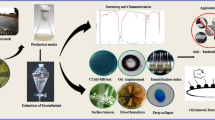
Abstract
Marine crude oil spill is the most alarming environmental issue and the dreadful instance of petroleum hydrocarbon pollution around the world. Successful remediation of these toxic pollutants demands for the ample production of biosurfactant and biocatalysts by the native hydrocarbon-degrading microbes. The main focus of this investigation is to determine the potential of a halo-tolerant and biosurfactant-producing hydrocarbonoclastic bacterium, Enterobacter hormaechei, for the effective bioremediation of marine oil spill. The strain had the ability to produce biosurfactant with excellent surface and emulsification activities along with biocatalysts. The activity of the extracellular enzymes such as lipase and laccase was found to be 160 U/ml and 38 U/ml, respectively, whereas the intracellular enzymes like alkane hydroxylase, alcohol dehydrogenase and esterase showed the corresponding activities of 48 U/ml, 86 U/ml and 102 U/ml. Overall, E. hormaechei could be able to degrade nearly 85% of petroleum hydrocarbons present in crude oil within 10 days of incubation. The biosurfactant was characterized to be an anionic, high molecular weight (48 kDa) lipoprotein-type biosurfactant. The biosurfactant was further characterized by Fourier transform infrared spectroscopy. The E. hormaechei was employed for the treatment of simulated marine oil spill and the degradation followed pseudo-second-order kinetics with rate constant k2 0.2775 and R2 0.9923. The crude oil degradation was confirmed by gas chromatography–mass spectrometry. The study suggested that the E. hormaechei is a potential biosurfactant and biocatalysts producer with the effective management of accidental marine oil spills.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crude oil-derived petroleum hydrocarbons are the ubiquitously found hazardous contaminants of the marine environments (de Silva et al. 2014; Kumari et al. 2016). Crude oil is the blend of four different classes of petroleum hydrocarbons comprising saturates, aromatics, resins and asphaltenes (Gurav et al. 2017; Sekkour et al. 2019). Saturates or aliphatic hydrocarbons include easily biodegradable n-alkanes (C7–C34) and less degradable isomeric alkane structures (Yanto and Tachibana 2014), whereas polycyclic aromatic hydrocarbons (PAHs) contain more than two benzene rings which are more recalcitrant in nature (Tahhan et al. 2011; Zhang et al. 2011; Pasumarthi et al. 2013). Moreover, global Environmental Protection Agencies enlisted petroleum hydrocarbons as perilous contaminants because of lethal and oncogenic nature (Wu et al. 2016). During the process of crude oil exploitation, transportation and refining, there is a chance of entry of these dangerous compounds into the marine environment which would cause catastrophic effects to all biotic life (Zhang et al. 2016). On January 28th 2017, a major crude oil spillage occurred along the coastal/shoreline area in Chennai, in which around 75000 tons of heavy petroleum was spilled over the Bay of Bengal and causes severe environmental impairment (Han et al. 2018). Another tragic oil spill in Columbia in March 2018 caused the death of more than 2400 animals and damage of more than 1000 tree species and also affected the health of local communities. Even though several combative methods such as skimming, floating boom and dispersal by chemical surfactants are followed to manage oil spills, still there is an inevitable need for efficient and environmental-friendly strategies for the effective management of disastrous marine oil spills.
In recent years, an emerging technology “bioremediation using indigenous microorganisms” is globally employed as an alternative sustainable strategy for the elimination of fatal environmental pollutants (Asemoloye et al. 2017; Chen et al. 2017; Imam et al. 2019). However, low solubility and hydrophobicity of the petroleum hydrocarbons (PHCs) become the major bottleneck in the successful bioremediation of crude oil (Patowary et al. 2016). The presence of surface acting or emulsifying molecules could improve the solubility of PHCs, thereby making them readily available for the biodegradation process (Plociniczak et al. 2011). Biosurfactants are the surface active, amphipathic biomolecules having a hydrophobic tail and a hydrophilic head part which allow these molecules to work at surface interfaces, reduce the surface tension, form stable emulsions and increase the solubility of weak solutes in water (Qiao and Shao 2010). Thus, these microbial-based surfactants are widely used as a promising active molecule in industries as well as in environment particularly for the bioremediation of oil-contaminated sites because of their expertise and low toxicity (Joy et al. 2017).
Many biodegradative enzymes belong to class of oxidoreductases are served as the major key components in the metabolic transformation of toxic PHCs into simpler non-toxic compounds (Muthukamalam et al. 2017). The biodegradation or biotransformation of PHCs is initiated by a key enzyme called alkane hydroxylase, which converts alkane to alkanols (Salam and Idris 2019). The subsequent conversion of alkanol to its corresponding aldehyde and carboxylic acids will be mediated by the series of dehydrogenases like alcohol dehydrogenase, aldehyde dehydrogenase and esterase or fatty acid hydroxylase, respectively (Kadri et al. 2018a). Also, the activity of some of the extracellular enzymes like laccase and lipase has also been observed during the remediation of PHCs-contaminated sites (Liu et al. 2017; Azin et al. 2018; Kadri et al. 2018b).
