistical optimization of Gracilaria edulis for adsorption removal of dye from textile effluents (Venkataraghavan et al. 2020).
Artificial intelligence
Owing to current technological advancements, there has been upsurge in the application of artificial intelligence (AI) model for various applications in different fields including water purification, catalysis, medical, etc. Interconnected assembly of units called artificial neurons or nodes is used for the design of AI and this concept is premised of biological nervous system. Currently, this concept enjoys global attention as a computational-based modelling tool which is highly efficient for predicting rate of pollutant removal using sets of data. This provides avenue for comparative study of the correlation coefficient obtained from experimental and expected data. This leads to a reduction in the cost and time of an experiment. Furthermore, AI has been shown to be capable of identifying complex and complicated nonlinear relationships among several parameters and variables in addition to showing correlations of simulated output and the interactive influence among variables (Nasr et al. 2017; Mazloom et al. 2020; Liao and Yao 2021; Martini and Roni 2021).
For example, use of artificial neutral network (ANN) has been reported to model pseudo-second-order kinetic model for adsorptive removal of paracetamol using modified orange peels from aqueous media at different adsorption conditions such as initial concentration (10–50 mg/L), contact time (0–330 min) and temperature (30–50 °C) (Afolabi et al. 2020b). It was revealed that the ANN with hyperbolic tangent sigmoid transfer function at the input layer and linear transfer function at the output layer, eighteen (18) hidden neurons and Levenberg–Marquardt as its backpropagation algorithm showed the optimal prediction ability. It further demonstrates the effective application in modelling pseudo-second-order kinetic model using modified orange peels in removing paracetamol (Afolabi et al. 2020b). Several, other report on the application of AI for modelling the efficiency of organic adsorption process has been reported in the past few studies. Table 3 presents various adsorption performance comparison of different adsorbents using Artificial intelligent.
Concluding remarks
The purification of water and wastewater using adsorption technique is a globally recognized and reliable scientific procedure to overcome the predicament of contaminant with most traditional techniques applied in wastewater treatment operation. Adsorption has been ascertained to be economical, safe, and stress-free to employ. These characteristics have abetted it to prevail over the impediments that include enormous consumption of space, extravagant, commercially unappealing, and operational toil, in addition to the disposal glitches which is common with conventional techniques. The obtainability of enormous quantities of naturally existing materials in addition to wastes generated from both agricultural activities has gotten numerous considerations in contemporary time. The extensive employment of these potential adsorbents after subsequent modification by means of more than a few techniques that include chemical, mechanical, thermal, gasification, or blend of techniques.
Efficient biomass-derived adsorbent is expected to be well-designed and serves as good candidate for efficient removal of organic pollutants from wastewater treatment plant. Based on the survey of literature, use of unmodified biomass adsorbents for decontamination of organic pollutants from aqueous mixture is not certain and suffers poor surface area, pore volume and sizes while their adsorption performance is also low. However, it has been demonstrated by previous studies that several types of modifications seemed to enhance the biomass adsorbents for better adsorptive performance towards organic pollutants (including pesticides, insecticides. pharmaceutical and dyes). Furthermore, literature has shown that many of these studies based on the adsorption of organic contaminants using biomass adsorbents were carried out in aqueous systems and on a laboratory scale. This did not undermine the potentiality of these studies. However, for commercial and practical applications, adsorbents from biomass origin should be effectively applied directly into effluents of wastewater. Therefore, more studies should be carried out to elucidate the design and synthesis of biomass-based adsorbents which can be used successfully in wastewater treatment systems for decontaminating organic pollutants.
Additionally, focus of future studies should be on sustainable measures on how spent biomass adsorbents that have lost their performance/ability on organic pollutants would be effectively disposed. This is to ensure proper understanding of the stability of biomass-based adsorbent materials. Also, it is necessary to corroborate the commercial values and the practical applications of modified biomass materials towards organic pollutants removal in wastewater and their vital associated issues in the environment.
Furthermore, in recent years, use of combined processes has received increased attention for decontaminating organic pollutants from aqueous mixtures. There is, however, insufficient knowledge and understanding on combined adsorption process and existing treatment technologies like reverse osmosis, nanofiltration, ozonation, Fenton-oxidation, and photocatalytic degradation. Hence, investigation on the efficiency of removing organic pollutants using hybridized adsorption process and other treatment methods is necessary. Future studies should be centred on designing and production costs of modified adsorbents from biomass source and the corresponding cost evaluation analysis for organic pollutants contaminant treatment from laboratory, pilot, and industrial scales. This should help to establish viable method(s) that should be implemented for disposing the spent adsorbents. Such technique is expected to be environmentally sustainable, and hybridized adsorption with other treatment processes (methods) to ensure highly and better removal performance. The limitations that prevent most biomass adsorbents from becoming commercially viable can be reduced by investigating these knowledge gaps.
In agreement with the green/sustainable chemistry, since chemical modification significantly enhances the sorption capacity, when compared with the unmodified counterpart, therefore use of environmentally friendly reagents should be focused on with aim of minimizing the use of these chemicals and minimization of the wastes generated and released into the environment.
The effects of interfering ions present in the environment laden with organic pollutants are not considered in many chemically modified biomass materials. Presence of interfering ions such as NO3−, Cl−, SO42−, H2PO4−,Ca2+, and Fe2+ have been previously reported to interference with the organic contaminants (pesticides including endosulphan, 2,4-dichlorophenoxyacetic acid, humic acid and atrazine) (Das et al. 2009). More investigation on the use of these sorbents for systems with the multiple organic pollutants in the same environments is required to examine the influence of one organic pollutant adsorption over the other. Similarly, further studies are needed on the chemical(s) that can introduce certain functional group(s) that are capable of adsorbing different contaminants same way they are present in the real wastewater system and the corresponding influence of one contaminant on the uptake of the others.
Lastly, the utilization of biological (biomass) materials as precursor for the fabrication of nanoparticles (NPs) is currently attracting wider popularity as a secondary metabolite owing to their inherent properties as reducing agents for the NPs and stabilizing/capping agents. Interestingly, most of the resultant NPs have reduced particle sizes, higher pore volumes and surface areas that were better than their precursors (Yu et al. 2016; Iwuozor et al. 2021). This suggests that more research in the area is promising to optimize the usage of chemical substances on the sorbents for sorption process.
Data availability
All data and materials used in this study are available within this article.
