Abstract
In the current study, the Layered Double Hydroxide (LDH) containing combinations of iron, magnesium-iron and zinc-iron as a modified Fenton-Like reaction to investigate its catalytic activity as a solar photocatalyst is investigated. In this regard, magnetic Fe2+/Fe3+, Mg2+/Fe3+ and Zn2+/Fe3+ catalysts were successfully fabricated via a simple co-precipitation technique and then characterized through X-ray diffraction and a high-resolution Transmission Electron Microscope. The prepared catalysts evaluated as heterogeneous solar Fenton-Like treatment facility. The results revealed that the application of LDH catalysts incorporated with H2O2, promoting Fenton-Like reaction for synthetic textile wastewater effluents loaded with Remazol Brilliant Blue R dye mineralization. The catalytic activity of LDH system was systematically evaluated through testing the reaction conditions including initial pH, LDH catalyst and H2O2 doses, initial load of dye in wastewater and finally the solar radiation time. Under the optimal conditions that are optimized through factorial design, the LDH-Fenton-Like system could be reached to 74% removal within 15-min of solar irradiance time in acidic pH (about 3.0). For the all studied systems, the reaction is exothermic, non-spontaneous in nature and works at low-energy barrier. The catalysts are being reused after recovery for six reaction cycles with only 10% reduction in its activity. Hence, the recycling approach is effective and highlighting the potential of recovering and reusing magnetized nanoparticles for economic large-scale water treatment applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Major water pollution is occurred from the textile dying release to the environment since 15% of the used dyes are vanished after processing and contaminate the biosphere. To add up, their toxicity and persistence posses a threat damage to the ecosystem, marine life and also to human health through the release of such carcinogenic effluents to the drinking water resources (Tony 2019; Zhao et al. 2009). Reactive dyes are anionic dyes that are extensively used in the textile dying processes since they include reactive groups to bind onto the fibers to be dyed through the formation of the covalent bonds (Sajab e al. 2019; Ashour et al. 2014). Remazol Brilliant blue, is a type of reactive dyes that is used extensively in cellulosic fibers’ dying facilities, such dye is categorized as one of the most stable and resistant dyes (Khan et al. 2015; Tony 2020a, b). Its fixation effectiveness is about 80% because of the formation of vinyl sulfone and of 2-hydroxyethyl-sulfone besides the hydrolysis reactions (Taha 2010; Rezaee et al. 2008). However, due to the presence of anthraquinone aromatic composition structure, such dye displays a resistance to degradation and prevents sunlight transmission into the streams (Unal et al. 2019). Thus, the presence of such dyes in aqueous effluents causes series damage, and it is imperative to be treated before the final disposal to the aquatic system (Ashour and Tony 2017).
During the last decades, several chemical (Thabet et al. 2021), biological (Feng & Le-Cheng, 2004) and/or physical treatments (Mitsika et al. 2013) were introduced for textile effluents elimination for toxicity control. However, the majority of those techniques were unsuccessful since they transfer the pollutants phase rather than eliminating them, thus the end products after treatments may be more toxic or need further treatments (Guo et al. 2018; Tony 2021a). The crucial goal of scientists is searching for harmless end products technologies for eliminating the persistent and toxic substances. Recently, superior oxidation techniques, namely, advanced oxidation processes (AOPs) have been emerged as a of powerful oxidizing tool through the formation of high reactive intermediates like hydroxyl radicals (˙OH) which are signified as the second most oxidant following fluorine that could be successfully transform the effluent to harmless end products. AOPs are including numerous methodologies such as Fenton (Van et al. 2020), TiO2 (Tony and Mansour 2019), ozonation, photo catalysis (Tony 2020a) and other treatment methods. The non-specific ˙OH radicals are attacking a wide range of pollutants, i.e., phenolic compounds (Feng & Le-Cheng, 2004), aromatic hydrocarbons (Guo et al. 2018), dyestuff wastewater (Feng et al. 2013), pesticides (Mitsika et al. 2013) and pharmaceuticals (Changotra et al. 2019) and mineralizing them. Among AOPs, Fenton reaction that depends on producing the high reactive hydroxyl radicals with a high oxidation rate compared to other AOPs (Sharma et al. 2015). Due to such advantages Fenton system (Fe2+/3+/H2O2) as one of the environmentally benign catalysts has been frequently applied for treating industrial effluents.
On the other hand, the most significant feature for practical application in industry is the possibility of green catalysts for recovery to suggest a sustainable use (Ashour and Tony 2020). Conventional catalyst segregation techniques that are signified as monotonous methods including chromatography, filtration and centrifugation are inconvenient. Since they could not be efficiently recovered after reaction. Therefore, magnetically separable nanocatalysts is a superior option to overcome such drawbacks as robust and sustainable alternatives. Their magnetic characteristic allows the facility of simple and significant separation from the reaction media, hence diminishing the inherent work up techniques. Subsequently, those easy to reuse magnetic catalysts satisfy the concept of green chemistry. Considerable attention has been focused onto magnetite (Fe3O4) as an engineered magnetic nanomaterial and is categorized among the simplest Ferro-spinel materials (Sharma et al. 2015). Modifications could be applied for such substances as Ferro-spinel materials commonly known as ferrites by substituting one of iron molecules with a second metal for enhancing both magnetic properties and catalytic activity to be introduced as Layered Double Hydroxide (LDH) catalyst. Such modifications allow to narrow band gap of ferrites to could utilize the visible region of the solar spectrum (Casbeer et al. 2012). This modification improves the oxidizing power of Fenton type reactions. However, it is essential to integrate the wastewater treatment system for practical applications with a recoverable and reusable nanoparticles. As a result of the inability to separate nanoparticles from the treated wastewater streams, hesitation has surrounded the large-scale application for the commercial remediation. Thus, magnetic nanoparticles are the answer. To alleviate such concerns, Layered Double Hydroxide nanoparticles, which could be magnetically separated, are the promising green treatment technology that can facilitate the practical wastewater treatment technology in an economic and green facility. Therefore, the application of magnetic nanoparticles is a superior option for both removing the nanoparticles from the treated effluent and for reusing the nanoparticles in further treatments. Hence, the nanoparticles concentrations and dissolved matter do not exceed the environmental regulation limits, i.e., total iron <300 ppb for potable water (Powell et al. 2020).