Even though various researches have been reported on the crude oil-assimilating microbial strains and suggested their application in the oil spill management, no successful and effective outcomes have been achieved (Milic et al. 2012). The extreme marine conditions like salinity and nutrients scarcity are the major hurdles in attaining prospective results in the field of oil spill bioremediation (Nwankwegu et al. 2017). Hence, the present study is focussed on isolation of potential hydrocarbon-degrading strain and its subsequent application in the marine oil spill remediation using agricultural waste rice husk as the biomatrix to improve microbial growth and stability in harsh environmental conditions. In addition, the effects of biostimulants or nutritional amendments such as glucose and urea on crude oil degradation were investigated.
Moreover, the present study is addressing the solution for the global issue and the research could be widely applied for the bioremediation of marine oil spill. The biosurfactant produced by E. hormaechei forms the basis for successful bioremediation of these hazardous pollutants.
This study was conducted during 2018–2019 at Biomolecules and Biocatalysis Laboratory, Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Tamil Nadu, India.
Materials and methods
Screening, isolation and identification of crude oil-assimilating microorganisms
Marine-spilled crude oil and the polluted seawater were collected from an affected coastal area in Ennore, Tamil Nadu, India. Soil source for the isolation of hydrocarbonoclastic microorganisms was sampled from five different sites like activated sludge from the sewage treatment plant, diesel-contaminated soil, petroleum-polluted soil around Chennai Petroleum Corporation Ltd. (CPCL) Manali, soil acclimatized with crude oil sludge and petroleum-contaminated soil around automobile workshop. The enrichment medium comprised of polluted sea water added with marine-spilled crude oil 1%, glucose 1% and ammonium sulphate 1% was used for the isolation of microbes. Each soil sample, 1 g, was separately added into enrichment medium, 100 ml, and incubated for 7 days at 120 rpm and at 35 °C. Then, 2 ml of culture was again inoculated into fresh enrichment medium and incubated at 35 °C and the subculturing was repeated twice for every 7 days. Next, the enriched microbial culture was serially diluted, spread on nutrient agar plates and incubated till the visible colonies appear. Each colony of different morphologies was streaked on nutrient agar plates to get a pure isolate and stored for future studies. To select a potential hydrocarbon-degrading and biosurfactant-producing strain, a pure culture of each isolate was inoculated into mineral medium (g/L), 100 mL, containing NaH2PO4, 0.2; K2HPO4, 0.8; CaCl2, 0.5; FeSO4, 0.05; MgSO4, 0.1; and NaCl, 1.0; and the major energy source of 1% crude oil was supplied. Then, culture flasks were incubated for 10 days at 120 rpm and at 35 °C.
All isolates were evaluated related to its cell surface hydrophobicity, surface tension reduction and degradation efficiency (Wang et al. 2014; Elumalai et al. 2017). The cell surface hydrophobicity of all isolates was determined by Bacterial Adhesion to Hydrocarbon (BATH) assay (Panjiar et al. 2015). Each isolate was inoculated in Luria–Bertani (LB) medium and grown overnight at 30 °C. Then, the culture was centrifuged at 10,000 rpm for 10 min to separate the bacterial cells. The collected pellet was washed twice, resuspended in LB medium and adjusted to absorbance values between 0.1 and 0.4 (at OD600nm). The adjusted cell suspension, 2 ml, was mixed with n-hexadecane, 1 ml, followed by vortexing at maximum speed for 2 min and kept undisturbed for 30 min to achieve the hydrophobic phase separation. Then, the bottom layer was carefully collected and the turbidity was observed using UV–Vis spectrophotometer at OD600nm. LB medium without bacterial inoculation was used as a blank. The percentage of cell adherence (%A) was estimated as per the following equation.
The BS production during degradation was studied by measuring the surface tension reduction of culture medium in all experimental flasks. Cells were removed by centrifugation at 10,000 rpm for 10 min, and the culture-free supernatant was subjected to measurement of the surface tension using a tensiometer (K11, Kruss, Germany)(Zheng et al. 2012). The percentage of crude oil degradation was measured in terms of residual crude oil present in the flasks after biodegradation. Residual crude oil was extracted from both control and experimental flasks by mixing the entire culture free medium with the equal volume of hexane. In the separating funnel, hexane/residual crude oil mixture was mixed continuously for 15 min and allowed for phase separation. Again, hexane was added with the aqueous layer to collect any residual oil present and all the samples were pooled together. Using the rotary evaporator, hexane was separated out from the crude oil mixture and weighed gravimetrically. The degradation efficiency was calculated using the following formula (Sarkar et al. 2016).
The selected crude oil-degrading bacterial isolate was then identified by 16S rRNA sequencing analysis.
Biosurfactant and biocatalysts assays
Biosurfactant–emulsification activity
The efficiency of the produced BS was assessed by calculating emulsification index (E24). Two ml of culture-free medium was vortexed with the equal volume of kerosene at maximum speed and kept undisturbed for 24 h. The height of the emulsified phase was then measured, and the emulsification index was calculated using the given formula (Colin et al. 2013).
