Abstract
Polycyclic aromatic hydrocarbons (PAHs) are the toxic and persistent micro-pollutants recalcitrant to biodegradation. Photo-Fenton process is a commonly adopted advanced oxidation process. Advanced oxidation processes generate highly reactive hydroxyl radicals (OH·) which completely mineralise the organic contaminants. This study aims to find the efficiency of photo-Fenton oxidation process in the removal of PAHs and COD from landfill leachate, and investigate its effect on 16 PAHs according to their number of aromatic rings. Experiments were designed using central composite design, a module of response surface methodology (RSM) in the Design-Expert software. pH, Fe2+ concentration, H2O2 concentration, reaction time and UV intensity were the five experimental variables which were optimised and modeled successfully. The statistical analysis proved that all the variables have significant effect on the model. The value of R2 (0.94) showed a high reliability in the estimation of chemical oxygen demand and polycyclic aromatic hydrocarbons removal efficiency. Optimum experimental conditions of pH 6.5, Fe2+ 1.1 g/L, H2O2 concentration 5.5 g/L, reaction time 40 min and UV intensity 13.5 W resulted in the maximum chemical oxygen demand and polycyclic aromatic hydrocarbons removal efficiency of 84.43% and 92.54%, respectively. Validation was carried out by conducting additional set of experiments, and the small gap between observed and predicted values confirmed that central composite design is the effective tool to optimise the photo-Fenton oxidation process in the degradation of chemical oxygen demand and polycyclic aromatic hydrocarbons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The significant issue in landfilling is management of leachate. It is well known that leachate contains many contaminants which pollute various environmental media; hence, adequate treatment of leachate is necessary before its discharge into the environment. Many of the advanced countries still pose difficulty in the complete treatment of the leachate. Apart from the conventional contaminants present in the leachate, studies show that it may also contain many of the micro-pollutants (poly-aromatic hydrocarbons and phthalate acid esters) in ppb range (Asakura et al. 2004; Fang et al. 2009; Zheng et al. 2009).
Polycyclic aromatic hydrocarbons (PAHs) are the group of compounds which are ubiquitous contaminants produced from both natural and anthropogenic activities such as pyrolysis, automobile exhaust and coal refining (Lyche et al. 2009; Ranc et al. 2016). These are used in the production of several plastic products, personal care products, cosmetics and pesticides. Disposal of these products in landfill leads to presence of these micro-pollutants in the leachate (Fang et al. 2018; Li et al. 2017). Studies have reported their presence in the several contaminated environmental compartments such as surface water, soil, sediments and also in airborne particulate matter (Niu et al. 2014; Selvaraj et al. 2015; Sha et al. 2007); Wang et al. (2008). The contamination occurs in various ways like municipal and industrial wastewater, rain or runoff water. They have also been detected in drinking water. PAHs concentration in surface water ranges from 0.1 to 830 ng/dm3 (Kwon et al. 2009). PAHs are extremely mutagenic, carcinogenic and teratogenic substances, which is the reason why they have gained a lot of attention in the studies of water, air and soil pollution. They affect the endocrinal activities both in humans and animals. Hence, they are known to be endocrine-disrupting compounds (Gomez-Hens and Aguilar-Caballos 2003; He et al. 2009). PAHs are persistent in nature due to the chemical stability and resistance to biodegradation. Hence, strict restrictions are imposed by law in many of the developed nations (Chen et al. 2017; Lamichhane et al. 2016).
PAHs have been classified by US-EPA as priority pollutants, to restrict the release of the compounds into the environment. Additionally, a maximum emission concentration is set for the most dangerous PAHs (Pandey et al. 2011). European Union (EU/2005/84/EC, 2005) has restricted the use of PAHs in children’s toys to be less than 0.1%, and World Health Organization (WHO 2004) has mentioned the limit of PAHs in drinking water to be less than 10 ppb (Organization 2004). Quantification and degradation of these micro-pollutants (PAHs) are burning issues. Conventional treatment plants are usually designed to remove only few of the pollutants such as BOD, COD, ammoniacal nitrogen and heavy metals, which can be easily detected and removed, whereas elimination of these micro-pollutants from the leachate is a challenging issue.