To the best of the authors’ knowledge, there is a lack in the literature cited on modulating the solar photo-Fenton by altering the cation within the ferrite to Mg or Zn as a magnetic bimetallic Layered Double Hydroxide (LDH) catalyst for solar energy applications for treating Remazol Brilliant Blue R effluents. Herein, the main goal of the present work is introducing a simple cost efficient sustainable catalyst as a source of solar photo-Fenton technique. In this context, simple and facile synthesis of magnetite via an efficient co-precipitation technique is conduced. Additionally, magnetite nanoparticles could be engineered through introducing Mg or Zn cations into its structure for improving its catalytic activity for Remazol Brilliant Blue R dye oxidation. The influence of the operating parameters i.e., catalyst and H2O2 reagents concentration, pH of the aqueous solution, dye loading and wastewater temperature on the oxidation reaction is assessed. The ability of the catalyst recyclability is examined via successive application was also evaluated to assure the good stability of the material.
Materials and methods
Experimental section
Synthesis of nanostructures photocatalysts
Fe(III)/Fe(II), Zn(II)/Fe(II) and Mg(II)/Fe(II) Samples with chemical formula of MFe2O4 (where M = Mg2+, Zn2+ and Fe2+) have been synthesized. MgFe2O4 and ZnFe2O4 were prepared using the sol-gel method while the Fe3O4 was prepared using the co-perception method. Analytical grade Mg(NO3)2.6H2O, Zn(NO3)2.6H2O, Fe(NO3)3.3H2O and monohydrate citric acid with a purity of 99.98% were used without further purification. 1:1 molar ratio of salts to citric acid was applied. All the chemicals were at high-grade and supplied by Sigma-Aldrich (Chemical Cp. St. Loius, Mo, USA). The metal nitrate and citric acid were dissolved in double-distilled water (sol), using magnetic agitator, to form clear solution. The boiling temperature of water was used to evaporate the excess water and form gel. The gel dried and completely burnt to form a crunchy powder. The powder was manually milled using an agate grinder. The Fe3O4 was prepared accordion the previous work (Thabet et al. 2021).
Characterization of the prepared nanomaterials
The phase structure of the prepared samples was analyzed by X-ray diffractometer (XRD), (X-lab Shimadzu X-6000), and identified with Cu-Kα radiation. Additionally, the particle size was detected using a High-Resolution Transmission Electron Microscope (HR-TEM, Tecnai G20, FEI, Netherland).
Remazol Brilliant dye wastewater and oxidation procedure
Remazol Brilliant Blue R (RBBR) is used as the synthetic reactive textile dying wastewater source (high-grade dye, supplied by DyStar Ltd., German) and used as received with no extra purification. The desired concentrations are prepared and used for treatments. The solution pH is adjusted, if required, via NaOH and/or H2SO4 addition using Adwa digital pH meter (AD1030, Adwa instrument, Hungary). All chemicals were supplied by Sigma-Aldrich and used with no further purification.
Solar pyrometer (Model, CMP3) was used for the solar intensity measurement during the experimental time. This sensor is installed in AMSEES laboratory in Shebin El-Kom city during the months of experimental work.
Initially, a 1000-mL aqueous dye contaminating water was used to be treated through circulation in the photo-rector. The as-synthesized catalysts were added separately at different sets of experiments as the source of Fenton-Like reaction, and then H2O2 (30% w/v, supplied by Sigma-Aldrich) was added to the aqueous solution to initiate the oxidizing reaction. Subsequently, the solution was subjected to a pilot scale photochemical reactor for treatment, and a magnetic stirring was conducted through the experiments to assure mixing.
Afterward, aliquot samples were periodically withdrawn at various time intervals to examine the dye removal by analysis after the catalyst is separated. A UV-vis spectrophotometer, Unico UV-2100 model, USA, was used for color removal analysis to investigate the residual dye concentration in the solution.
The solar photochemical reactor is based on the focus line of reflective concentrating parabolic-trough collector through the sunrays are concentrated. Collector is established by bending a reflective sheet into parabolic shape from reflective and highly polished aluminum. The reflector collects the sun radiation parallel to the axis of the parabola toward the focal line where the tubular reactor is installed. The aqueous matrix flows through a tubular reactor mounted on the focal line of the collector. A full illustration of the photo reactor is reported before by the author (Tony 2021b). The experimental procedures and the photochemical reactor are schematically presented in Fig. 1.
Various process parameters are investigated including: LDH and H2O2 doses, effluent pH, initial dye loading, circulating flow rate, temperature and solar illumination intensity in order to fully understand the system performances. Furthermore, the system variables are optimized, and the optimum values are located to maximize the process performances.
Nanoparticles recovery
To recover the nanoparticles following the treatment process, the LDH nanoparticles in the treated effluent are subjected to filtration. Thereafter, the LDH was washed with distilled water and then oven dried to 105ºC. Afterward, it is returned for the dye oxidation system for successive use, and the process is repeated for continual use till it lose its activity.
Factorial design
The effect of process parameters in the LDH-Fenton-Like reaction and the optimum conditions for the maximal RBBR dye removal were investigated using a Box-Behnken design of experiments based on Response Surface Methodological analysis, RSM, with three factors run by the software SAS version (SAS, 1990). The experimental parameters studied here were selected on the basis of the most effective variables on the LDH-Fenton-Like reaction according to the experimental results preliminary obtained from checking the process parameters on the oxidation efficiency. Hence, the selected variables are: pH, LDH catalyst dose and H2O2 concentration. Table 1 displays the coded and original values of the process parameters used in the matrix designed, which is exhibited in Table 2 for each model studies. Additionally, in order to avoid experimental errors, the experiments were accomplished in random order, and all of them were conducted in duplicates. Furthermore, the second order polynomial quadratic models for the three Fenton-Like systems according to the Eq. (1) are investigated. Then, to explore the significance of the attained models, the statistical analysis using analysis of variance (ANOVA) were performed via SAS software (SAS 1990). Also, Mathematica software (V 5.2) is applied to locate the numerical values of the optimized variable and responses.