Abbreviations
- ACs:
-
Activated carbons
- ACFs:
-
Activated carbon fibres
- EDA:
-
Electron donor–acceptor
- BSAC:
-
Banana stalk activated carbon
- CBS:
-
Cob bio-waste sorbents
- PUHs:
-
Phenylurea herbicides
- FAO:
-
Food and Agricultural Organization
- RSB:
-
Raw straw biochar
- IS:
-
Ionic strength
- PVA:
-
Polyvinyl alcohol
- I.C:
-
Initial conditions
- NA:
-
Not applicable
- Ps1:
-
Pseudo-first order
- Ps2:
-
Pseudo-second order
- RSM:
-
Response surface methodology
- CCD:
-
Central composite design
- BBD:
-
Box–Behnken design
- MB:
-
Methylene blue
- PFPAC:
-
Pomegranate fruit peel activated carbon
- MG:
-
Malachite green
- AI:
-
Artificial intelligence
- ANN:
-
Artificial neural network
- NPs:
-
Nanoparticles
References
Adebusuyi AT, Sojinu SO, Aleshinloye AO (2022) The prevalence of persistent organic pollutants (POPs) in West Africa—a review. Environ Chall 7:100486. https://doi.org/10.1016/j.envc.2022.100486
Adegoke KA, Bello OS (2015) Dye sequestration using agricultural wastes as adsorbents. Water Resour Ind 12:8–24. https://doi.org/10.1016/j.wri.2015.09.002
Adegoke KA, Oyewole RO, Lasisi BM, Bello OS (2017) Abatement of organic pollutants using fly ash based adsorbents. Water Sci Technol 76:2580–2592. https://doi.org/10.2166/wst.2017.437
Adegoke KA, Iqbal M, Louis H, Bello OS (2019) Synthesis, characterization and application of CdS/ZnO nanorod heterostructure for the photodegradation of Rhodamine B dye. Mater Sci Energy Technol 2:329–336. https://doi.org/10.1016/j.mset.2019.02.008
Adegoke KA, Adegoke OR, Araoye AO et al (2022a) Engineered raw, carbonaceous, and modified biomass-based adsorbents for Rhodamine B dye removal from water and wastewater. Bioresour Technol Reports 18:101082. https://doi.org/10.1016/j.biteb.2022.101082
Adegoke KA, Akinnawo SO, Ajala OA et al (2022b) Progress and challenges in batch and optimization studies on the adsorptive removal of heavy metals using modified biomass-based adsorbents. Bioresour Technol Rep 19:101115. https://doi.org/10.1016/j.biteb.2022.101115
Adegoke KA, Olagunju AO, Alagbada TC et al (2022c) Adsorptive removal of endocrine-disrupting chemicals from aqueous solutions: a review. Water Air Soil Pollut 233:38. https://doi.org/10.1007/s11270-021-05405-8
Afolabi IC, Popoola SI, Bello OS (2020a) Machine learning approach for prediction of paracetamol adsorption efficiency on chemically modified orange peel. Spectrochim Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2020.118769
Afolabi IC, Popoola SI, Bello OS (2020b) Modeling pseudo-second-order kinetics of orange peel-paracetamol adsorption process using artificial neural network. Chemom Intell Lab Syst 203:104053. https://doi.org/10.1016/j.chemolab.2020.104053
Agboola OS, Bello OS (2020) Enhanced adsorption of ciprofloxacin from aqueous solutions using functionalized banana stalk. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01038-9
Agboola OS, Akanji SB, Bello OS (2021) Functionalized banana stalk for lumefantrine drug removal. Phys Chem Res 9:483–507. https://doi.org/10.22036/pcr.2021.261506.1865
Ahmad T, Rafatullah M, Ghazali A et al (2010) Removal of pesticides from water and wastewater by different adsorbents: a review. J Environ Sci Heal Part C Environ Carcinog Ecotoxicol Rev 28:231–271. https://doi.org/10.1080/10590501.2010.525782
Ahmad M, Rajapaksha AU, Lim JE et al (2014a) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Ahmad MA, Ahmad Puad NA, Bello OS (2014b) Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resour Ind 6:18–35. https://doi.org/10.1016/j.wri.2014.06.002
Ahmad MA, Afandi NS, Adegoke KA, Bello OS (2016) Optimization and batch studies on adsorption of malachite green dye using rambutan seed activated carbon. Desalin Water Treat 57:21487–21511. https://doi.org/10.1080/19443994.2015.1119744
Ahmad MA, Ahmed NB, Adegoke KA, Bello OS (2019) Sorption studies of methyl red dye removal using lemon grass (Cymbopogon citratus). Chem Data Collect 22:100249. https://doi.org/10.1016/j.cdc.2019.100249
Ahmad MA, Eusoff MA, Oladoye PO et al (2020) Statistical optimization of Remazol Brilliant Blue R dye adsorption onto activated carbon prepared from pomegranate fruit peel. Chem Data Collect. https://doi.org/10.1016/j.cdc.2020.100426
Ahmad MA, Ahmed NB, Adegoke KA, Bello OS (2021a) Adsorptive potentials of lemongrass leaf for methylene blue dye removal. Chem Data Collect 31:100578. https://doi.org/10.1016/j.cdc.2020.100578
Ahmad MA, Eusoff MA, Adegoke KA, Bello OS (2021b) Sequestration of methylene blue dye from aqueous solution using microwave assisted dragon fruit peel as adsorbent. Environ Technol Innov 24:101917. https://doi.org/10.1016/j.eti.2021.101917
Ahmad MA, Eusoff MA, Oladoye PO et al (2021c) Optimization and batch studies on adsorption of Methylene blue dye using pomegranate fruit peel based adsorbent. Chem Data Collect 32:100676. https://doi.org/10.1016/j.cdc.2021.100676
Ahmad MA, Ahmed NA, Adesina Adegoke K, Bello OS (2022) Trapping synthetic dye molecules using modified lemon grass adsorbent. J Dispers Sci Technol 43:583–597. https://doi.org/10.1080/01932691.2020.1844016
Ahmaruzzaman M, Ahmed MJK, Begum S (2015) Remediation of Eriochrome Black T-contaminated aqueous solutions utilizing H3PO4-modified berry leaves as a non-conventional adsorbent. Desalin Water Treat 56:1507–1519. https://doi.org/10.1080/19443994.2014.950995
Akhtar M, Hasany SM, Bhanger MI, Iqbal S (2007) Low cost sorbents for the removal of methyl parathion pesticide from aqueous solutions. Chemosphere 66:1829–1838. https://doi.org/10.1016/j.chemosphere.2006.09.006
Alireza S, Arezoo M, Samira M et al (2022) Methylene blue removal using grape leaves waste: optimization and modeling. Appl Water Sci 12:1–11. https://doi.org/10.1007/s13201-022-01648-w
Ambaye TG, Vaccari M, van Hullebusch ED et al (2021) Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int J Environ Sci Technol 18:3273–3294. https://doi.org/10.1007/s13762-020-03060-w
Amini M, Younesi H, Bahramifar N et al (2008) Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. J Hazard Mater 154:694–702. https://doi.org/10.1016/j.jhazmat.2007.10.114
Arthur M, Liu G, Hao Y et al (2019) Urban food-energy-water nexus indicators: a review. Resour Conserv Recycl 151:104481