Extracellular enzymes—lipase and laccase assay
The extracellular enzyme assays were performed with the crude enzyme molecules released in the culture medium during crude oil degradation. The oil and culture-free extracellular enzymes were extracted by the centrifugation of culture medium at 10,000 × g for 10 min at 4 °C [cooling centrifuge (Remi C-24 plus)]. Lipase activity was performed using olive oil as substrate, for which hydrophobic oil substrate was provided in the form of emulsion. The olive oil 10% (v/v) in hot water-soluble polyvinyl alcohol (PVA) 2% (w/v) was emulsified by ultrasonication using ultrasonicator. The enzymatic reaction mixture consisting of olive oil emulsion, 5 ml; 0.03% Triton X-100, 2 ml; 3 M NaCl, 1 ml; 0.075% CaCl2, 2 ml; and 0.01 M potassium phosphate buffer (pH 7.0) 4 ml was allowed for mixing in shaker for 5 min. The reaction was initiated by adding crude enzyme extract, 1 ml, and incubated for 15 min in shaker. Finally, the enzyme activity was stopped using 1:1 ratio of acetone/ethanol mixture, 15 ml, and titrated against 0.02 N NaOH using phenolphthalein indicator. One unit activity of lipase is defined as the amount of enzyme that released 1 µmol of fatty acid per min under reaction conditions (Ramani et al. 2012). The laccase activity was assayed through the oxidation of 2,2-azinobis(3-ethylbenzothiozoline-6-sulphonate) (ABTS). The reaction mixture containing 5 mM ABTS, 100 µl; crude enzyme, 100 µl; and 0.1 M sodium acetate buffer (pH 4.5) 2.8 ml, was incubated, and an increase in absorbance was measured after 5 min at 420 nm. One unit activity of laccase is the amount of enzyme required to oxidize 1 µmol of ABTS per minute (Kalcikova et al. (2014).
Intracellular enzymes—alkane hydroxylase, alcohol dehydrogenase and esterase assay
The activities of alkane hydroxylase, alcohol dehydrogenase and esterase enzymes were determined after the extraction of crude intracellular enzymes. The resulted cell pellet from the centrifugation of culture medium was rinsed twice and resuspended in Tris–HCl buffer (20 mM, pH 7.4), 2 ml. The suspended bacterial cells were subjected to intermittent sonication at 4 °C with the pulse rate of 30 s for 3 min using ultrasonicator. Further, the intracellular enzymes were collected after the centrifugation of sonicated cells for 10 min at 10,000 × g. The reaction mixture of alkane hydroxylase assay is comprised of 20 mM Tris–hydrochloride, 0.1 mM of nicotinamide adenine dinucleotide hydrogen (NADH) along with CHAPS buffer (0.15%, pH 7.4) and crude enzyme extract (50 µl). The assay was started by adding 10 µl of PHC substrate (1% n-hexadecane prepared in 80% dimethylsulphoxide (DMSO)), and reduction in absorbance was read at 340 nm (Meng et al. 2017). One unit activity of alkane hydroxylase was expressed as the amount of enzyme needed for the oxidation of one mmol of NADH per minute. The reaction mixture for the alcohol dehydrogenase assay consisted of 4 mM of NAD+, 1 M Tris–HCl buffer (pH 8.8) and crude enzyme extract, 50 µl. The reaction was carried out for 3 min after the addition of 99% ethanol, 100 µl, and increase in absorbance was measured at 340 nm. One unit activity of alcohol dehydrogenase is defined as the amount of enzyme required to reduce 1 NAD+ per min (Jauhari et al. 2014). The assay mixture of esterase was prepared by adding 50 mM potassium phosphate buffer (pH 7.5), 2.8 ml; 100 mM o-nitrophenyl butyrate solution (ONPB) diluted in DMSO, 0.1 ml; and crude enzyme extract, 0.1 ml. The esterase activity in terms of increased absorbance at 420 was observed per minute for 5 min. One unit activity of esterase is represented as the enzyme used to produce 1 μmol of esters per minute (Sekhon et al. 2012).
Optimization of environmental parameters
The effect of different external carbon sources such as glucose, fructose, sucrose, maltose and lactose and nitrogen sources like peptone, yeast extract, beef extract, urea and ammonium sulphate, pH and crude oil concentration was studied. Response Surface Methodology (RSM), a statistical and mathematical modelling technique, was used to evaluate the relationship between different experimental conditions during crude oil degradation. The optimization experiments were designed according to central composite design (CCD) using Design-Expert Stat-Ease version 12. The degradation efficiency and emulsification index were considered as the responses for the study (Saranya et al. 2014).
Extraction and characterization of biosurfactant
Crude biosurfactant from the culture medium was extracted on the day of maximum emulsification index achieved. The pH of the cell-free culture medium was adjusted with 6 N HCl till pH 2.0 and then kept for overnight incubation at 4 °C. The precipitate was collected by centrifugation and neutralized to pH 7.0. The crude BS was lyophilized and weight was measured.
The surface charge of the BS was determined using agar double diffusion method and zeta potential analysis as mentioned (Saranya et al. 2015). Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) was performed to determine the molecular weight of the biosurfactant in accordance with the protocol of Laemmli (1970), on a 5% stacking gel and 10% separating gel using 20 to 250 KiloDaltons (KDa). The emulsification index of the BS against different hydrocarbons such as kerosene, diesel, petrol, hexadecane, crude oil and olive oil was determined. The functional groups of BS were determined using the Fourier transform infrared (FT-IR) spectroscopy (PerkinElmer). The moisture-free crude BS was pelleted into the size of 13 mm diameter and 1 mm thickness by adding spectroscopic grade potassium bromide, and the analysis was performed between 400 and 4000 cm−1 spectral range. The fatty acid composition of BS was analysed after the esterification of fatty acids. 5 mg of crude BS was dissolved in 1 ml of chloroform, and esterification was carried out with 20 ml of methanol and 1 ml of H2SO4 at 60 °C under reflux. After 5 h, retentate containing fatty acid methyl esters were collected with the same amount of hexane (1:1) and subjected for analysis after solvent evaporation. The GC–MS analysis of esterified BS was carried out using Agilent 6890 GC equipped with a 5973C mass spectrum. A 5% phenyl methyl siloxane capillary column was run with a temperature program of 50–120 °C for 20 min and raised to 280 °C, 2 min.