Results and discussions
XRD analysis
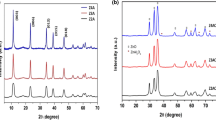
For the X-ray diffraction analysis, Magnetic nanoparticles were dried and analyzed using X-ray diffractometer. Fig. (2) shows the XRD spectra of MgFe2O4, ZnFe2O4 and FeFe2O4 nanoparticles. The peaks of all investigated samples showed single spinel phase ferrites. It is observed that the (311) miller plane appears at (2θ) = 35.52, 35.28 and 35.49 for MgFe2O4, ZnFe2O4 and FeFe2O4, respectively. This is due to the variation of the ion radius of the magnetic ions. The increase in the ionic radius caused the peaks to shift to low 2θ (El-Sayed et al. 2017).
Particle size and morphology
The average particle size of the prepared nanoferrites has been obtained using digital TEM Images. The histograms of the magnetic nanoparticles are shown in Fig. (3). From the TEM micrographs, the particles are nearly spherical in shape. The digital image was analyzed using the IMAGEJ 1.48V program to determine the particles size distribution. The average particle size for MgFe2O4, ZnFe2O4 and FeFe2O4, are 15, 14 and 8 nm, respectively.
Catalytic oxidation of different LDH-Fenton-Like systems
Initially, to investigate the optimum oxidation reaction time of the various applied systems simultaneously with investigating the comparison of their different oxidizing abilities, the dye removal (RBBR) from wastewater was tested and compared. The oxidizing systems investigated are: Fenton-Like systems, H2O2 system and coagulating systems all based on initiating the reaction through UV radiation supplied from solar energy. Fenton-Like reaction is investigated using Fe-Fe-LDH/H2O2/Solar, Zn-Fe-LDH/H2O2/Solar and Mg-Fe-LDH/H2O2/Solar at the starting natural pH values of wastewater using 40 and 400 mg/L of LDH catalyst and H2O2, respectively. Additionally, the coagulating system is based on using various LDH systems (Fe-Fe, Zn-Fe and Mg-Fe) augmented with solar radiation for dye oxidation, and finally, the solo hydrogen peroxide initiated with solar radiation is also attained for the object of comparison. For the all applied systems, the 40 and 400 mg/L concentrations for catalyst and H2O2, respectively, were chosen to attain a reasonable comparison. The results in Fig. 4 demonstrated that for the all systems, the dye removal decreased with increasing the reaction time. Initially, rapid oxidation rate was observed within the initial 5 min of solar irradiance time followed by steady oxidation rate within the following 10 min. After 15-min of solar irradiation time, the dye removal attained were ranged from 20 to 28% removal for the Fenton-Like-based systems arranged in the order of 28, 26 and 20 for LDH-Fe-Fe-, LDH-Zn-Fe- and LDH-Mg-Fe Fenton-Like, respectively, when the pH of wastewater was kept as its natural value with no adjustment and using the same Fenton doses. However, such oxidation values are only between 9 and 18% for the coagulating LDH systems with no H2O2 addition in the reaction medium. Further, solo hydrogen peroxide addition only achieved 17% of the dye removal. Noteworthy, the optimal oxidizing reaction time for the dye removal using solar energy systems was recorded after 15 min regardless the applied system used, and thus, this time is adjusted in the further experiments.
The dye oxidation detected through its oxidation-reduction is explained via the formation of the hydroxyl radicals (·OH) that is categorized as the highly oxidation species through the Fenton-Like oxidation reaction. Such ·OH radicals’ posses high oxidation strength for attacking the pollutants in the treated wastewater for oxidizing and mineralizing them (Saravanan et al. 2013; Tony and Lin, 2020a, b, c; Hao et al. 2020).
Still, an oxidation is achieved using the solo systems of H2O2 or LDHs. This could be due to the ·OH radicals production that are the responsible in the oxidation (Saravanan et al. 2013). Besides, coagulation is taking place in the solo the LDH (Fe-Fe, Zn-Fe and Mg-Fe) systems. However, Fenton-Like solar oxidizing systems is much more efficient in dye removal from wastewater compared to the other studied reactions. This is probably demonstrated by the fact that higher hydroxyl radicals are accumulated in the reaction medium in the case of the LDH-Fenton-Like reagent rather than the other solar oxidation systems. It depicted that the oxidizing (·OH) radicals are very hard to be generated if only with solo oxidation systems (LDH or H2O2) at least though our experimental conditions (Tayeb et al. 2019). Hence, the yield of the hydroxyl radicals generated is insufficient for textile Remazol Brilliant Blue R dye oxidation.
Effect of RBBR loading on the LDH-fenton-like reaction
Fig. 5 shows the effect of the initial Remazol Brilliant Blue R dye concentration in order to simulate the real world since high loads are discharged from the textile industry. LDH nanoparticles were investigated to conduct the Fenton-Like reaction based on the solar photocatalytic oxidation induced via 400 mg/L of H2O2 reagent. At a certain constant (40 mg/L) of the various LDH systems and pH 3.0 of the aqueous solution, it is clear from the data in Fig. 5 that the decrease of the initial RBBR dye load increases the oxidation rate. The percentage removals of the Remazol Brilliant Blue R dye were decreased from (83 to 40%), (87-49%) and (90-52%) with increasing the initial dye load from 10 to 100 mg-RBBR/L, for the systems LDH-Fe-Fe-, LDH-Zn-Fe- and LDH-Mg-Fe-Fenton–Like, respectively. For the all Fenton-Like systems, this decrease in the RBBR dye removal rate with increase in the initial dye concentration could be attributed by the increase in the shadowing effect of the high wastewater load with Remazol Brilliant Blue R that hinders the solar radiation the source of the UV illumination from penetrating the aqueous media and generating the oxidizing OH radicals species. This observation regarding the reduction in removal rate with the high loaded wastewater is previously reported by Cetinkaya et al. (2018) in oxidizing wastewater suing Fenton reaction.