Aryee AA, Mpatani FM, Zhang X et al (2020) Iron (III) and iminodiacetic acid functionalized magnetic peanut husk for the removal of phosphate from solution: characterization, kinetic and equilibrium studies. J Clean Prod 268:122191. https://doi.org/10.1016/j.jclepro.2020.122191
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18. https://doi.org/10.1007/s11104-010-0464-5
Babaei AA, Khataee A, Ahmadpour E et al (2016) Optimization of cationic dye adsorption on activated spent tea: equilibrium, kinetics, thermodynamic and artificial neural network modeling. Korean J Chem Eng 33:1352–1361. https://doi.org/10.1007/s11814-014-0334-6
Bagheri A, Abu-Danso E, Iqbal J, Bhatnagar A (2020) Modified biochar from Moringa seed powder for the removal of diclofenac from aqueous solution. Environ Sci Pollut Res 27:7318–7327. https://doi.org/10.1007/s11356-019-06844-x
Bai Y, Dou Y, Xie LH et al (2016) Zr-based metal-organic frameworks: design, synthesis, structure, and applications. Chem Soc Rev 45:2327–2367. https://doi.org/10.1039/c5cs00837a
Balarak D, Bazrafshan E, Mostafapour F (2016) Equilibrium, kinetic studies on the adsorption of acid green 3 (Ag3) dye onto Azolla filiculoides as adosorbent. Am Chem Sci J 11:1–10. https://doi.org/10.9734/acsj/2016/22048
Balci B, Keskinkan O, Avci M (2011) Use of BDST and an ANN model for prediction of dye adsorption efficiency of Eucalyptus camaldulensis barks in fixed-bed system. Expert Syst Appl 38:949–956. https://doi.org/10.1016/j.eswa.2010.07.084
Beheary M, El-matary F, El-hamid HTA et al (2018) Removal of some pesticides from contaminated water using low cost materials. Adv Environ Biol. https://doi.org/10.22587/aeb.2018.12.7.1
Bello OS, Adegoke KA, Olaniyan AA, Abdulazeez H (2015a) Dye adsorption using biomass wastes and natural adsorbents: overview and future prospects. Desalin Water Treat 53:1292–1315. https://doi.org/10.1080/19443994.2013.862028
Bello OS, Ahmad MA, Semire B (2015b) Scavenging malachite green dye from aqueous solutions using pomelo (Citrus grandis) peels: kinetic, equilibrium and thermodynamic studies. Desalin Water Treat 56:521–535. https://doi.org/10.1080/19443994.2014.940387
Bello IA, Bello OS, Adegoke KA (2017a) Effects of modifying agents on the dyeability of cotton fabric using malachite green dye. Ann Sci Technol 2:26–36. https://doi.org/10.2478/ast-2018-0005
Bello OS, Adegoke KA, Akinyunni OO (2017b) Preparation and characterization of a novel adsorbent from Moringa oleifera leaf. Appl Water Sci 7:1295–1305. https://doi.org/10.1007/s13201-015-0345-4
Bello OS, Lasisi BM, Adigun OJ, Ephraim V (2017c) Scavenging Rhodamine B dye using Moringa oleifera seed pod. Chem Speciat Bioavailab 29:120–134. https://doi.org/10.1080/09542299.2017.1356694
Bello OS, Owojuyigbe ES, Babatunde MA, Folaranmi FE (2017d) Sustainable conversion of agro-wastes into useful adsorbents. Appl Water Sci 7:3561–3571. https://doi.org/10.1007/s13201-016-0494-0
Bello OS, Adegoke KA, Fagbenro SO, Lameed OS (2019a) Functionalized coconut husks for rhodamine-B dye sequestration. Appl Water Sci. https://doi.org/10.1007/s13201-019-1051-4
Bello OS, Adegoke KA, Sarumi OO, Lameed OS (2019b) Functionalized locust bean pod (Parkia biglobosa) activated carbon for Rhodamine B dye removal. Heliyon 5:e02323. https://doi.org/10.1016/j.heliyon.2019.e02323
Bello OS, Alao OC, Alagbada TC, Olatunde AM (2019c) Biosorption of ibuprofen using functionalized bean husks. Sustain Chem Pharm. https://doi.org/10.1016/j.scp.2019.100151
Bello OS, Alabi EO, Adegoke KA et al (2020a) Rhodamine B dye sequestration using Gmelina aborea leaf powder. Heliyon 6:e02872. https://doi.org/10.1016/j.heliyon.2019.e02872
Bello OS, Alagbada TC, Alao OC, Olatunde AM (2020b) Sequestering a non-steroidal anti-inflammatory drug using modified orange peels. Appl Water Sci. https://doi.org/10.1007/s13201-020-01254-8
Bello OS, Moshood MA, Ewetumo BA, Afolabi IC (2020c) Ibuprofen removal using coconut husk activated biomass. Chem Data Collect. https://doi.org/10.1016/j.cdc.2020.100533
Bello OS, Adegoke KA, Inyinbor AA, Dada AO (2021) Trapping Rhodamine B dye using functionalized mango (Mangifera indica) pod. Water Environ Res 93:2308–2328. https://doi.org/10.1002/wer.1606
Benyoucef S, Harrache D, Djaroud S et al (2020) Preparation and characterization of novel microstructure cellulosic sawdust material: application as potential adsorbent for wastewater treatment. Cellulose 27:8169–8180. https://doi.org/10.1007/s10570-020-03349-6
Binh QA, Nguyen V-H, Kajitvichyanukul P (2022) Influence of pyrolysis conditions of modified corn cob bio-waste sorbents on adsorption mechanism of atrazine in contaminated water. Environ Technol Innov 26:102381. https://doi.org/10.1016/j.eti.2022.102381
Bulgariu L, Escudero LB, Bello OS et al (2019) The utilization of leaf-based adsorbents for dyes removal: a review. J Mol Liq 276:728–747. https://doi.org/10.1016/j.molliq.2018.12.001
Cara I-G, Rusu B-G, Raus L, Jitareanu G (2017) Sorption potential of alkaline treated straw and a soil for sulfonylurea herbicide removal from aqueous solutions: an environmental management strategy. Chemosphere 186:360–366. https://doi.org/10.1016/j.chemosphere.2017.07.140
Cavas L, Karabay Z, Alyuruk H et al (2011) Thomas and artificial neural network models for the fixed-bed adsorption of methylene blue by a beach waste Posidonia oceanica (L.) dead leaves. Chem Eng J 171:557–562. https://doi.org/10.1016/j.cej.2011.04.030
Cazetta AL, Vargas AMM, Nogami EM et al (2011) NaOH-activated carbon of high surface area produced from coconut shell: kinetics and equilibrium studies from the methylene blue adsorption. Chem Eng J 174:117–125. https://doi.org/10.1016/j.cej.2011.08.058
Çelekli A, Birecikligil SS, Geyik F, Bozkurt H (2012) Prediction of removal efficiency of Lanaset Red G on walnut husk using artificial neural network model. Bioresour Technol 103:64–70. https://doi.org/10.1016/j.biortech.2011.09.106
Çelekli A, Bozkurt H, Geyik F (2016) Artificial neural network and genetic algorithms for modeling of removal of an azo dye on walnut husk. Desalin Water Treat 57:15580–15591. https://doi.org/10.1080/19443994.2015.1070759
Chakraborty S, Chowdhury S, Das SP (2013) Artificial neural network (ANN) modeling of dynamic adsorption of crystal violet from aqueous solution using citric-acid-modified rice (Oryza sativa) straw as adsorbent. Clean Technol Environ Policy 15:255–264. https://doi.org/10.1007/s10098-012-0503-4