Bioremediation of marine oil spill—a laboratory-scale study
Preparation of rice husk-laden E. hormaechei
The microbial inoculum for the bioremediation was prepared using the agricultural industry by-product rice husk. The use of rice husk improves the growth of microorganisms and also increases the durability of microbes in the toxic environment (Nwankwegu et al. 2017). Five grams of overnight grown microbial biomass was added to the 10 g of sterilized rice husk, and 40% of moisture was provided to ensure microbial growth. The microbial growth on the rice husk was analysed using scanning electron microscopy.
Experimental design
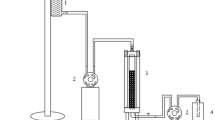
The experimental setup for the bioremediation consisted of a rectangular tank of about 40 × 20 × 10 cm dimensions with a total capacity of 8 l. The consistent supply of aeration was provided at the bottom of the tank to promote the even distribution of microbes throughout the medium. Five hundred millilitres of crude oil were spilled over five litres of seawater, and about 10 g of rice husk-laden E. hormaechei (RH-EH) was spread on the spill. In addition, glucose and urea were added to the seawater as the biostimulants (Xu et al. 2018). The percentage of TPH removal was determined at the end of the experiment.
Kinetics of biodegradation of crude oil
In order to determine the rate kinetics for the crude oil biodegradation, nonlinear kinetic models were employed (Ramavandi et al. 2018). The biodegradation kinetics was expressed by the following equation.
where C is the amount of TPH present in the residual crude oil (g); C0, amount of TPH present in the initial crude oil (g); t, incubation time (day); k, rate constant (day−1).
Composition-wise degradation analysis of crude oil
The composition of initial and degraded crude oil was estimated gravimetrically using saturates, aromatics, resins and asphaltenes (SARA) analysis. The total PHCs present in the crude oil, 1 ml, were first extracted with dichloromethane and kept for solvent evaporation. Then, PHC was dissolved in n-hexane, and insoluble asphaltenes were separated by using Whatman filter paper and estimated gravimetrically. The silica gel of 60–120 mesh was used to pack the column, and the packed column was prewashed with n-hexane, and concentrated soluble PHC fraction was loaded. Saturates were eluted using n-hexane, 1:1(v/v) ratio of n-hexane and trichloromethane mixture were used to collect aromatic fraction, and the resins were eluted by a mixture of trichloromethane and methanol (1:1, v/v). All eluted fractions were quantified gravimetrically (Abouee Mehrizi et al. 2019).
GC–MS analysis of initial and degraded crude oil
The hydrocarbon composition of both initial and degraded crude oil was analysed by following the GC–MS conditions mentioned by Suganthi et al. (2018). The obtained initial and degraded crude oil mass spectra were interpreted for the presence or the absence of peaks corresponding to PHCs identified using NIST Library.
Results and discussion
Screening, isolation and identification of the most efficient crude oil-degrading strain
After enrichment for 3 weeks, a total of 10 different bacterial isolates from the various hydrocarbon contaminated soil samples were collected, viz. 2 isolates (AS1 and AS2) from activated sludge from sewage treatment plant, 2 isolates (DS1 and DS2) from diesel contaminated soil nearby the generator, 1 isolate (MS) from petroleum-contaminated soil, 3 isolates (STP1, STP2 and STP3) from soil acclimatized with crude oil sludge and 2 isolates (WS1 and WS2) from PHC-contaminated soil around automobile workshop. Out of all the ten isolates, the isolate STP-3 showed maximum growth as well as high TPH removal than the other strains. In BATH assay, the isolate STP-3 showed highest percentage of cell adherence 61% towards the hydrophobic substrate, hexadecane (Fig. 1). In the same way, the surface tension of the experimental flask inoculated with the isolate STP-3 was greatly reduced to 35.44 mN/m (Supplementary table S1). Thus, from the results obtained, it was concluded that isolate STP-3 effectively utilized the petroleum hydrocarbons present in the crude oil, thereby achieving maximum degradation. Through 16 s rRNA sequencing technique, the strain STP-3 was identified as Enterobacter hormaechei. The resulted gene sequence was submitted in GenBank under accession number of MK603175. A phylogenetic tree for the E. hormaechei is shown in Supplementary Fig. S2.
Production of biosurfactant and biocatalysts during biodegradation
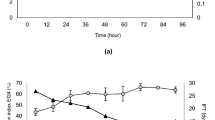
Figure 2 illustrates the day-wise profile of growth and the degradation efficiency the selected isolate Enterobacter hormaechei STP-3. Both profiles follow the same trend during the incubation. The rate of degradation was slow in the lag phase till 3rd day and was progressively increased to a higher degree and achieved around 80% at the end of the log phase. Finally, it reached to saturation point at 84% of PHC degradation after the 9th day of incubation. The biosurfactant production was indicated by its emulsification activity and the reduction in the surface tension of culture medium (Ohadi et al. 2017). From graph (Fig. 3), it could be observed that the emulsification activity was steadily increased until day 5, reaching maximum activity of 46.34%, afterwards the activity decreased. Likewise, there was surface tension reduction in the crude oil degraded culture medium from 86 to 35 mN/m on the 5th day, and there was no further decline in the surface tension. Therefore, it can be predicted that the biosurfactant was produced as a primary metabolite as its production was associated with the microbial growth during crude oil degradation.