Effects of experimental parameters on RBBR oxidation
Effect of solar illumination
As previously stated in literature (Marcelino et al. 2015; Cetinkaya et al. 2018), solar experiments are extensively solar radiation intensity dependent reactions. Thus, from such regard, experiments were done at various levels of natural solar intensities at different daytime periods to monitor the suitable radiance time for conducting experiments. Meanwhile through the oxidation reaction, the solar intensity is recorded at the location of the solar reactor (AMSEES Laboratory, Menoufia University, Shebin El-Kom city at the north of Egypt). The location is well endowed with solar radiation especially at the summer season that suggesting a place is a good option for conducting solar treatment system. This city is considered as one of the most Egyptian abundant solar radiation cities according to the previous reports (Tony and Mansour 2020). The average sunshine over the city is over 10 h/day. During the experimental days, the attained higher solar radiation value was about 1034.5 W/m2 that is attained around the solar noon. This value is reduced in the early morning or before the sunset to the average value about 230.3 W/m2 through the time of the current study the average incident solar radiation, Is,av is presented in the inset of Fig. 6.
Several sets of oxidation experiments at various daytime were conducted for the various LDH-Fenton-Like reactions, and the results in Fig. 6 illustrates even the solar radiation is low that there is still oxidation reaction is taking place, however the dye reduction is low as seen from the results. However, it noteworthy to mention that at the acidic the pH 3.0 during the high solar radiation periods using 100 H2O2 and 80 mg/L of LDH, the maximal attained removal of 72% was achieved for Mg-Fe type catalyst. Thus, as expected, the intensity of solar radiation affects the rate of dye oxidation for the all studied LDH system. The solar irradiation is playing a vital role in initiating the catalyst and H2O2 reaction to generate the highly oxidizing reactive intermediates, ·OH species, that representing the main reaction responsible of mineralizing the dye molecules. The aromatic rings in the dye structure are hydroxylated and produce a hydroycyclohexadienyl resulted from attacking the aromatic ring via hydroxyl radicals (He et al. 2015).
By far, from the experimental data, the application of clean and renewable energy source in the photocatalysis is a viable option for wastewater treatment. Hence, solar energy utilization to drive an induction source for advanced oxidation processes is a potential sustainable solution that substitutes the artificial ultraviolet radiation from the sustainable and economic point of view.
Effect of flow rate
The circulation flow rate of the aqueous solution in the solar reactor is attained since its role is significant due to the extent of time that the solution is exposed into solar radiation in the tubular reactor. A steady flow rate in the range of 250–1200 mL/min was tested at constant other variables (pH 3.0; 40 and 400 mg/L of catalyst and H2O2, respectively, for 20 ppm of the dye concentration). The results displayed in Fig. 7 show that enhancement of the RBBR dye removal is attained (40–72%) in the three studied systems, Fe-Fe, Zn-Fe and Mg-Fe Fenton-Like with the circulation flow rate increase from up to 750 mL/min. However, further increase in the solution circulation rate leading to a reduction in the dye removal. This is as expected since at the higher flow of the aqueous solution in the tubular reactor, the length of exposure of to the solar irradiance is decreased and subsequently, the ·OH radicals yield is deduced. Thus, the oxidation rate is afterward reduced. Similarly, the very slow flow of the aqueous solution in the tubular reactor is also unfavorable as well as the too fast flow rate. This is due to the at very high flow rate, the solution exposure time to the solar radiation that helps in producing the hydroxyl radicals is not sufficient for oxidizing the dye molecules. However, the too slow rate is helping in catalyst precipitation in the tubular reactor instead of reacting with the hydrogen peroxide to produce the oxidizing radicals. Thus, the overall reaction yield is reduced. Previous reported literature showed a similar trend in treating wastewater polluted with Orange II dye effluents via such technique (Robles et al. 2017).
Effect of pH and Fenton-like doses
Commonly, Fenton-Like oxidation reaction is a too sensitive process for the starting pH of the aqueous matrix, besides the reagent doses (H2O2 and catalyst). Hydroxyl radicals are considered the workhorse of H2O2-based oxidation reactions, and such radicals’ yield is affected with the optimal system parameters (Shen et al. 2008; Zhang et al. 2017). Fenton reaction has a preferred optimum pH region that is sharply affects the hydroxyl radicals’ generation. From this regard, the pH effect was examined in the range of: the wastewater original pH (6.8), acidic pH (3.0 and 4.0) and alkaline pH 8.0. Results in Fig. 8 A demonstrate that for the all Fenton-Like systems, the preferred pH was 3.0, which is corresponding to the maximal dye removals (keeping all parameters constant: flow rate 250 mL/min, dye load 20 ppm, H2O2 400 and LDH catalyst 40 mg/L, respectively).
This investigation could be illustrated by at alkaline pH, the catalyst complexes precipitate in the wastewater instead of producing the radicals that are the responsible of the reaction. Thus, the reaction involves the organometallic complex. In contrast, the ·OH radicals’ that produced in the acidic pH values was high. Additionally, the presence of inorganic carbon in the aqueous matrix further scavenging the ·OH radicals’ effect which is easily terminated at the acidic pH value (Zhang et al. 2017).
On the other hand, H2O2 amount plays a vital role in producing (·OH) radicals’ species (Eq. 2), therefore, its effect on the RBBR removal is investigated for the all LDH studied system with keeping the other parameters constant (pH 3.0, dye load 20 ppm, 750 mL/min of circulation flow rate and 40 mg/L of LDH catalyst). The results in Fig. 8 B reveal that increasing the hydrogen peroxide for the all LDH-Fenton-Like systems results in an increase in the dye removal. However, further H2O2 increase, observed dye removal efficiency is declined. Hence, the higher H2O2 doses that are more than the optimal value inhibits the dye oxidation rate. This could be illustrated as the high H2O2 concentration acts as a hydroxyl radical scavenger instead of generating them as presented in Eqs. (3, 4). Besides, recombination reaction occurs between OH• radicals and likewise OH• radical, and H2O2 is produced which is also OH• radicals’ scavenger (Eq. (5)) (Schrank et al. 2007; He et al. 2015).