Chen B, Chen Z, Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol 102:716–723. https://doi.org/10.1016/j.biortech.2010.08.067
Choi J, Won W, Capareda SC (2019) The economical production of functionalized Ashe juniper derived-biochar with high hazardous dye removal efficiency. Ind Crops Prod 137:672–680. https://doi.org/10.1016/j.indcrop.2019.05.006
Chowdhury S, Das SP (2013) Artificial neural network (ANN) modeling of adsorption of methylene blue by NaOH-modified rice husk in a fixed-bed column system. Environ Sci Pollut Res 20:1050–1058. https://doi.org/10.1007/s11356-012-0912-2
Chowdhury ZZ, Chandran RRR, Jahan A (2019) Symmetry extraction of cellulose nano-whiskers using ionic mathematical modelling using Box–Behnken design. Symmetry 11:1148. https://doi.org/10.3390/sym11091148
Cocos C (2021) Adsorption of azo-anionic dyes in a solution using modified. Water 13:1–20
Çoruh S, Gürkan H, Kilic E, Geyikci F (2014) Prediction of adsorption efficiency for the removal malachite green and acid blue 161 dyes by waste marble dust using ANN. Glob Nest J 16:676–689. https://doi.org/10.30955/gnj.001366
Daffalla SB, Mukhtar H, Shaharun MS (2020) Preparation and characterization of rice husk adsorbents for phenol removal from aqueous systems. PLoS ONE. https://doi.org/10.1371/journal.pone.0243540
Dai Y, Zhang N, Xing C et al (2019) The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: a review. Chemosphere 223:12–27
Dan Y, Ji M, Tao S et al (2021) Impact of rice straw biochar addition on the sorption and leaching of phenylurea herbicides in saturated sand column. Sci Total Environ 769:144536. https://doi.org/10.1016/j.scitotenv.2020.144536
Das AK, Saha S, Pal A, Maji SK (2009) Surfactant-modified alumina: an efficient adsorbent for malachite green removal from water environment. J Environ Sci Health Part A Toxic Hazard Subst Environ Eng 44:896–905. https://doi.org/10.1080/10934520902958708
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40. https://doi.org/10.1016/j.susmat.2016.06.002
Demirhan E (2020) Response surface methodology approach for adsorptive removal of Reactive Blue 19 onto green pea pod. Water Sci Technol 19:1137–1147. https://doi.org/10.2166/wst.2020.199
Deshmukh SK (2012) Indigo dye removal by using coconut shell adsorbent and performance evaluation by artificial neural network. Lect Notes Mech Eng. https://doi.org/10.1007/978-81-322-1007-8_61
Dolatabadi M, Mehrabpour M, Esfandyari M et al (2018) Modeling of simultaneous adsorption of dye and metal ion by sawdust from aqueous solution using of ANN and ANFIS. Chemom Intell Lab Syst 181:72–78. https://doi.org/10.1016/j.chemolab.2018.07.012
Dutta M, Ghosh P, Basu JK (2012) Prediction of adsorption capacity of microwave assisted activated carbon for the decolorization of direct blue 86 by using artificial neural network. Asian J Appl Sci 5:414–422. https://doi.org/10.3923/ajaps.2012.414.422
Dutta M, Das U, Mondal S et al (2015) Adsorption of acetaminophen by using tea waste derived activated carbon. Int J Environ Sci 6:270–281. https://doi.org/10.6088/ijes.6031
El-Geundi MS, Nassar MM, Farrag TE, Ahmed MH (2013) Methomyl adsorption onto cotton stalks activated carbon (CSAC): equilibrium and process design. Procedia Environ Sci 17:630–639. https://doi.org/10.1016/j.proenv.2013.02.079
Elhag Elhussien M (2017) Removal of ciprofloxacin hydrochloride from aqueous solution by pomegranate peel grown in Alziedab agricultural scheme—river Nile State. Sudan Adv Biochem 5:89. https://doi.org/10.11648/j.ab.20170505.12
Elmorsi RR, El-Wakeel ST, Shehab El-Dein WA et al (2019) Adsorption of methylene blue and Pb2+ by using acid-activated Posidonia oceanica waste. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-39945-1
Fagbohungbe MO, Herbert BMJ, Hurst L et al (2017) The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag 61:236–249. https://doi.org/10.1016/j.wasman.2016.11.028
Falconer IR, Chapman HF, Moore MR, Ranmuthugala G (2006) Endocrine-disrupting compounds: a review of their challenge to sustainable and safe water supply and water reuse. Environ Toxicol 21:181–191
Faria PCC, Órfão JJM, Pereira MFR (2004) Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res 38:2043–2052. https://doi.org/10.1016/j.watres.2004.01.034
Fegousse A, El Gaidoumi A, Miyah Y et al (2019) Pineapple bark performance in dyes adsorption: optimization by the central composite design. J Chem. https://doi.org/10.1155/2019/3017163
Fernandez ME, Nunell GV, Bonelli PR, Cukierman AL (2014) Activated carbon developed from orange peels: batch and dynamic competitive adsorption of basic dyes. Ind Crops Prod 62:437–445. https://doi.org/10.1016/j.indcrop.2014.09.015
Franco D, Silva LFO, da Boit Martinello K et al (2021) Transforming agricultural waste into adsorbent: application of Fagopyrum esculentum wheat husks treated with H2SO4 to adsorption of the 2,4-D herbicide. J Environ Chem Eng 9:106872. https://doi.org/10.1016/j.jece.2021.106872
Gehlot P, Daga K, Pallavi V (2009) Adsorptive treatment of Azo dyes from aqueous solution using PVA coated carbon black as an adsorbent. Asian J Chem 21:5941–5946. https://doi.org/10.15242/iicbe.c0315115
Gehlot P, Daga K, Mehta R (2011) Adsorption study of dye water using poly vinyl alcohol coated carbon black as an effective and low cost adsorbent. Int J Chem 3:56–61. https://doi.org/10.5539/ijc.v3n3p56
George Nche N-A, Bopda A, Raoul Tchuifon Tchuifon D et al (2017) Removal of paracetamol from aqueous solution by adsorption onto activated carbon prepared from rice husk. J Chem Pharm Res 9:56–68
Ghaedi M, Ghaedi AM, Ansari A et al (2014) Artificial neural network and particle swarm optimization for removal of methyl orange by gold nanoparticles loaded on activated carbon and Tamarisk. Spectrochim Acta Part A Mol Biomol Spectrosc 132:639–654. https://doi.org/10.1016/j.saa.2014.04.175
Ghaedi AM, Ghaedi M, Karami P (2015) Comparison of ultrasonic with stirrer performance for removal of sunset yellow (SY) by activated carbon prepared from wood of orange tree: artificial neural network modeling. Spectrochim Acta Part A Mol Biomol Spectrosc 138:789–799. https://doi.org/10.1016/j.saa.2014.11.019