Enzyme activity during crude oil degradation
The activity profile of lipase and laccase is shown in Fig. 4. Lipases are the important hydrolysing catalysts that mediate the conversion of triglycerides to free fatty acids and glycerol. In the study, the lipase activity was about 60 U/ml on 1st day and gradually increased to 130 U/ml on the 5th day. Then, there was a slow drop in the activity during the later phase of growth. Laccase is an oxidoreductase enzyme that catalyses the aromatic ring cleavage of phenolic compounds and prevalently found in fungi. In this study, the activity of laccase was progressed along with the time and maximum activity, 58U/ml, was found on the day 7. The delayed production of laccase enzyme might be due to the complex nature of the aromatics.
Alkane hydroxylase is the key enzymes that initiate the alkane conversion through oxidation reaction in which oxygen molecules are substituted at the terminal sites of hydrocarbon chain. In our study, the enzyme activity was continuously monitored for 10 days and found to be maximum at 5th day around 52 U/ml in 10-day incubation. The activity was steadily increased along with growth until day five and started to decrease in the subsequent days. The maximum activity of intracellular alcohol dehydrogenase was 118 U/ml on day seven, and it decreased later. The activity of alcohol dehydrogenase enzyme was comparatively greater than the alkane hydroxylase. Meng et al. (2017) also observed higher alcohol dehydrogenase activity than alkane hydroxylase in Pseudomonas synxantha LSH-7 during n-hexadecane biodegradation. The activity of esterase was found to be higher as130 U/ml on the 5th day when compared to the other two intracellular enzymes. The combined activity profiles of intracellular enzymes are depicted in Fig. 5. The time-varied activities of hydrocarbon-degrading enzymes might be due to the changes in the PHCs concentration during crude oil degradation. Overall, crude oil PHCs were first oxidized by alkane hydroxylase and converted into primary alcohol that was transformed to respective aldehyde. In the end, fatty acid was released by the activity of esterase enzyme. The resulting fatty acids are converted to dicarboxylic acids through β-oxidation and enter into the tricarboxylate cycle for microbial growth. Hence, it could be understand that crude oil was first emulsified by the biosurfactant and then subjected to stepwise biocatalytic degradation by the sequential production of both extracellular and intracellular enzymes secreted by the E. hormaechei strain.
Optimization of process parameters
Using RSM analysis, optimal values of the significant factors which are involved in crude oil degradation and BS production were found using the central composite design. In Table S2, the high and low values of the selected factors are given. The relationship between the significant factors related to crude oil degradation and BS production was studied by the regression analysis using the second-order polynomial in Eqs. (5) and (6), respectively.
Analysis of variance–partial sum of squares: type III (ANOVA)
The statistical fit of the coded equation given was analysed by F test and second-order polynomial ANOVA. The F test analysis proved that the experimental data were correlated with the designed second-order polynomial model. The lower value of the coefficient of variation (CV) for crude oil degradation (0.3201) and BS production (1.09) showed well precision and reliability of experiments. The regression coefficient (R2) for degradation and for BS production was observed to be 0.9831 and 0.9941, respectively, explaining that the proposed model showed 99.41% and 98.31% variability in the response.
The three-dimensional representation of the contour plots (Figs. 6, 7) represented using regression model was given to determine the influence of the independent factors and relationship among each independent factor on the resultant factors. It indicated the optimal process parameters for the highest crude oil degradation and the BS production were pH, 7.0; concentration of glucose, 2%; concentration of urea, 2%; and concentration of crude oil, 3%. Each plot illustrated the influence of two factors, when the other factor was kept zero.
Characterization of biosurfactant
In agar double diffusion assay, the diffusion of two distinct molecules of same or different ionic charge in a solid agar medium occurs. The visible arc was observed between the cationic surfactant (CTAB) and the BS, whereas no arc was seen between anionic SDS and BS (Supplementary Fig. S2). Thus, it can be confirmed that the biosurfactant produced by E. hormaechei was anionic. The zeta potential of the biosurfactant was found to be at − 31 mV, which suggests the polarized nature of the compound. This confirms the biosurfactant produced in this study is anionic in nature (Supplementary Fig. S3). From SDS–PAGE (Fig. S4), the molecular mass was determined to be 48 kDa, which confirms the BS belongs to the high molecular weight class of lipopeptide. The emulsification activity of the biosurfactant was evaluated against different hydrocarbons such as n-hexadecane, olive oil, kerosene, petrol, diesel and crude oil, and their respective E24 were calculated as 46, 38, 42, 40 and 44% (Supplementary Fig. S5). The maximum emulsification activity of the biosurfactant was 46% for hexadecane, followed by crude oil, diesel, kerosene, petrol and olive oil. The FT-IR spectrum of partially purified biosurfactant displays major bands at 3435, 2926, 1651 and 1551 cm−1 (Supplementary Fig. S6). The FT-IR spectrum was interpreted for various functional groups present to determine the molecular nature of the BS. The peak 3435 cm−1 indicates N–H bonding corresponds to the stretching vibration of the amides. The C–H stretching vibrations at 2926 cm−1 denote the presence of alkyl (CH2–CH3) hydrocarbon chain. The characteristic band at 1651 cm−1 confirms the presence of amide bond (C–O=N–H) in BS. The peak 1550 cm−1 denotes the N–H bending vibration and C–N stretching vibrations of protein. Hence, it can be confirmed that this biosurfactant is a lipopeptide that comprises a chain of amino acids attached covalently with a long hydrocarbon chain. In the GC–MS chromatogram of esterified fatty acids, the prominent peaks of 15.624, 17.969, 20.278, 20.321 and 20.547, which corresponds to tetradecanoic acid, hexadecanoic acid, octadecanoic acid, octadecenoic acid and eicosanoic acid, respectively, have appeared (Supplementary Fig. S7).