LDH-based Fenton-Like oxidation catalyst activate H2O2 for generating ˙OH free radicals in water and thereby mineralize the contaminants. Therefore, the optimal catalyst presences in the solution maximizing the ˙OH yield in the reaction medium. To add up, it is crucial for keeping minimal catalyst dose form the prospective of process efficiency and economic cost for the treatment. Moreover, the higher dosage of the catalyst could decline the reaction efficiency. Fig. 8 C illustrates the effective dye oxidation process by varying the concentration over the range 10–80 mg/L for all the LDH systems (Fe-Fe, Zn-Fe and Mg-Fe). It is noted from the data of the experimental results that for all the LDH systems, a same trend is attained via oxidation removal since increasing catalyst dose results in an increase dye removal (Soares et al. 2007). However, in most cases within the experimental range, further increase in the LDH concentration declines the reaction yield. But the optimal LDH concentration is differs according to the system used. The maximum Remazol Brilliant Blue oxidation is corresponding to 72 and 50% when 40 mg/L of Mg-Fe or Fe-Fe LDH systems, respectively, is the source of the Fenton-Like reaction. Such values of the dye oxidation are corresponding to 60% when the catalyst dose is 80 mg/L for the Zn-Fe LDH system.
LDH catalysts, Mg, Zn or Fe ions are critical for creating the photoactive hydroxo complexes, thereby absorb the photons in the solar ultraviolet arrays to produce the highly reactive species radicals (·OH). Further, solar illumination irradiating the catalyst (\(M{Fe}_{2}{O}_{4}, Zn or Mg)\), the result is generating on their surfaces an electron (e)/hole (h) pair (Eq. 6) to further promoting a series of reactions (Eq. (7, 8) that yielding ·OH radicals (He et al. 2015).
Then, the generated iron ions in the reaction solution react with H2O2 to form more ·OH radicals and metal ions again in a series of successive reactions according to the classical Fenton type reactions (Eqs. 9–14). Remazol Brilliant Blue structure contains aromatic rings which are attacked via ·OH radicals to hydroxylate them and build up a hydroycyclohexadienyl radical (Eq. 15). Overall, the reaction should be conducted in optimal doses for maximizing the yield of radicals and hence strongly oxidizing the dye molecules (He et al. 2015). However, non-optimal concentrations could result in system unbalance, and ·OH radicals recombines together to produce H2O2 instead of oxidizing the dye molecules and hinders the reaction yield (Eq. 16).
It is noteworthy to mention, final sludge removal and disposal after the Fenton’s reactions could pose a problem. Since, such final sludge separation and disposal are problem for the environment and from the economic prospective. Of note, after the Fenton reaction the recyclable catalyst that is easily introduced as magnetisable separable option extend the Fenton’s practical application life.
Factorial design and ANOVA testing
In order to optimize and analyze the effect of the reaction parameters, pH and Fenton reagent doses, on the dye removal, factorial experimental design has been carried out. This investigation is essential in elucidating the optimal conditions and minimizing the reagent doses to attain a high system performance. A 15-runs are conducted for the each proposed model, namely Model γ1 (LDH-Fe-Fe/H2O2/Solar, Model γ2 (LDH-Zn-Fe/H2O2/Solar) and Model γ3 (LDH-Mg-Fe/H2O2/Solar) according the experimental design tabulated in Table 2. The data collected from these experiments were processed and fitted to quadratic polynomial model Eqs. (17–19) using the SAS software and the models’ multivariate statistical analysis (ANOVA), allowing to determine the correlation between the studied variables and the maximized response (dye removal).
According to the ANOVA results (Table 3), the quadratic polynomial models are highly significant with a satisfied correlation coefficient values, r2. Generally, from the statistics point of view, the coefficient of determination (r2) is acceptable if it is more than 80% (SAS 1990). Besides the fitness of the model, the model is also verified when a large value of Fisher test that is much greater than unity and a small p-value (< 0.05) is attained. For the three proposed models, the coefficient of determination values are 94, 98 and 96% for γ1, γ2 and γ1, respectively, that signify the fitness of the model. The ANOVA results reveal a high correlation between the response and the variables since a low probability (Pr > F) of 0.017, 0.0017 and 0.006 for γ1, γ2 and γ1, respectively. It is concluded that there is a good fit between the proposed simulated models and experimental data, as can be also observed in the results of the ANOVA test.
The type of interactions between the selected variables, pH and Fenton doses are required to be identified. To do so, the 3D surface and contour graphs described by the three regression models have been plotted and displayed in Fig. 9A–C. These graphs show the influence of each two independent variables studied on the Remazol Brilliant Blue R dye removal.
According to Fig. 9A i, ii and iii, the Remazol Brilliant oxidation efficiency evaluated through its concentration removal is steadily improved with the increased Fenton-Like reagent dosage of both reagents LDH catalyst and H2O2. The chief attribution of this trend is the ·OH radicals’ yield in the aqueous matrix is elevated with the catalyst dosage increase. However, after a certain reagent limit, the dye removal is declined. This significant relation could be related to the over catalyst dosing acts as a (·OH) radical scavenger rather than a generator (He et al. 2015). Further, the extent of curvature of a surface plot is signal for the degree of exaggerated on the response (γ1, %) since the more circular contour curvature identifies a weaker interaction effect. Additionally, similar trends are attained for the other models γ2 and γ3 as seen in the 3D surface and 2D contour plots in Fig. 9 B (i, ii and iii) and C (i, ii and iii). To add up, as seen in the graphs the system is highly sensitive to the pH change in comparison to the other variables as seen in graphs in Fig. 8 A (iii), B (iii) and C (iii). It is notable to mention that degree of dye removal is different according to the LDH system applied as seen from the response value in each case.
Also, the numerical simulated optimization of the possible combinations LDH, H2O2 and solar energy is further attained through Mathematica software (version V 5.2), and the optimum values (displayed in Table 4) are used to conduct real experiments. The predicted values are then confirmed through duplicates of experiments, and original response values are compared with the simulated ones. High correlation between the model predictions and the experimental results for the three models is attained as seen in Table 4 that further confirms the goodness-of-fitness of the models for the results besides the ANOVA test.