Ghorbani F, Kamari S (2017) Application of response surface methodology for optimization of methyl orange adsorption by Fe-grafting sugar beet bagasse. Adsorpt Sci Technol 35:317–338. https://doi.org/10.1177/0263617416675625
Ghosh SK, Bandyopadhyay A (2017) Adsorption of methylene blue onto citric acid treated carbonized bamboo leaves powder: equilibrium, kinetics, thermodynamics analyses. J Mol Liq 248:413–424. https://doi.org/10.1016/j.molliq.2017.10.086
Gong L, Sun W, Kong L (2013) Adsorption of methylene blue by NaOH-modified Dead leaves of plane trees. Comput Water Energy Environ Eng 02:13–19. https://doi.org/10.4236/cweee.2013.22b003
González-García P (2018) Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew Sustain Energy Rev 82:1393–1414. https://doi.org/10.1016/j.rser.2017.04.117
González-García P, Centeno TA, Urones-Garrote E et al (2013) Microstructure and surface properties of lignocellulosic-based activated carbons. Appl Surf Sci 265:731–737. https://doi.org/10.1016/j.apsusc.2012.11.092
Gunasekar V, Ponnusami V (2013) Kinetics, equilibrium, and thermodynamic studies on adsorption of methylene blue by carbonized plant leaf powder. J Chem. https://doi.org/10.1155/2013/415280
Güzel F, Sayğılı H, Akkaya Sayğılı G et al (2017) Optimal oxidation with nitric acid of biochar derived from pyrolysis of weeds and its application in removal of hazardous dye methylene blue from aqueous solution. J Clean Prod 144:260–265. https://doi.org/10.1016/j.jclepro.2017.01.029
Hayati B, Mahmoodi NM (2012) Modification of activated carbon by the alkaline treatment to remove the dyes from wastewater: mechanism, isotherm and kinetic. Desalin Water Treat 47:322–333. https://doi.org/10.1080/19443994.2012.696429
Heibati B, Rodriguez-Couto S, Ozgonenel O et al (2016) A modeling study by artificial neural network on ethidium bromide adsorption optimization using natural pumice and iron-coated pumice. Desalin Water Treat 57:13472–13483. https://doi.org/10.1080/19443994.2015.1060906
Hoo HM, Mohamad M, Wannahari R (2022) Optimization study of malachite green dye adsorption by eggshell using optimization study of malachite green dye adsorption by eggshell using response surface methodology. https://doi.org/10.4028/p-66g2qs
Hu Y (2018) Research and development of regeneration technology of activated carbon. Coal Chem Ind 41:136–139
Hussin ZM, Talib N, Hussin NM et al (2015) Methylene blue adsorption onto NaOH modified durian leaf powder: isotherm and kinetic studies. Am J Environ Eng 5:38–43. https://doi.org/10.5923/c.ajee.201501.07
Ibrahim AO, Adegoke KA, Adegoke RO et al (2021) Adsorptive removal of different pollutants using metal-organic framework adsorbents. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.115593
Islam MT, Hyder AG, Saenz-Arana R et al (2019) Removal of methylene blue and tetracycline from water using peanut shell derived adsorbent prepared by sulfuric acid reflux. J Environ Chem Eng 7:102816. https://doi.org/10.1016/j.jece.2018.102816
Iwuozor KO, Ogunfowora LA, Oyekunle IP (2021) Review on sugarcane-mediated nanoparticle synthesis: a green approach. Sugar Tech. https://doi.org/10.1007/s12355-021-01038-7
Jain SN, Gogate PR (2017a) NaOH-treated dead leaves of Ficus racemosa as an efficient biosorbent for Acid Blue 25 removal. Int J Environ Sci Technol 14:531–542. https://doi.org/10.1007/s13762-016-1160-7
Jain SN, Gogate PR (2017b) Acid Blue 113 removal from aqueous solution using novel biosorbent based on NaOH treated and surfactant modified fallen leaves of Prunus dulcis. J Environ Chem Eng 5:3384–3394. https://doi.org/10.1016/j.jece.2017.06.047
Jain SN, Gogate PR (2017c) Adsorptive removal of acid violet 17 dye from wastewater using biosorbent obtained from NaOH and H2SO4 activation of fallen leaves of Ficus racemosa. J Mol Liq 243:132–143. https://doi.org/10.1016/j.molliq.2017.08.009
Jain R, Mathur M, Sikarwar S (2006b) Removal of indigocarmine from industrial effluents using low cost absorbent. J Sci Ind Res (india) 65:258–263
Jain H, Bartzis C, Clarke E (2006a) Satisfiability checking of non-clausal formulas using general matings. In: Lecture notes in computer science (including subseries lecture notes in artificial intelligence and lecture notes in bioinformatics), vol 4121 LNCS, pp 75–89. https://doi.org/10.1007/11814948_10
Jawad AH, Rashid RA, Ishak MAM, Wilson LD (2016) Adsorption of methylene blue onto activated carbon developed from biomass waste by H2SO4 activation: kinetic, equilibrium and thermodynamic studies. Desalin Water Treat 57:25194–25206. https://doi.org/10.1080/19443994.2016.1144534
Jawad AH, Abdulhameed AS, Mastuli MS (2020) Acid-factionalized biomass material for methylene blue dye removal: a comprehensive adsorption and mechanism study. J Taibah Univ Sci 14:305–313. https://doi.org/10.1080/16583655.2020.1736767
Jia M, Wang F, Bian Y et al (2013) Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Bioresour Technol 136:87–93. https://doi.org/10.1016/j.biortech.2013.02.098
Jocić A, Breitenbach S, Pašti IA et al (2022) Viscose-derived activated carbons as adsorbents for malathion, dimethoate, and chlorpyrifos—screening, trends, and analysis. Environ Sci Pollut Res 29:35138–35149. https://doi.org/10.1007/s11356-022-18721-1
Jung C, Son A, Her N et al (2015) Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: a review. J Ind Eng Chem 27:1–11
Kabir ER, Rahman MS, Rahman I (2015) A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 40:241–258
Kassegne AB, Okonkwo JO, Berhanu T et al (2020) Ecological risk assessment of organochlorine pesticides and polychlorinated biphenyls in water and surface sediment samples from Akaki River catchment, central Ethiopia. Emerg Contam 6:396–404. https://doi.org/10.1016/j.emcon.2020.11.004
Khan MA, Saeed K et al (2012) In vitro adsorption of drugs using modified sugarcane bagasse. J Sci Ind Res (india) 71:161–167
Khan A, Wang X, Gul K et al (2018) Microwave-assisted spent black tea leaves as cost-effective and powerful green adsorbent for the efficient removal of Eriochrome black T from aqueous solutions. Egypt J Basic Appl Sci 5:171–182. https://doi.org/10.1016/j.ejbas.2018.04.002
Khasri A, Bello OS, Ahmad MA (2018) Mesoporous activated carbon from Pentace species sawdust via microwave-induced KOH activation: optimization and methylene blue adsorption. Res Chem Intermed 44:5737–5757. https://doi.org/10.1007/s11164-018-3452-7