Bioremediation of simulated marine oil spill
The electron micrograph showing microbial growth on the surface of the rice husk is given in Figure S8, and the experimental setup for the bioremediation of simulated marine oil spill is illustrated in Figure S9. After 10 days of bioremediation experiment, the percentage removal of TPH present in crude oil was determined by oil and grease analysis, and it was about 83.7%. The rate kinetics of the biodegradation of crude oil by using only E. hormaechei and RH-E. hormaechei were studied. The first-order (k1) and second-order (k2) rate constants of both treatments are determined and provided in Table 1. The results confirmed that both treatments followed pseudo-second-order reaction as corresponds to the observed greater R2 values. The SARA analysis of both initial and degraded crude oil is shown in Fig. 8. Saturates and aromatics portions were degraded to a higher percentage, whereas there was an negligible reduction in the asphaltenes content as it is more complex in nature to assimilate. From the GC–MS analysis, it has been inferred that there was a reduction of PHC in the treated sample when compared with the initial sample (Fig. 9). It authenticates the gravimetric results of both oil and grease analysis and SARA analysis. The hydrocarbons ranging from C8 to C34, including octane, nonane, decane, dodecane, tetradecane, pentadecane, hexadecane, heptadecane, octadecane, pristane, nonacosane, eicosane, heneicosane and tetratriacontane, were present in the untreated sample. In the treated sample, 9-octadecenoic acid, octadecyl ester, octadecane, 3-ethyl-5-(2-ethyl butyl)-, pentadecane, 2,6,10-trimethyl-, carbonic acid, ethyl pentafluorobenzyl ester and benzene, (1,3,3-trimethylnonyl) were detected as the degradation intermediates. The results confirmed the degradation of crude oil by E. hormaechei.
Conclusion
The study reported the crude oil-degrading efficiency of a rarely found bacterium Enterobacter hormaechei, isolated from petroleum hydrocarbon acclimatized soil. Isolate STP-3 was confirmed as potential BS producer and crude oil remediating agent, and most importantly, the role of different biocatalysts such as alkane hydroxylase, alcohol dehydrogenase, esterase lipase and laccase in the degradation of petroleum hydrocarbons was also investigated. Finally, the demonstration of marine oil spill remediation by E. hormaechei coated rice husk showed the pronounced efficiency in the remediation of an oil spill in the marine conditions. Hence, this strain could be effectively employed as a bioremediation agent for the recovery of petroleum hydrocarbon-polluted marine environment, and further exploration of the genetic characteristics of Enterobacter hormaechei could provide betterment in the usage of this proficient strain.
References
Abouee Mehrizi E, Kermani M, Farzadkia M et al (2019) Study of improvement of bioremediation performance for the degradation of petroleum hydrocarbons in oily sludge by a chemical pretreatment strategy. J Mater Cycles Waste Manag 21:1052–1062. https://doi.org/10.1007/s10163-019-00848-y
Asemoloye MD, Ahmad R, Jonathan SG (2017) Synergistic action of rhizospheric fungi with Megathyrsus maximus root speeds up hydrocarbon degradation kinetics in oil polluted soil. Chemosphere 187:1–10. https://doi.org/10.1016/j.chemosphere.2017.07.158
Azin E, Moghimi H, Heidarytabar R (2018) Petroleum degradation, biosurfactant and laccase production by Fusarium neocosmosporiellum RH-10: a Microcosm study. Soil Sediment Contam 27:329–342. https://doi.org/10.1080/15320383.2018.1473334
Chen Q, Li J, Liu M et al (2017) Study on the biodegradation of crude oil by free and immobilized bacterial consortium in marine environment. PloS ONE 12:1–23. https://doi.org/10.1371/journal.pone.0174445
Colin VL, Pereira CE, Villegas LB et al (2013) Production and partial characterization of bioemulsifier from a chromium-resistant actinobacteria. Chemosphere 90:1372–1378. https://doi.org/10.1016/j.chemosphere.2012.08.002
Elumalai P, Parthipan P, Parthiba Karthikeyan O, Rajasekar A (2017) Enzyme-mediated biodegradation of long-chain n-alkanes (C32 and C40) by thermophilic bacteria. 3 Biotech 7:1–10. https://doi.org/10.1007/s13205-017-0773-y
Gurav R, Lyu H, Ma J et al (2017) Degradation of n-alkanes and PAHs from the heavy crude oil using salt-tolerant bacterial consortia and analysis of their catabolic genes. Environ Sci Pollut Res 24:11392–11403. https://doi.org/10.1007/s11356-017-8446-2