PAHs are bio-recalcitrant, low aqueous soluble and toxic in nature due to which they cannot be completely eliminated by conventional physicochemical and biological systems. Additionally, low concentrations of these pollutants in the leachate pose challenge to their removal efficiency. Hence, advanced oxidation processes (AOPs) are known to be highly effective, as they generate highly reactive hydroxyl radicals that can eliminate almost all the types of organic compounds leading to total mineralisation (He et al. 2009; Rubio-Clemente et al. 2014).
In this study, photo-Fenton oxidation process is adopted. This process uses Fenton reagents (H2O2 and Fe2+) along with UV–vis radiation (λ < 600 nm) producing additional OH· radicals via two reaction pathways: (1) conversion of Fe3+–Fe2+ ions using UV light [Eq. (1)] (Faust and Hoigné 1990) and (2) photolysis of peroxide via shorter wavelengths [Eq. (2)].
Additional hydroxyl radicals are generated when the ferrous ions yielded by UV light go through the Fenton reaction. Studies have reported that photo-Fenton process uses lesser amount of iron and generates lower amount of sludge. Additionally, the use of UV light in this system kills the microorganisms in polluted water-bodies (Gárcia-Fernández et al. 2012).
In recent years, many researchers studied photo-Fenton oxidation process for the management of landfill leachate (Amor et al. 2015; Ebrahiem et al. 2017; Li et al. 2016; Primo et al. 2008). However, most of the studies evaluated the color, COD and turbidity removal. Therefore, this study also focuses on degradation of PAHs, in addition to COD, from leachate of hazardous waste landfill using photo-Fenton process.
The objectives of this study aim (1) to find optimum variables (pH, Fe2+, H2O2, reaction time and UV intensity), and (2) to find removal efficiency of total PAHs, COD and effect on PAHs on basis of their aromatic rings. Central composite design (CCD), a module in design of experiments (DOE), was adopted to design, analyze, optimise and validate the experiments. This study was conducted at Malaysia during the year 2019.
Materials and methods
Reagents
All the reagents obtained were of analytical grade obtained from Merck. The chemicals used were hydrogen peroxide (H2O2, 30% w/w), sulfuric acid (H2SO4), sodium hydroxide (NaOH), ferrous (II) sulfate heptahydrate (Fe2SO4.7H2O), anhydrous sodium sulfate, dichloromethane and acetonitrile (HPLC grade). All chemicals were used without drying nor further purification. The 16 PAHs mixture were obtained from Sigma-Aldrich USA. Standards of various concentrations for PAHs were prepared using acetonitrile as the solvent for calibration use.
Gas chromatography–mass spectrometry
Quantification of the 16 PAHs in leachate samples (before and after treatment) was performed using gas chromatography–mass spectroscopy (Perkin Elmer Clarus 600s). Elite 5MS column with dimensions of 30 m × 0.25 mm ID × 0.25 μm was used. The temperature of the column was adjusted between 60 and 175 °C at 6 °C/min, later elevated at 3 °C/min until 240 °C and finally it is made constant at 300 °C for 7 min. Selected ion monitoring (SIM) mode was used for acquiring the data.
Sampling
Leachate samples were obtained from a local landfill containing hazardous waste. An official agreement was signed with the local landfill authorities to collect the raw leachate samples to be used for the study. This landfill receives 100 metric tons of hazardous waste daily, which generates 150 m3/d of hazardous leachate that is treated in the facility before being discharged to the environment. The characterisation of hazardous waste leachate is presented in Table 1. Leachate samples from this landfill were collected in new PVC containers and stored at 4 °C in the laboratory. Standard Methods for the Examination of Water and Wastewater (APHA 2005) were adopted for measuring all the parameters (Eaton et al. 2005).