Khodaie M, Ghasemi N, Moradi B, Rahimi M (2013) Removal of methylene blue from wastewater by adsorption onto ZnCl2 activated corn husk carbon equilibrium studies. J Chem. https://doi.org/10.1155/2013/383985
Khonde RD, Pandharipande SL (2012) Artificial neural network modeling for adsorption of dyes from aqueous solution using rice husk carbon. Int J Comput Appl 41:1–5. https://doi.org/10.5120/5526-7567
Kooh MRR, Dahri MK, Lim LBL et al (2016) Batch adsorption studies of the removal of methyl violet 2B by soya bean waste: isotherm, kinetics and artificial neural network modelling. Environ Earth Sci 75:1–14. https://doi.org/10.1007/s12665-016-5582-9
Lai JY, Li YT, Wang TP (2010) In vitro response of retinal pigment epithelial cells exposed to chitosan materials prepared with different cross-linkers. Int J Mol Sci 11:5256–5272. https://doi.org/10.3390/ijms11125256
Le Bellec F, Vélu A, Fournier P et al (2015) Helping farmers to reduce herbicide environmental impacts. Ecol Indic 54:207–216. https://doi.org/10.1016/j.ecolind.2015.02.020
Li H, Cao Y, Zhang D, Pan B (2018a) pH-dependent KOW provides new insights in understanding the adsorption mechanism of ionizable organic chemicals on carbonaceous materials. Sci Total Environ 618:269–275. https://doi.org/10.1016/j.scitotenv.2017.11.065
Li J, Yu G, Pan L et al (2018b) Study of ciprofloxacin removal by biochar obtained from used tea leaves. J Environ Sci (china) 73:20–30. https://doi.org/10.1016/j.jes.2017.12.024
Li Z, Wang M, Jin C et al (2020) Synthesis of novel Co3O4 hierarchical porous nanosheets via corn stem and MOF-Co templates for efficient oxytetracycline degradation by peroxymonosulfate activation. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123789
Liao M, Yao Y (2021) Applications of artificial intelligence-based modeling for bioenergy systems: a review. GCB Bioenergy 13:774–802. https://doi.org/10.1111/gcbb.12816
Mahmoudi F, Amini MM, Sillanpää M (2020) Hydrothermal synthesis of novel MIL-100(Fe)@SBA-15 composite material with high adsorption efficiency towards dye pollutants for wastewater remediation. J Taiwan Inst Chem Eng 116:303–313. https://doi.org/10.1016/j.jtice.2020.11.025
Makeswari M, Santhi T, Ezhilarasi MR (2016) Adsorption of methylene blue dye by citric acid modified leaves of Ricinus communis from aqueous solutions. J Chem Pharm Res 8:452–462
Maleki A, Daraei H, Khodaei F et al (2013) Investigation of potato peel-based bio-sorbent efficiency in reactive dye removal: artificial neural network modeling and genetic algorithms optimization. J Adv Environ Health Res 1:21–29
Mandal A, Singh N, Purakayastha TJ (2017) Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal. Sci Total Environ 577:376–385. https://doi.org/10.1016/j.scitotenv.2016.10.204
Martini S, Roni KA (2021) The existing technology and the application of digital artificial intelligent in the wastewater treatment area: a review paper. J Phys Conf Ser. https://doi.org/10.1088/1742-6596/1858/1/012013
Mashayekh-Salehi A, Moussavi G (2016) Removal of acetaminophen from the contaminated water using adsorption onto carbon activated with NH4Cl. Desalin Water Treat 57:12861–12873. https://doi.org/10.1080/19443994.2015.1051588
Mazloom MS, Rezaei F, Hemmati-Sarapardeh A et al (2020) Artificial intelligence based methods for asphaltenes adsorption by nanocomposites: application of group method of data handling, least squares support vector machine, and artificial neural networks. Nanomaterials 10:1–29. https://doi.org/10.3390/nano10050890
Mendes KF, Freguglia RMDO, Martins BAB et al (2017) Cow bonechar for pesticide removal from drinking water. Sch J Agric Vet Sci 4:504–512. https://doi.org/10.21276/sjavs.2017.4.11.11
Moeinian K, Mehdinia SM (2019) Removing methylene blue from aqueous solutions using rice husk silica adsorbent. Polish J Environ Stud 28:2281–2288. https://doi.org/10.15244/pjoes/91044
Mohammad SG, Ahmed SM (2017) Preparation of environmentally friendly activated carbon for removal of pesticide from aqueous media. Int J Ind Chem 8:121–132. https://doi.org/10.1007/s40090-017-0115-2
Moreno-Castilla C (2004) Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon N Y 42:83–94. https://doi.org/10.1016/j.carbon.2003.09.022
Mu’azu ND, Jarrah N, Zubair M, Alagha O (2017) Removal of phenolic compounds from water using sewage sludge-based activated carbon adsorption: a review. Int J Environ Res Public Health 14:1094
Nasr M, Mahmoud AED, Fawzy M, Radwan A (2017) Artificial intelligence modeling of cadmium(II) biosorption using rice straw. Appl Water Sci 7:823–831. https://doi.org/10.1007/s13201-015-0295-x
Netto MS, Georgin J, Franco DSP et al (2022) Effective adsorptive removal of atrazine herbicide in river waters by a novel hydrochar derived from Prunus serrulata bark. Environ Sci Pollut Res 29:3672–3685. https://doi.org/10.1007/s11356-021-15366-4
Ngakou CS, Anagho GS, Ngomo HM (2019) Non-linear regression analysis for the adsorption kinetics and equilibrium isotherm of phenacetin onto activated carbons. Curr J Appl Sci Technol 36:1–18. https://doi.org/10.9734/cjast/2019/v36i430246
Niad M, Rasoolzadeh L, Zarei F (2014) Biosorption of copper (II) on Sargassum angostifolium C.Agardh phaeophyceae biomass. Chem Speciat Bioavailab 26:176–183. https://doi.org/10.3184/095422914X14039722451529
Ogunlalu O, Oyekunle IP, Iwuozor KO et al (2021) Trends in the mitigation of heavy metal ions from aqueous solutions using unmodified and chemically-modified agricultural waste adsorbents. Curr Res Green Sustain Chem 4:100188. https://doi.org/10.1016/j.crgsc.2021.100188
Ogunmodede J, Akanji SB, Bello OS (2021) Moringa oleifera seed pod-based adsorbent for the removal of paracetamol from aqueous solution: a novel approach toward diversification. Environ Prog Sustain Energy 40:1–11. https://doi.org/10.1002/ep.13615
Ojedokun AT, Bello OS (2017a) Liquid phase adsorption of Congo red dye on functionalized corn cobs. J Dispers Sci Technol 38:1285–1294. https://doi.org/10.1080/01932691.2016.1234384
Ojedokun AT, Bello OS (2017b) Kinetic modeling of liquid-phase adsorption of Congo red dye using guava leaf-based activated carbon. Appl Water Sci 7:1965–1977. https://doi.org/10.1007/s13201-015-0375-y
Oyelude E (2015) Adsorption of methylene blue from aqueous solution using acid modified calotropis procera leaf powder. J Appl Sci Environ Sanit 6(4):477–484