Han Y, Nambi IM, Prabhakar Clement T (2018) Environmental impacts of the Chennai oil spill accident—a case study. Sci Total Environ 626:795–806. https://doi.org/10.1016/j.scitotenv.2018.01.128
Imam A, Suman SK, Ghosh D, Kanaujia PK (2019) Analytical approaches used in monitoring the bioremediation of hydrocarbons in petroleum-contaminated soil and sludge. TrAC Trends Anal Chem 118:50–64. https://doi.org/10.1016/j.trac.2019.05.023
Jauhari N, Mishra S, Kumari B, Singh SN (2014) Bacteria-mediated aerobic degradation of hexacosane in vitro conditions. Bioresour Technol 170:62–68. https://doi.org/10.1016/j.biortech.2014.07.091
Joy S, Rahman PKSM, Sharma S (2017) Biosurfactant production and concomitant hydrocarbon degradation potentials of bacteria isolated from extreme and hydrocarbon contaminated environments. Chem Eng J 317:232–241. https://doi.org/10.1016/j.cej.2017.02.054
Kadri T, Magdouli S, Rouissi T, Brar SK (2018a) Ex-situ biodegradation of petroleum hydrocarbons using Alcanivorax borkumensis enzymes. Biochem Eng J 132:279–287. https://doi.org/10.1016/j.bej.2018.01.014
Kadri T, Rouissi T, Magdouli S et al (2018b) Production and characterization of novel hydrocarbon degrading enzymes from Alcanivorax borkumensis. Int J Biol Macromol 112:230–240. https://doi.org/10.1016/j.ijbiomac.2018.01.177
Kalcikova G, Babic J, Pavko A, Gotvajn AZ (2014) Fungal and enzymatic treatment of mature municipal landfill leachate. Waste Manag 34:798–803. https://doi.org/10.1016/j.wasman.2013.12.017
Kumari B, Singh SN, Singh DP (2016) Induced degradation of crude oil mediated by microbial augmentation and bulking agents. Int J Environ Sci Technol 13:1029–1042. https://doi.org/10.1007/s13762-016-0934-2
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Liu B, Liu J, Ju M et al (2017) Bacteria-white-rot fungi joint remediation of petroleum-contaminated soil based on sustained-release of laccase. RSC Adv 7:39075–39081. https://doi.org/10.1039/c7ra06962f
Meng L, Li H, Bao M, Sun P (2017) Metabolic pathway for a new strain Pseudomonas synxantha LSH-7′: from chemotaxis to uptake of n-hexadecane. Sci Rep 7:1–13. https://doi.org/10.1038/srep39068
Milic JS, Solevic TM, Beskoski VP et al (2012) Biodegradation of petroleum sludge and petroleum polluted soil by a bacterial consortium: a laboratory study. Biodegradation 23:1–14. https://doi.org/10.1007/s10532-011-9481-1
Muthukamalam S, Sivagangavathi S, Dhrishya D, Rani SS (2017) Characterization of dioxygenases and biosurfactants produced by crude oil degrading soil bacteria. Brazilian J Microbiol 48:637–647. https://doi.org/10.1016/j.bjm.2017.02.007
Nwankwegu AS, Onwosi CO, Azi F et al (2017) Use of rice husk as bulking agent in bioremediation of automobile gas oil impinged agricuktural soil. Soil Sediment Contam Int J 26:96–114. https://doi.org/10.1080/15320383.2017.1245711
Ohadi M, Dehghan-Noudeh G, Shakibaie M et al (2017) Isolation, characterization, and optimization of biosurfactant production by an oil-degrading Acinetobacter junii B6 isolated from an Iranian oil excavation site. Biocatal Agric Biotechnol 12:1–9. https://doi.org/10.1016/j.bcab.2017.08.007
Panjiar N, Sachan SG, Sachan A (2015) Screening of bioemulsifier-producing micro-organisms isolated from oil-contaminated sites. Annu Microbiol 65:753–764. https://doi.org/10.1007/s13213-014-0915-y
Pasumarthi R, Chandrasekaran S, Mutnuri S (2013) Biodegradation of crude oil by Pseudomonas aeruginosa and Escherichia fergusonii isolated from the Goan coast. Mar Pollut Bull 76:276–282. https://doi.org/10.1016/j.marpolbul.2013.08.026
Patowary K, Patowary R, Kalita MC, Deka S (2016) Development of an efficient bacterial consortium for the potential remediation of hydrocarbons from contaminated sites. Front Microbiol 7:1–14. https://doi.org/10.3389/fmicb.2016.01092
Płociniczak MP-, Płaza GA, Piotrowska-seget Z, Cameotra SS (2011) Environmental applications of biosurfactants: recent advances. Int J Mol Sci 12:633–654. https://doi.org/10.3390/ijms12010633
Qiao N, Shao Z (2010) Isolation and characterization of a novel biosurfactant produced by hydrocarbon-degrading bacterium Alcanivorax dieselolei B-5. J Appl Microbiol. https://doi.org/10.1111/j.1365-2672.2009.04513.x
Ramani K, Jain SC, Mandal AB, Sekaran G (2012) Microbial induced lipoprotein biosurfactant from slaughterhouse lipid waste and its application to the removal of metal ions from aqueous solution. Colloids Surfaces B Biointerfaces 97:254–263. https://doi.org/10.1016/j.colsurfb.2012.03.022
Ramavandi B, Ghafarizadeh F, Alavi N et al (2018) Biotreatment of total petroleum hydrocarbons from an oily sludge using co-composting approach. Soil Sediment Contam 27:524–537. https://doi.org/10.1080/15320383.2018.1489371