Photo-Fenton experiments
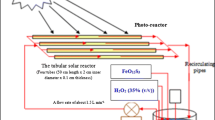
A series of batch experiments were conducted for the photo-Fenton process using the leachate from hazardous waste landfill, which adopted UV lamps of three different intensities (11, 14 and 16 W). The size of the sample used was 500 mL. To begin with, the sample was adjusted to the required pH using 1 M sulfuric acid. Necessary amounts of Fenton reagents (Fe2+ and H2O2) were added to the sample, while continuously stirring the sample. UV lamp of desired intensity was enclosed in a quartz tube and immersed in a glass beaker containing the sample. In order to maximise the exposure of the leachate sample to UV light, the UV lamp was submerged to the bottom of the beaker, without causing disturbance to the movement of stirrer. To avoid exposure to the UV rays, the arrangement was enclosed in a casing of aluminum foil. After completion of the required reaction time, the UV lamp was switched off, followed by the removal of the aluminum foil. To cease the reaction, the pH of the sample was adjusted to above pH 10. The sample was then subjected to sedimentation for half an hour at the end of the experiment, and the supernatant was collected for analysis of COD, followed by the extraction process for PAHs analysis.
Determination of PAHs
PAHs were obtained by liquid–liquid extraction (Net et al. 2015); Yaqub et al. (2014). 100 mL of supernatant and 12 mL of dichloromethane were taken in a separatory funnel placed on a retort stand. The funnel was then vigorously shaken a few times and regularly vented to release the pressure generated in the funnel. The funnel was then placed on the retort stand undisturbed to allow the formation of two layers of solution. After few minutes, the two layers were observed in the funnel. The upper portion contained water to be discarded and the lower portion contained an organic solution, which was transferred to a separate beaker. The collected organic solution was then mixed with desired amount of anhydrous sodium sulfate to get rid of water content. The solution was then filtered to get a clear sample, which was further concentrated to 2 mL in a rotary evaporator that was set at a pressure of 660 mg of Hg, temperature 60 °C and rotation 40 rpm. The concentrated sample was then collected in vials for analysis of PAHs using GC–MS. Table 2 presents the characteristics and quantification of PAHs. Equation 3 shows the determination of PAHs removal percentage.
where \({\left(\sum 16\mathrm{PAHs}\right)}_{i}=initial \:PAHs\: concentration\)
Results and discussion
Statistical analysis
“Design expert 10” software was used for the mathematical modeling, design, statistical analysis and to optimise the independent variables. The variables which are used in this study were: pH (A), Fe2+ concentration (B), H2O2 concentration (C), reaction time (D) and UV intensity (E). COD (Y1) and PAHs removal efficiencies (Y2) were considered as the responses. The five variables were changed to dimensionless quantities (A, B, C, D and E). The low, center and high levels of each independent variable are assigned correspondingly to the face-centered central composite design as − 1, 0 and + 1, respectively. In order to achieve greater accuracy in the polynomial fit and to ensure comparison of factors of various natures with various units, the independent variables were given dimensionless coded values (Eq. 4).
\({x}_{i}\) is the dimensionless coded value of the ith independent variable and \({x}_{0}\) denotes the value of \({x}_{i}\) at the center point and Δ\(x\) denotes the step change value (Montgomery 2017). The independent variables and their corresponding levels for the CCD used in the study can be seen in Table 3. Table 4 shows the experimental design matrix and response for COD and PAHs. The narrow ranges of each independent variable were fixed by conducting the preliminary experiments using factorial design, which is the first step in RSM.
Regression models and statistical testing
Equation 5 is an empirical second-order polynomial model which describes the behavior of the system.