Comparing the oxidizing efficiency from the current investigation using the modified Fenton-Like type reactions with other recent literature based on heterogeneous Fenton treatments are conducted and displayed in Table 5. It could be concluded that the modified Fenton-Like system that posses high magnetic properties which facilitate the magnetic separation of the nanoparticles after oxidation reaction and before the final effluent disposal show reasonable removal efficiency reached to 74%. Although some other Fenton-Like-based heterogeneous treatments that reported previously in literature (Table 5) such as microwave enhanced systems or ultraviolet radiation have exhibited high potential for pollutants removal from wastewater as seen in Table 5, they posses some drawbacks such as the need of electric cost and in some cases high doses of chemical agents are required. To add up, the magnetized nanoparticles is efficient and economically acceptable since they are a recyclable catalyst that could be used for successive use before the final disposal. However, such option is not present in non-magnetized catalyst, which makes the process costly also the formation of toxic effluents. Moreover, the current systems presented in this study also have the advantage of using economic, renewable and environmentally friendly solar energy source for initiating the Fenton-Like reaction. Hence, these groups of disadvantages are not related to the current magnetized system. Therefore, this suggested method is much better, cheaper and environment benign compared to the other heterogeneous Fenton-Like systems listed in Table 5. Furthermore, from the economic prospective, such LDH catalyst could be easily recoverable and could be introduced for a successive sustainable use such advantages that verify the catalyst has potential application in real environment and could overcome the low oxidation efficiency compared to other materials in Table 5.
Temperature effect on kinetics and thermodynamics of oxidation
To further understand the LDH solar oxidation process that is taking place between various combinations of Fe-Fe, Zn-Fe or Mg-Fe catalysts and H2O2 as a Fenton-Like reaction source, temperature effect is investigated to subsequently study the kinetics and thermodynamics of the reaction. Thus, the temperature increased from room temperature to 40, 50 and 60°C, and the oxidation reaction is monitored in all cases of the Fenton-Like system. The results displayed in Fig. 10 show that for the all studied Fenton-Like-based systems (LDH-Fe-Fe, -Zn-Fe and –Mg-Fe), the temperature increase results in higher oxidation yield and thereby higher dye removal is attained at higher reaction temperature. The removal reached to 87, 89 and 91% for Layered Double Hydroxide Fe-Fe Fenton-Like, -Zn-Fe Fenton-Like and –Mg-Fe Fenton-Like, respectively, at 60°C in comparison to 50, 61 and 72% for the same systems at room temperature. This phenomenon could be related to the high reaction temperature accelerates the generation of the reaction radicals. Hence, a superior generation of ·OH radicals is attained with temperature elevation. The effective oxidation rate is achieved with the rise in temperature within the studied range. Previous reports cited in literature (Soares et al. 2007) explored that the reaction using the catalytic oxidation is associated with an optimum temperature value, and it is a limiting stage for the oxidation reaction.
Fully understanding of the Fenton-Like oxidation reaction is important for practical applications and design aspects. To do so, kinetic study is important; however, it is not simple since Fenton oxidation reactions are a complex reaction including various species with a simultaneous oxidation and coagulation existence (He et al. 2015). The experimental results at different temperatures including room temperature, 40, 50 and 60 ºC are applied for the zero-, first- and second-kinetic models, and the overall system performances is investigated via dye removal, and the corresponding kinetic constants are estimated in each system. The data listed in Table 6 reveals that the coefficients of regression (r2) values that used to evaluate each model is the highest for the first-order kinetic model. This means the first-order kinetic model is well fitted the experimental data. Also, the lowest value of half-life time (t0.5) is corresponding to the 60ºC reaction temperature. Previous reports in accordance with the current study in using Fenton reaction for wastewater treatment contaminated with reactive black 5 dye (Argun and Karatas 2011).
Since the kinetic modeling of the all Fenton-Like studied oxidation kinetics exhibited the first-order model, the first-order rate constants are expressed as a function of temperature by the Arrhenius equation. Consequently, the temperature dependence of the kinetic parameters of LDH-Fenton-Like system is calculated via Arrhenius equation (Eq. (20)). Further, other thermodynamic variables including Gibbs free energy of activation (∆G°), enthalpy of activation (∆H°) and entropy of activation (∆S°) for the Fenton-Like oxidation system were also investigated through Eqs. (21–23) (Argun and Karatas, 2011; Ahmadi et al. 2016) (where A is the Arrhenius constant, T is the wastewater absolute temperature, Ea is the energy of activation of the Fenton-Like systems, and R is the universal gas constant). Furthermore, the energy of activation is attained from the slope of plotting -Ea/R versus 1/T (Fig. 11).
The evaluated thermodynamic parameters for the all studied Fenton-Like systems are given in Table 7. According to the data tabulated in Table (7), all the system shows positive Gibbs free energy of activation values, which signify the possibility of non-spontaneity oxidation reaction principal to occur. Also, negative results of the enthalpy of activation reaction indicate the LDH-Fenton-Like systems are occurring in exothermic nature. To add up, the negative values of entropy of activation attained are verifying the non-spontaneity nature of the systems. Thermodynamic results validate the decrease in the degree of freedom of the dye molecules in the wastewater effluent and maintained a high oxidation yield. A low energy barrier is required for the all LDH-FL systems 5.31, 29.51 and 36.14 kJ/mol for Fe-Fe, Zn-Fe or Mg-Fe Fenton-Like systems. Earlier results in the literature suggested the oxidation of the Fenton system proceed at low energy barrier (Ahmadi et al. 2016; Tony and Lin, 2020a).
Stability and reuse assays
Reuse of the LDH catalysts used in the Fenton-Like reactions was investigated using the catalysts (Fe-Fe, Zn-Fe and Mg-Fe) for successive cycles for Remazol Brilliant Blue R oxidation as shown in Fig. 12. After each step of Fenton-Like use, the LDH was washed with distilled water and oven dried (105 ℃), then it is returned for the dye oxidation system. It is observed from the experimental results, the all catalysts used showed a significant dye removal remained almost invariant. After the 6th cycle, the Remazol Brilliant Blue R removal percentage due to the oxidation reaction is marginally decreased from 72 to 61% for Mg-Fe-LDH catalyst after the sixth cycles of use and from 61 to 49% for the ZnFe-LDH and form 50 to 42% for the Fe-LDH, probably due to the decline in their activities. Hence, LDH catalysts offer stable performance in Fenton-like oxidation reactions that enabling prolonged application of these LDH catalyst materials in the treatment of wastewaters containing dyes. Additionally, it is noteworthy to mention that Mg-Fe-LDH catalyst besides its high catalytic activity it posses the high magnetic properties that enabling and facilitating the magnetic separation and thus reduce its losses. Finally, the recovered catalysts were reduced by 0.5–1% from their initial values. Hence, this current study is designed to generate and apply LDH nanoparticles as a novel magnetized heterogeneous catalyst as an easily removable, recoverable and recyclable material for the sustainable opportunity in oxidizing textile dye effluents. For real application world, such magnetized catalyst posses the facility of simple collection from aqueous stream after treatment facility via magnetic separation that expand its range of application for reusing in successive cycles. These viable options, easily removable and could be recycled explore the advantages of economic cost.