Salam LB, Idris H (2019) Consequences of crude oil contamination on the structure and function of autochthonous microbial community of a tropical agricultural soil. Environ Sustain 2:167–187. https://doi.org/10.1007/s42398-019-00058-0
Saranya P, Kumari HS, Jothieswari M et al (2014) Novel extremely acidic lipases produced from Bacillus species using oil substrates. J Ind Microbiol Biotechnol 41:9–15. https://doi.org/10.1007/s10295-013-1355-6
Saranya P, Bhavani P, Swarnalatha S, Sekaran G (2015) Biosequestration of chromium(III) in aqueous solution using cationic and anionic biosurfactants produced from two different Bacillus sp.—a comparative study P. RSC Adv 5:80596–80611
Sarkar J, Kazy SK, Gupta A, Dutta A, Mohapatra B, Roy A, Bera P, Mitra A, Sar P (2016) Biostimulation of indigenous microbial community for bioremediation of petroleum refinery sludge. Front Microbiol 7:1407. https://doi.org/10.3389/fmicb.2016.01407
Sekhon KK, Khanna S, Cameotra SS (2012) Biosurfactant production and potential correlation with esterase activity. J Pet Environ Biotechnol 03:1–10. https://doi.org/10.4172/2157-7463.1000133
Sekkour S, Abdelkader B, Bouchiba Z et al (2019) The diversity of cultivable hydrocarbon-degrading bacteria isolated from crude oil contaminated soil and sludge from Arzew Refinery in Algeria. J Microbiol Biotechnol Food Sci 9:70–77. https://doi.org/10.15414/jmbfs.2019.9.1.70-77
Silva RDCFS, Almeida DG, Rufino RD et al (2014) Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int J Mol Sci 15:12523–12542. https://doi.org/10.3390/ijms150712523
Suganthi SH, Murshid S, Sriram S, Ramani K (2018) Enhanced biodegradation of hydrocarbons in petroleum tank bottom oil sludge and characterization of biocatalysts and biosurfactants. J Environ Manag 220:87–95. https://doi.org/10.1016/j.jenvman.2018.04.120
Tahhan RA, Ammari TG, Goussous SJ, Al-Shdaifat HI (2011) Enhancing the biodegradation of total petroleum hydrocarbons in oily sludge by a modified bioaugmentation strategy. Int Biodeterior Biodegrad 65:130–134. https://doi.org/10.1016/j.ibiod.2010.09.007
Wang W, Cai B, Shao Z (2014) Oil degradation and biosurfactant production by the deep sea bacterium Dietzia maris As-13-3. Front Microbiol 5:1–11
Wu M, Dick WA, Li W et al (2016) Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int Biodeterior Biodegradation 107:158–164. https://doi.org/10.1016/j.ibiod.2015.11.019
Xu X, Liu W, Tian S et al (2018) Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: a perspective analysis. Front Microbiol 9:1–11. https://doi.org/10.3389/fmicb.2018.02885
Yanto DHY, Tachibana S (2014) Potential of fungal co-culturing for accelerated biodegradation of petroleum hydrocarbons in soil. Elsevier, Amsterdam
Zhang Z, Hou Z, Yang C et al (2011) Degradation of n-alkanes and polycyclic aromatic hydrocarbons in petroleum by a newly isolated Pseudomonas aeruginosa DQ8. Bioresour Technol 102:4111–4116. https://doi.org/10.1016/j.biortech.2010.12.064
Zhang J, Xue Q, Gao H et al (2016) Production of lipopeptide biosurfactants by Bacillus atrophaeus 5-2a and their potential use in microbial enhanced oil recovery. Microb Cell Fact 15:1–11. https://doi.org/10.1186/s12934-016-0574-8
Zheng C, Li Z, Su J et al (2012) Characterization and emulsifying property of a novel bioemulsifier by Aeribacillus pallidus YM-1. J Appl Microbiol 113:44–51. https://doi.org/10.1111/j.1365-2672.2012.05313.x
Acknowledgement
The authors are grateful to the Department of Biotechnology, Ministry of Science and Technology, New Delhi, for sanctioning the project (BT/PR20297/BBE/117/193/2016) under “Biosystems and Bioprocess Engineering scheme”. We are also extending our acknowledgment to SRM-DBT Partnership Platform for Contemporary Research Services and Skill Development in Advanced Life Sciences Technologies and Department of Biotechnology, School of Bioengineering, SRMIST (No. BT/PR12987/INF/22/205/2015), for providing instrumental facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The research does not involve human volunteers or animals for any experiments conducted by any of the authors.
Additional information
Editorial responsibility: Xu Han.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muneeswari, R., Swathi, K.V., Sekaran, G. et al. Microbial-induced biosurfactant-mediated biocatalytic approach for the bioremediation of simulated marine oil spill. Int. J. Environ. Sci. Technol. 19, 341–354 (2022). https://doi.org/10.1007/s13762-020-03086-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-03086-0