‘Y’ denotes the response (COD and PAH removal efficiency), \({\beta }_{0}\) denotes offset coefficient, βi, βii and βij denote the coefficients of the linear, quadratic and interaction effect. \({x}_{i}\) and \({x}_{j}\) are the independent factors pH, Fe2+ concentration, H2O2 concentration, reaction time and UV intensity. ‘k’ indicates total number of independent variables in the experiment and ‘ε’ denotes the random error (Montgomery 2017).
Equations 6 and 7 are the models for predicting the COD and PAHs removal percentage with reasonable accuracy. The negative and positive signs represent the impact of the variable on the removal efficiency. In both the regression equations, the positive sign of the coefficient reveals the synergistic effect, whereas the negative sign represents the antagonistic effect.
ANOVA was conducted for the responses. The analysis confirms that all the five variables and few interactions among them are significant, showing their significant role in the COD and PAHs removal from landfill leachate by photo-Fenton process. The obtained F value of 21.40 and 46.53 implies that the model is significant for efficient removal of COD and PAHs, respectively. The ‘P’ values that are less than 0.05 specify that the independent variables are significant, and the results show that all the individual parameters and few of the interactions are significant model terms. On the other hand, the values of ‘Lack of Fit’ are nonsignificant having values greater than 0.05. A model is well fitted to the experimental data if it presents a significant regression and a nonsignificant lack of fit.
The statistical analysis proves that all the variables have a significant effect on the models. The summary of the RSM model fit output is illustrated in Table 5. All the values obtained are well within the permissible limits, which ensure that the results obtained from the experiments are reliable and satisfactory.
Diagnostic plots
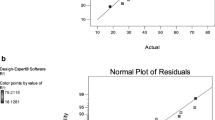
Figure 1 demonstrates that the predicted values of the responses from the model and the observed values are spread almost closer to the straight line (y = x), which signifies that observed and predicted values agree with each other.
Interactions between variables
The graphical interactions among the variables are shown by three-dimensional response surface plots of pH, and H2O2 concentration, Fe2+ concentration, reaction time and UV intensity were plotted. Most of the interactions among the various variables were significant making the plateau curvature of the 3D surfaces noticeable, as can be seen in Figs. 2 and 3. Most of the graphs show peaks indicating the optimum conditions for the responses with maximum value.
Effect of pH on COD and PAHs
Figure 2 illustrates the interactions of pH with other variables, where it can be seen that the graph declines as the pH increases beyond 5 resulting in a decrease in the COD removal efficiency from 80 to 60%. Similarly, Fig. 3 shows that the PAHs removal efficiency decreased as the pH increased beyond 5, confirming that acidic pH is more effective in the photo-Fenton process. It is due to the fact that at acidic pH, production of hydroxyl radicals is higher, which increases the efficiency of the process. Previous studies using photo-Fenton process reported that hydroxyl radicals are generated constantly at pH values between 2 and 5, and high efficiency can be obtained within this pH range (Atmaca 2009; Nidheesh and Gandhimathi 2012). At pH values higher than 5, Fe (III) ions precipitate in the form of ferric hydroxide sludge and the ferrous ions tend to co-precipitate with Fe3+sludge. This leads to ferrous ion deficiency in the solution, which is the main Fenton reagent in the reaction. This might be another reason for the decrease in the COD and PAHs removal rates at pH values higher than 5 (Khajouei et al. 2019). Equations 6 and 7 show the negative impact of pH on both the COD and PAHs removal efficiency. However, the interactions of pH with both the Fenton reagents and reaction time have positive effect on the system.