Özer D, Dursun G, Özer A (2007) Methylene blue adsorption from aqueous solution by dehydrated peanut hull. J Hazard Mater 144:171–179. https://doi.org/10.1016/j.jhazmat.2006.09.092
Pathania D, Sharma S, Singh P (2017) Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab J Chem 10:S1445–S1451. https://doi.org/10.1016/j.arabjc.2013.04.021
Phan KA, Phihusut D, Tuntiwiwattanapun N (2022) Preparation of rice husk hydrochar as an atrazine adsorbent: optimization, characterization, and adsorption mechanisms. J Environ Chem Eng 10:107575. https://doi.org/10.1016/j.jece.2022.107575
Philippot G, Albino M, Chung UC et al (2015) Continuous BaTi1-yZryO3 (0 ≤ y ≤ 1) nanocrystals synthesis in supercritical fluids for nanostructured lead-free ferroelectric ceramics. Mater Des 86:354–360. https://doi.org/10.1016/j.matdes.2015.07.111
Putri KNA, Keereerak A, Chinpa W (2020) Novel cellulose-based biosorbent from lemongrass leaf combined with cellulose acetate for adsorption of crystal violet. Int J Biol Macromol 156:762–772. https://doi.org/10.1016/j.ijbiomac.2020.04.100
Qiao K, Tian W, Bai J et al (2018) Preparation of biochar from Enteromorpha prolifera and its use for the removal of polycyclic aromatic hydrocarbons (PAHs) from aqueous solution. Ecotoxicol Environ Saf 149:80–87. https://doi.org/10.1016/j.ecoenv.2017.11.027
Raja GL, Subhashree KD, Kantayya KE (2022) In utero exposure to endocrine disruptors and developmental neurotoxicity: implications for behavioural and neurological disorders in adult life. Environ Res. https://doi.org/10.1016/j.envres.2021.111829
Rashid RA, Jawad AH, Ishak MABM, Kasim NN (2018) FeCl3-activated carbon developed from coconut leaves: characterization and application for methylene blue removal. Sains Malaysiana 47:603–610. https://doi.org/10.17576/jsm-2018-4703-22
Sadaf S, Bhatti HN (2016) Response surface methodology approach for optimization of adsorption process for the removal of Indosol Yellow BG dye from aqueous solution by agricultural waste. Desalin Water Treat 57:11773–11781. https://doi.org/10.1080/19443994.2015.1048308
Sadaf S, Bhatti HN, Arif M et al (2015) Box–Behnken design optimization for the removal of Direct Violet 51 dye from aqueous solution using lignocellulosic waste. Desalin Water Treat 56:2425–2437. https://doi.org/10.1080/19443994.2014.968215
Safarik I, Safarikova M (2010) Magnetic fluid modified peanut husks as an adsorbent for organic dyes removal. Phys Procedia 9:274–278. https://doi.org/10.1016/j.phpro.2010.11.061
Saha P, Das Mishra R, Husk R (2012) Adsorption of safranin onto chemically modified rice husk in a upward flow packed bed reactor : artificial neural network modeling. Elixir Pollut 44:7579–7583
Salman JM, Hameed BH (2010) Removal of insecticide carbofuran from aqueous solutions by banana stalks activated carbon. J Hazard Mater 176:814–819. https://doi.org/10.1016/j.jhazmat.2009.11.107
Samuel SM, Abigail MEA, Chidambaram R (2015) Isotherm modelling, kinetic study and optimization of batch parameters using response surface methodology for effective removal of Cr (VI) using fungal biomass. PLoS ONE 10:1–15. https://doi.org/10.1371/journal.pone.0116884
Schug TT, Janesick A, Blumberg B, Heindel JJ (2011) Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol 127:204–215. https://doi.org/10.1016/j.jsbmb.2011.08.007
Sham AYW, Notley SM (2018) Adsorption of organic dyes from aqueous solutions using surfactant exfoliated graphene. J Environ Chem Eng 6:495–504. https://doi.org/10.1016/j.jece.2017.12.028
Shang JG, Kong XR, He LL et al (2016) Low-cost biochar derived from herbal residue: characterization and application for ciprofloxacin adsorption. Int J Environ Sci Technol 13:2449–2458. https://doi.org/10.1007/s13762-016-1075-3
Shen W, Li Z, Liu Y (2010) Surface chemical functional groups modification of porous carbon. Recent Patents Chem Eng 1:27–40. https://doi.org/10.2174/1874478810801010027
Singh H, Chauhan G, Jain AK, Sharma SK (2017) Adsorptive potential of agricultural wastes for removal of dyes from aqueous solutions. J Environ Chem Eng 5:122–135. https://doi.org/10.1016/j.jece.2016.11.030
Sivakumar P, Palanisamy PN (2009) Adsorptive removal of reactive and direct dyes using non-conventional adsorbent–column studies. J Sci Ind Res (india) 68:894–899
Skinner MK (2011) Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res Part C Embryo Today Rev 93:51–55. https://doi.org/10.1002/bdrc.20199
Song Y, Ding S, Chen S et al (2018) Removal of malachite green in aqueous solution by adsorption on sawdust removal of malachite green in aqueous solution by adsorption on sawdust. Korean J Chem Eng. https://doi.org/10.1007/s11814-015-0103-1
Sunsandee N, Ramakul P, Phatanasri S, Pancharoen U (2020) Biosorption of dicloxacillin from pharmaceutical waste water using tannin from Indian almond leaf: Kinetic and equilibrium studies. Biotechnol Rep 27:e00488. https://doi.org/10.1016/j.btre.2020.e00488
Tang Y, Li Y, Zhao Y et al (2017) Enhanced removal of methyl violet using NaOH-modified C. camphora leaves powder and its renewable adsorption. Desalin Water Treat 98:306–314. https://doi.org/10.5004/dwt.2017.21569
Tapia-Orozco N, Ibarra-Cabrera R, Tecante A et al (2016) Removal strategies for endocrine disrupting chemicals using cellulose-based materials as adsorbents: a review. J Environ Chem Eng 4:3122–3142
Thambiraj S, Sharmila G, Ravi Shankaran D (2018) Green adsorbents from solid wastes for water purification application. In: Materials today: proceedings, pp 16675–16683
Tursi A, Chatzisymeon E, Chidichimo F et al (2018) Removal of endocrine disrupting chemicals from water: adsorption of bisphenol-A by biobased hydrophobic functionalized cellulose. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15112419
Üner O, Geçgel Ü, Kolancilar H, Bayrak Y (2017) Adsorptive removal of rhodamine B with activated carbon obtained from okra wastes. Chem Eng Commun 204:772–783. https://doi.org/10.1080/00986445.2017.1319361
Urbain KY, Fodjo EK, Ardjouma D et al (2017) Removal of imidacloprid using activated carbon produced from Ricinodendron heudelotii shells. Bull Chem Soc Ethiop 31:397–409. https://doi.org/10.4314/bcse.v31i3.4
Utomo HD, Phoon RYN, Shen Z et al (2015) Removal of methylene blue using chemically modified sugarcane bagasse. Nat Resour 06:209–220. https://doi.org/10.4236/nr.2015.64019