Conclusion
In this study, a sustainable alternative to Fenton-Like catalyst is suggested through LDH catalysts augmented with hydrogen peroxide for synthetic wastewater containing textile dying. Coupling of solar radiation and photocatalysis improve the RBBR dye through the supportive effect between the photocatalysis and ultraviolet radiation sourced from the sun. Fe-Fe-, Zn-Fe- and Mg-Fe-LDH catalysts showed a good treatability reached to 72% acquired after 15-min of the irradiation time in the solar collector. It was found that, for the all systems, Fenton’s reaction is well worked around the acidic pH 3.0. The results well fitted the first-order reaction kinetic model, and the process is exothermic and non-spontaneous in nature working at low level of energy barrier. Effective removal of nanoparticles from treated water stream after treatment technology is cost efficient treatment facility forms the commercial prospective for real world applications. Magnetic separation and recycling of effluent magnetized LDH catalyst used in the Fenton-Like systems revealed and showed high attempt for dye removal for a long-term treatment. This presented a significant advantage for the practical application in the real industry world. Thus, it is concluded that the magnetic layered double hydroxide nanomaterials is introducing a safe use of the nanomaterials in the water treatment systems.
References
Ahmadi M, Behin J, Mahnam AR (2016) Kinetics and thermodynamics of peroxydisulfate oxidation of reactive yellow 84. J Saudi Chem Soc 20(6):644–650
Argun ME, Karatas M (2011) Application of Fenton process for decolorization of reactive black 5 from synthetic wastewater: Kinetics and thermodynamics. Environ Progr Sustain Energy 30(4):540–548
Ashour EA, Tony MA (2017) Equilibrium and kinetic studies on biosorption of iron (II) and iron (III) Ions onto eggshell powder from aqueous solution. Appl Eng. 1(3):65–73
Ashour EA, Tony MA (2020) Eco-friendly removal of hexavalent chromium from aqueous solution using natural clay mineral: activation and modification effects. SN Appl Sci 2(12):1–13
Ashour EA, Tony MA, Purcell PJ (2014) Use of agriculture-based waste for basic dye sorption from aqueous solution: kinetics and isotherm studies. Am J Chem Eng. 2(6):92–98
Casbeer E, Sharma VK, Li X-Z (2012) Synthesis and photocatalytic activity of ferrites under visible light: a review. Sep Purif Technol 87:1–14
Cetinkaya SG, Morcali MH, Akarsu S, Ziba CA, Dolaz M (2018) Comparison of classic Fenton with ultrasound Fenton processes on industrial textile wastewater. Sustain Environ Res 28(4):165–170
Changotra R, Rajput H, Dhir A (2019) Treatment of real pharmaceutical wastewater using combined approach of Fenton applications and aerobic biological treatment. J Photochem Photobiol: Chem 376:175–184
El-Sayed HM, Ali IA, Azzam A, Sattar AA (2017) Influence of the magnetic dead layer thickness of Mg-Zn ferrites nanoparticle on their magnetic properties. J Magnet Magnetic Mater 424:226–232
Feng X, Mao GY, Bu FX, Cheng XL, Jiang DM, Jiang JS (2013) Controlled synthesis of monodisperse CoFe2O4 nanoparticles by the phase transfer method and their catalytic activity on methylene blue discoloration with H2O2. J Magnet Magnetic Mater 343:126–132
Feng HE, Le-Cheng LEI (2004) Degradation kinetics and mechanisms of phenol in photo-fenton process. J Zhejiang Univ Sci A 5(2):198–205
Guo Y, Xue Q, Zhang H, Wang N, Chang S, Wang H, Pang H, Chen H (2018) Treatment of real benzene dye intermediates wastewater by the Fenton method: characteristics and multi-response optimization. RSC Adv 8(1):80–90
Hao M, Qiu M, Yang H, Hu B, Wang X (2020) Recent advances on preparation and environmental applications of MOF-derived carbons in catalysis. Sci Total Environ 58:143333
He HP, Zhong YH, Liang XL, Tan W, Zhu JX, Wang CY (2015) Natural Magnetite: an efficient catalyst for the degradation of organic contaminant. Scientif Rep 5:10
Homem V, Alves A, Santos L (2013) Microwave-assisted Fenton’s oxidation of amoxicillin. Chem Eng J 220:35–44
Khan MAN, Siddique M, Wahid F, Khan R (2015) Removal of reactive blue 19 dye by sono, photo and sonophotocatalytic oxidation using visible light. Ultrason Sonochem 26:370–377
Marcelino RBP, Queiroz MTA, Amorim CC, Leão MMD, Brites-Nóbrega FF (2015) Solar energy for wastewater treatment: review of international technologies and their applicability in Brazil. Environ Sci Pollut Res 22(2):762–773
Mitsika EE, Christophoridis C, Fytianos K (2013) Fenton and Fenton-like oxidation of pesticide acetamiprid in water samples: kinetic study of the degradation and optimization using response surface methodology. Chemosphere 93(9):1818–1825
Powell CD, Atkinson AJ, Ma Y, Marcos-Hernandez M, Villagran D, Westerhoff P, Wong SM (2020) Magnetic nanoparticle recovery device (MagNERD) enables application of iron oxide nanoparticles for water treatment. J Nanopart Res 22:48
Rezaee A, Ghaneian MT, Khavanin A, Hashemian SJ, Moussavi GH (2008) Photochemical oxidation of reactive blue 19 dye (RB19) in textile wastewater by UV/K2S2O8 process. J Environ Health Sci Eng 5(2):95–100
Robles I, Rodríguez-Valadez FJ, Castaño E, Godínez LA (2017) Study of the influence of the operational parameters on the photoelectro-Fenton performance of an industrial wastewater treatment prototype using Orange II as a model pollutant. Sustain Environ Res 27(1):24–31