Effect of Fenton reagents on COD and PAHs
Optimising the dosage of Fenton reagents is vital in achieving a higher yield from the system. Figures 2 and 3 show the interaction of Fenton reagents, illustrating that the Fe2+ has the highest COD efficiency at 1.1 g/L, and higher and lower concentrations lead to decrease in the efficiency. Similarly, H2O2 concentration of 5.5 g/L showed maximum efficiency. In case of PAHs removal, the Fe2+ and H2O2 concentration of 1.1 and 5.5 g/L spiked the efficiency to the maximum. This may be due to lower dosages of Fenton reagents leading to lower production of oxidants, thus producing a low concentration of OH· radicals. In contrast, higher dosages of Fenton reagents have a scavenging effect on the hydroxyl radicals, leading to reduction in the efficiency of the process. Excess dosage results in the generation of hydroperoxyl radicals, which have a much weaker oxidising potential as compared to hydroxyl radicals. Furthermore, high dosages of hydrogen peroxide result in the auto-decomposition of hydrogen peroxide to form simpler compounds of water and oxygen, thereby lowering the hydroxyl radical concentration and decreasing the efficiency of the process. Equations 6 and 7 show positive impact of Fe2+ reagent with H2O2, reaction time and UV intensity both in the removal of COD and PAHs. Optimum UV intensity might help Fenton reagents to react efficiently to produce higher amount of hydroxyl radicals required for the degradation of organic compounds.
Effect of reaction time on COD and PAHs
Reaction time or treatment time is a significant parameter which controls the efficiency of the reaction. Too short of a reaction time does not allow the organic compounds to react with the Fenton reagents completely. On the other hand, longer reaction time beyond the optimal point tends to produce toxic intermediates leading to reduction in the efficiency of the system. Additionally, longer reaction time will not be economically viable. Hence, it is necessary to find the optimum reaction time. From Fig. 2, it can be noticed that the COD removal efficiency increased from 70 to 90% with the increase in the time from 20 to 60 min. It can be seen that the COD and PAHs removal reached maximum at a reaction time of 60 min. Further increase in the reaction time did not have a major influence on the mineralisation. This may be due to formation of short-chain organic acids and hardly oxidisable by-products. Photo-assisted Fenton process helped to lower the reaction time to attain higher efficiency as compared to conventional Fenton process. Similar results have also been reported by many researchers (Umar et al. 2010). Buthiyappan et al. (2016) observed the decrease in the treatment efficiency beyond the optimal reaction time. Equation 7 predicts a negative impact between reaction time and UV intensity. This may be due to longer exposure of UV light hindering the Fenton reactions leading to a decreased efficiency in the system.
Effect of UV intensity on COD and PAHs
UV intensity has very high impact on the production of hydroxyl radicals required for oxidation of organic compounds and the photoreduction rate of Fe3+ to Fe2+ (Buthiyappan et al. 2016). The efficiency of the reaction increased with the intensity of radiation. However, beyond the optimum point, the rate of reaction has reduced. The use of UV light in Fenton process enhances the rate of reaction and mineralisation efficiency. As illustrated in Figs. 2 and 3, both the COD and PAHs removal efficiency increased from 60 to 80% as the UV intensity increased from 11 to 14 W, and further increase in the UV intensity decreased the process efficiency. Equation 6 indicates a negative impact between Fe2+ concentration and UV intensity for removal of PAHs. This is because higher dosages of Fenton reagents hinder the light penetration which eventually reduces the efficiency of the system. The quadratic model predicted a negative interaction between pH and UV intensity on PAHs degradation efficiency. At pH levels more than 8, coagulation process is found to be very effective after the addition of iron salts to the system. It is commonly known that turbidity is the major cause that hinders the efficiency of a UV system, which might be the reason for the negative relationship between UV and pH. In the study conducted by Lak et al. (2018), it was reported that the use of UV light radiation generated more Fe (II) from Fe (III) which in turn enhanced the COD removal efficiency as compared to conventional Fenton process. It was also observed that photo-Fenton process consumed lesser amount of Fe2+ reagent and generated less iron sludge as compared to Fenton process. Rocha et al. (2013) reported that 92% of PAHs were removed from petrochemical wastewater using solar photo-Fenton. In one of the photo-Fenton studies, using the synthetic water with the mixture of PAHs (85%), phenolic compounds (10%) and heterocyclic compounds (5%), it was found that PAHs with two and three rings were effectively degraded as compared to PAHs with four and five rings (Engwall et al. 1999). Lin et al. (2016) achieved a removal efficiency of 83.5% in the degradation of 16 PAHs found in textile dying sludge using Fenton process.