Venkataraghavan R, Thiruchelvi R, Sharmila D (2020) Statistical optimization of textile dye effluent adsorption by Gracilaria edulis using Plackett-Burman design and response surface methodology. Heliyon 6:e05219. https://doi.org/10.1016/j.heliyon.2020.e05219
Wang S, Gao B, Zimmerman AR et al (2015) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol 175:391–395. https://doi.org/10.1016/j.biortech.2014.10.104
Wang C, Liu L, Zhang Z et al (2016) Magnetic biomass activated carbon-based solid-phase extraction coupled with high performance liquid chromatography for the determination of phenylurea herbicides in bottled rose juice and water samples. Food Anal Methods 9:80–87. https://doi.org/10.1007/s12161-015-0181-z
Wang L, Chen G, Ling C, Zhang J, Szerlag K (2017) Adsorption of ciprofloxacin on to bamboo charcoal: effects of pH, salinity, cations, and phosphate. Environ Prog Sustain Energy 36:1108–1115. https://doi.org/10.1002/ep.12579
Wee SY, Aris AZ (2017) Endocrine disrupting compounds in drinking water supply system and human health risk implication. Environ Int 106:207–233
Xing R, Liu Y, Wang Y et al (2007) Active solid acid catalysts prepared by sulfonation of carbonization-controlled mesoporous carbon materials. Microporous Mesoporous Mater 105:41–48. https://doi.org/10.1016/j.micromeso.2007.06.043
Xu Y, Hu X (2010) Activated carbon adsorption of methylene blue based on artificial neural network. Mater Rev 24:23
Xu X, Hu X, Ding Z et al (2017) Waste-art-paper biochar as an effective sorbent for recovery of aqueous Pb (II) into value-added PbO nanoparticles. Chem Eng J 308:863–871. https://doi.org/10.1016/j.cej.2016.09.122
Yahya MA, Al-Qodah Z, Ngah CWZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sustain Energy Rev 46:218–235. https://doi.org/10.1016/j.rser.2015.02.051
Yang JX, Hong GB (2018) Adsorption behavior of modified Glossogyne tenuifolia leaves as a potential biosorbent for the removal of dyes. J Mol Liq 252:289–295. https://doi.org/10.1016/j.molliq.2017.12.142
Yang Y, Lin X, Wei B et al (2014) Evaluation of adsorption potential of bamboo biochar for metal-complex dye: equilibrium, kinetics and artificial neural network modeling. Int J Environ Sci Technol 11:1093–1100. https://doi.org/10.1007/s13762-013-0306-0
Yang G, Wu L, Xian Q et al (2016) Removal of congo red and methylene blue from aqueous solutions by vermicompost-derived biochars. PLoS ONE 11:e0154562
Yang Y, Ma X, Yang C et al (2022) Eco-friendly and acid-resistant magnetic porous carbon derived from ZIF-67 and corn stalk waste for effective removal of imidacloprid and thiamethoxam from water. Chem Eng J 430:132999. https://doi.org/10.1016/j.cej.2021.132999
Yaseen DA, Scholz M (2018) Treatment of synthetic textile wastewater containing dye mixtures with microcosms. Environ Sci Pollut Res 25:1980–1997. https://doi.org/10.1007/s11356-017-0633-7
Yu H, Wang R, Guo J et al (2016) Color-inducing elements and mechanisms in nephrites from Golmud, Qinghai, NW China: insights from spectroscopic and compositional analyses. J Miner Petrol Sci 111:313–325. https://doi.org/10.2465/jmps.151103
Yu J, Zhu J, Feng L et al (2019) Removal of cationic dyes by modified waste biosorbent under continuous model: competitive adsorption and kinetics. Arab J Chem 12:2044–2051. https://doi.org/10.1016/j.arabjc.2014.12.022
Yusop MFM, Mohd Johan Jaya E, Mohd Din AT et al (2022) Single-stage optimized microwave-induced activated carbon from coconut shell for cadmium adsorption. Chem Eng Technol. https://doi.org/10.1002/ceat.202200051
Zeng ZW, Tan XF, Liu YG et al (2018) Comprehensive adsorption studies of doxycycline and ciprofloxacin antibiotics by biochars prepared at different temperatures. Front Chem 6:1–11. https://doi.org/10.3389/fchem.2018.00080
Zheng C, Zhao L, Zhou X et al (2013) Treatment technologies for organic wastewater. Water Treat 11:250–286. https://doi.org/10.5772/52665
Zhou Y, Xing XH, Liu Z et al (2008) Enhanced coagulation of ferric chloride aided by tannic acid for phosphorus removal from wastewater. Chemosphere 72:290–298. https://doi.org/10.1016/j.chemosphere.2008.02.028
Zhou R, Zhang M, Zhou J, Wang J (2019) Optimization of biochar preparation from the stem of Eichhornia crassipes using response surface methodology on adsorption of Cd2+. Sci Rep 9:1–17. https://doi.org/10.1038/s41598-019-54105-1
Zhu J, Xiang S, Zhang B et al (2022) Oxygen-defective graphdiyne for ultra-efficient removal of sulfonylurea herbicides from aqueous solution. J Environ Chem Eng 10:107724. https://doi.org/10.1016/j.jece.2022.107724
Acknowledgements
K. A. Adegoke acknowledges the Global Excellence Stature (GES) 4.0 Postdoctoral Fellowships Fourth Industrial Revolution and the University of Johannesburg, South Africa. O. S. Bello acknowledges the support received from LAUTECH 2016 TET Fund Institution Based Research Intervention (TETFUND/DESS/UNI/OGBOMOSO/RP/VOL. IX). N. W. Maxakato acknowledges the supports received from the National Research Foundation of South Africa: Grant Number 118148, National Research Foundation of South Africa: Grant Number 138083, and Centre for Nanomaterials Science Research-University of Johannesburg, and University of Johannesburg, South Africa.
Funding
Open access funding provided by University of Johannesburg.
Author information
Authors and Affiliations
Contributions
KAA contributed to conceptualization, investigation, methodology, data curation, validation, visualization, writing—original draft preparation, and writing—review, and editing. SOA, OOA, TOA and ROA contributed to investigation, data curation, validation, visualization, writing—original draft preparation. NWM contributed to material, validation, and supervision. OSB contributed to material, data curation, validation, review, and editing and supervision. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies involving human or animal subjects.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adegoke, K.A., Akinnawo, S.O., Adebusuyi, T.A. et al. Modified biomass adsorbents for removal of organic pollutants: a review of batch and optimization studies. Int. J. Environ. Sci. Technol. 20, 11615–11644 (2023). https://doi.org/10.1007/s13762-023-04872-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04872-2