Sajab MS, Mohan D, Santanaraj J, Chia CH, Kaco H, Harun S, Kamarudin NHN (2019) Telescopic synthesis of cellulose nanofibrils with a stable dispersion of Fe(0) nanoparticles for synergistic removal of 5-fluorouracil [Article]. Scientific Rep 9:11
Saravanan R, Karthikeyan S, Gupta VK, Sekaran G, Narayanan V, Stephen A (2013) Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater Sci Eng: C. 33(1):91–98
SAS/STAT (1990) User’s Guide, SAS Institute, Inc., Cary, NC
Sharma R, Bansal S, Singhal S (2015) Tailoring the photo-fenton activity of spinel ferrites (mfe 2 o 4) by incorporating different cations (m= cu, zn, ni and co) in the structure. Rsc Adv 5(8):6006–6018
Shen W, Li Z, Wang H, Liu Y, Guo Q, Zhang Y (2008) Photocatalytic degradation for methylene blue using zinc oxide prepared by codeposition and sol–gel methods. J Hazard Mater 152(1):172–175
Soares ET, Lansarin MA, Moro CC (2007) A study of process variables for the photocatalytic degradation of rhodamine B. Brazilian J Chem Eng 24(1):29–36
Taha ATH (2010) Estimation of hourly global solar radiation in Egypt using mathematical model. Misr J Agric Eng 27(4):2033–2047
Tayeb AM, Tony MA, Ismaeel EK (2019) Engineered nanostructured ZnO for water remediation: operational parameters effect, box-behnken design optimization and kinetic determinations [Article]. Appl Water Sci 9(3):11
Thabet RH, Fouad MK, Ali IA, El Sherbiny SA, Tony MA (2021) Magnetite- based nanoparticles as an efficient hybrid heterogeneous adsorption/oxidation process for reactive textile dye removal from wastewater matrix. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.189671
Thabet RH, Tony MA, El Sherbiny SA, Ali IA, Fouad MK. (2020) Catalytic oxidation over nanostructured heterogeneous process as an effective tool for environmental remediation. IOP Conference Series: Materials Science and Engineering. 975:012004.
Tony MA (2019) An industrial ecology approach: green cellulose-based bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Int J Environ Anal Chem 101(2):167–183
Tony MA (2020) Central composite design optimization of bismarck dye oxidation from textile effluent with Fenton’s reagent. Appl Water Sci 10(5):1–9
Tony MA (2021) Low-cost adsorbents for environmental pollution control: a concise systematic review from the prospective of principles, mechanism and their applications. J Disp Sci Technol 25:1–23
Tony Maha A (2021) Solar concentration for green environmental remediation opportunity—International review: advances, constraints and their practice in wastewater treatment. 4 Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1895138
Tony MA, Lin L-S (2020) Iron recovery from acid mine drainage sludge as Fenton source for municipal wastewater treatment. Int J Environ Anal Chem 20:1–16
Tony MA, Lin LS (2020) Attenuation of organics contamination in polymers processing effluent using iron-based sludge: process optimization and oxidation mechanism. Environ Technol. https://doi.org/10.1080/09593330.2020.1803417
Tony MA, Lin LS (2020) Performance of acid mine drainage sludge as an innovative catalytic oxidation source for treating vehicle-washing wastewater. J Disp Sci Technol. https://doi.org/10.1080/01932691.2020.1813592
Tony MA, Lin L-S (2021) Iron coated-sand from acid mine drainage waste for being a catalytic oxidant towards municipal wastewater remediation. Int J Environ Res 15:1–11
Tony MA, Mansour SA (2019) Synthesis of nano-sized amorphous and nanocrystalline TiO2 for photochemical oxidation of methomyl insecticide in aqueous media. Water Environ J. https://doi.org/10.1111/wej.12522
Tony MA, Mansour SA (2020) Solar photo-Fenton reagent with nanostructured iron oxide for Bismarck dye oxidation: an Egyptian apparel case study. Int J Environ Sci Technol 17(3):1337–1350
Tony MA, Purcell PJ, Mansour SA (2020) Photodegradation and Box-Behnken design optimization for methomyl using Fenton process based on synthesized CuO nanocrystals via facile wet chemical technique. Chem Eng Commun 30:1–15
Zeolite-based adsorbent from alum sludge residue for textile wastewater treatment. International Journal of Environmental Science and Technology.1-14
Unal BO, Bilici Z, Ugur N, Isik Z, Harputlu E, Dizge N, Ocakoglu K (2019) Adsorption and aFenton oxidation of azo dyes by magnetite nanoparticles deposited on a glass substrate. J Water Process Eng 32:11
Van HT, Nguyen LH, Hoang TK, Nguyen TT, Tran TNH, Nguyen TBH, Vu XH, Pham MT, Tran TP, Pham TT (2020) Heterogeneous Fenton oxidation of paracetamol in aqueous solution using iron slag as a catalyst: Degradation mechanisms and kinetics. Environ Technol Innov 18:100670
Zhang H, Liu J, Ou C, Shen J, Yu H, Jiao Z, Han W, Sun X, Li J, Wang L (2017) Reuse of Fenton sludge as an iron source for NiFe2O4 synthesis and its application in the Fenton-based process. J Environ Sci 53:1–8
Zhao YQ, Keogh C, Tony MA (2009) On the necessity of sludge conditioning with non-organic polymer: AOP approach. J Resid Sci Technol 6(3):151–155
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Q. Aguilar-Virgen.
Rights and permissions
About this article
Cite this article
Tony, M.A., Ali, I.A. Mechanistic implications of redox cycles solar reactions of recyclable layered double hydroxides nanoparticles for remazol brilliant abatement. Int. J. Environ. Sci. Technol. 19, 9843–9860 (2022). https://doi.org/10.1007/s13762-021-03818-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03818-w