Effect of photo-Fenton oxidation on PAHs at optimum conditions
After discussing the effect of various operating conditions on total PAHs in "Interactions between variables" section, Fig. 4 shows the removal of PAHs at optimum operating conditions derived from the central composite design. Figure 4 shows that the removal efficiency is negatively affected with increase in the number of rings. This can be attributed to the fact that the two-ring and three-ring PAHs fall under low molecular weight (LMW) PAHs and four-, five-, six-ring PAHs are high molecular weight (HMW) PAHs. The LMW PAHs are more susceptible to degradation as compared to HMW PAHs (Yap et al. 2011). This can be confirmed with the reports of Souza et al. (Souza Duarte et al. 2011) who obtained removal efficiency of 85% and 60% for two-ring and six-ring hydrocarbons, respectively, using electrochemical oxidation.
Naphthalene is the low molecular weight PAH, and it is the only double-ringed aromatic hydrocarbon that attained the highest removal efficiency of 96.32%. This result is at par with Nam et al. (2001) and Psillakis et al. (2004) who obtained removal of 85% and 95%, respectively, for this compound. The compounds acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe) and anthracene (Ant) are the tricyclic low molecular aromatic hydrocarbons. The removal efficiency of this group is at par with two-ringed hydrocarbons. Further, the four-, five- and six-ring compounds attained removal efficiency of 87.99%, 65.88 and 59.27%, respectively. The lower removal efficiency of these compounds is attributed to HMW possessing high n-octanol/water partition coefficient (KOW), which makes them more resistant to degradation (Kong et al. 2018). Extending the discussion on operating conditions, pH played a significant role in the degradation of PAHs. In this study, pH 5 attained the highest removal efficiency. These results concur with Nadarajah et al. (2002) who achieved the highest removal efficiency at pH 4. Contradiction to these results, Beltran et al. (1998) reported highest removal of PAHs at pH 7. However, many studies using advanced oxidation process for the wastewater treatment reported acidic pH range as the best, irrespective of the target compounds (Mohajeri et al. 2010a, b; Mohajeri et al. 2011). The optimum Fenton reagents obtained were 1.07 g/L and 5.46 g/L for Fe2+ and H2O2, respectively, with a UV lamp of 14 W. Heng et al. (2012) used photo-Fenton process as a pretreatment for mature landfill leachate, it was observed that UV lamp of 6 W having wavelength emission of 365 nm was efficient enough to achieve COD removal of 70% and color removal of 80%. The optimum conditions vary with characteristics of the wastewater to be treated.
Optimisation and validation experiments
Numerical optimisation was adopted to find the optimum process parameters for the highest removal efficiency. Based on response surface and highest desirability functions, the optimum conditions for COD and PAHs removals were attained. All the variables were set to be ‘in range’ whereas the responses were maximised. The optimum operating conditions obtained were pH 6.5, Fe2+ concentration 1.1 g/L, H2O2 concentration 5.5 g/L, reaction time 40 min and UV intensity 13.5 W. Under the optimum conditions, the maximum COD and PAHs removal efficiency obtained was 84.43% and 92.54%, respectively. A set of additional experiments was performed to validate the experiments as shown in Table 6. The small error between the experimental results and predicted values of software confirms that CCD/RSM is an effective tool to optimise photo-Fenton oxidation process in the reduction of COD and PAHs.
Conclusion
Photo-Fenton process produced the highest removal efficiency of 84.43% and 92.54% for COD and total PAHs, respectively, under acidic pH 6.5. It was observed that pH values greater than 6.5 decreased the removal efficiency of both COD and PAHs. Among the three UV lamps used in the study, 14 W UV lamp obtained the highest removal efficiency compared to 11 W and 16 W. The optimum dosage of Fenton reagents was 1.1 g/L and 5.5 g/L for Fe2+ and H2O2, respectively. It was observed that removal efficiency of two- and three-ringed PAHs was higher than four-, five- and six-ringed PAHs. Therefore, it can be concluded that these experimental conditions are efficient in the removal of LMW PAHs. Further studies are required to obtain effective operating conditions in the removal of HMW PAHs from landfill leachate using advanced oxidation processes.
References
Amor C, De Torres-Socías E, Peres JA, Maldonado MI, Oller I, Malato S, Lucas MS (2015) Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J Hazard Mater 286:261–268
APHA (2005) Standard Methods for the Examination of Water and Waste Water, 21st ed. American Public Health Association, Washington, DC
Asakura H, Matsuto T, Tanaka N (2004) Behavior of endocrine-disrupting chemicals in leachate from MSW landfill sites in Japan. Waste Manage 24(6):613–622
Atmaca E (2009) Treatment of landfill leachate by using electro-Fenton method. J Hazard Mater 163(1):109–114
Beltrán FJ, González M, Ribas FJ, Alvarez P (1998) Fenton reagent advanced oxidation of polynuclear aromatic hydrocarbons in water. Water Air Soil Pollut 105(3–4):685–700
Buthiyappan A, Raman AAA, Daud WMAW (2016) Development of an advanced chemical oxidation wastewater treatment system for the batik industry in Malaysia. RSC Adv 6(30):25222–25241
Chen C-F, Chen C-W, Ju Y-R, Dong C-D (2017) Determination and assessment of phthalate esters content in sediments from Kaohsiung Harbor. Taiwan Mar Poll Bull 124(2):767–774
da Rocha ORS, Dantas RF, Bezerra Duarte MM, Lima Duarte MM, da Silva VL (2013) Solar photo-Fenton treatment of petroleum extraction wastewater. Desalination Water Treat 51(28–30):5785–5791
Eaton A, Clesceri L, Rice E, Greenberg A, Franson M (2005) Standard methods for the examination of water and wastewater American public health association, American Waterworks Association, water environmental federation, 21st edn. Port City Press, Pikeville
Ebrahiem EE, Al-Maghrabi MN, Mobarki AR (2017) Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab J Chem 10:S1674–S1679
Engwall MA, Pignatello JJ, Grasso D (1999) Degradation and detoxification of the wood preservatives creosote and pentachlorophenol in water by the photo-Fenton reaction. Water Res 33(5):1151–1158
Fang C-R, Long Y-Y, Shen D-S (2009) Comparison on the removal of phthalic acid diesters in a bioreactor landfill and a conventional landfill. Biores Technol 100(23):5664–5670
Fang C, Chu Y, Jiang L, Wang H, Long Y, Shen D (2018) Removal of phthalic acid diesters through a municipal solid waste landfill leachate treatment process. J Mater Cycles Waste Manage 20(1):585–591
Faust BC, Hoigné J (1990) Photolysis of Fe (III)-hydroxy complexes as sources of OH radicals in clouds, fog and rain. Atmos Environ Part A Gen Top 24(1):79–89
Gárcia-Fernández I, Polo-López MI, Oller I, Fernández-Ibáñez P (2012) Bacteria and fungi inactivation using Fe3+/sunlight, H2O2/sunlight and near neutral photo-Fenton: a comparative study. Appl Catal B 121:20–29
Gomez-Hens A, Aguilar-Caballos M (2003) Social and economic interest in the control of phthalic acid esters. TrAC, Trends Anal Chem 22(11):847–857
He P-J, Zheng Z, Zhang H, Shao L-M, Tang Q-Y (2009) PAEs and BPA removal in landfill leachate with Fenton process and its relationship with leachate DOM composition. Sci Total Environ 407(17):4928–4933
Heng GC, Elmolla ES, Chaudhuri M (2012) Optimization of photo-Fenton treatment of mature landfill leachate. Nat Environ Pollut Technol 11(1):65–72
Khajouei G, Mortazavian S, Saber A, Meymian NZ, Hasheminejad H (2019) Treatment of composting leachate using electro-Fenton process with scrap iron plates as electrodes. Int J Environ Sci Technol 16(8):4133–4142
Kong L, Gao Y, Zhou Q, Zhao X, Sun Z (2018) Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J Hazard Mater 343:276–284
Kwon SH, Kim JH, Cho D (2009) An analysis method for degradation kinetics of lowly concentrated PAH solutions under UV light and ultrasonication. J Ind Eng Chem 15(2):157–162
Lak MG, Sabour MR, Ghafari E, Amiri A (2018) Energy consumption and relative efficiency improvement of Photo-Fenton–optimization by RSM for landfill leachate treatment, a case study. Waste Manage 79:58–70
Lamichhane S, Krishna KB, Sarukkalige R (2016) Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: a review. Chemosphere 148:336–353
Li J, Zhao L, Qin L, Tian X, Wang A, Zhou Y, Chen Y (2016) Removal of refractory organics in nanofiltration concentrates of municipal solid waste leachate treatment plants by combined Fenton oxidative-coagulation with photo–Fenton processes. Chemosphere 146:442–449
Li R, Liang J, Duan H, Gong Z (2017) Spatial distribution and seasonal variation of phthalate esters in the Jiulong River estuary Southeast China. Mar Pollut Bull 122(1–2):38–46
Lin M, Ning X-A, An T, Zhang J, Chen C, Ke Y, Liu J (2016) Degradation of polycyclic aromatic hydrocarbons (PAHs) in textile dyeing sludge with ultrasound and Fenton processes: Effect of system parameters and synergistic effect study. J Hazard Mater 307:7–16
Lyche JL, Gutleb AC, Bergman Å, Eriksen GS, Murk AJ, Ropstad E, Skaare JU (2009) Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health, Part B 12(4):225–249
Mohajeri S, Aziz HA, Isa MH, Bashir MJ, Mohajeri L, Adlan MN (2010) Influence of Fenton reagent oxidation on mineralization and decolorization of municipal landfill leachate. J Environ Sci Health Part A 45(6):692–698
Mohajeri S, Aziz HA, Isa MH, Zahed MA, Adlan MN (2010) Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J Hazard Mater 176(1–3):749–758
Mohajeri S, Aziz HA, Zahed MA, Mohajeri L, Bashir MJ, Aziz IMH (2011) Multiple responses analysis and modeling of Fenton process for treatment of high strength landfill leachate. Water Sci Technol 64(8):1652–1660
Montgomery DC (2017) Design and analysis of experiments. Wiley, Hoboken
Nadarajah N, Van Hamme J, Pannu J, Singh A, Ward O (2002) Enhanced transformation of polycyclic aromatic hydrocarbons using a combined Fenton’s reagent, microbial treatment and surfactants. Appl Microbiol Biotechnol 59(4–5):540–544
Nam K, Rodriguez W, Kukor JJ (2001) Enhanced degradation of polycyclic aromatic hydrocarbons by biodegradation combined with a modified Fenton reaction. Chemosphere 45(1):11–20
Net S, Delmont A, Sempéré R, Paluselli A, Ouddane B (2015) Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): A review. Sci Total Environ 515:162–180
Nidheesh P, Gandhimathi R (2012) Trends in electro-Fenton process for water and wastewater treatment: an overview. Desalination 299:1–15
Niu L, Xu Y, Xu C, Yun L, Liu W (2014) Status of phthalate esters contamination in agricultural soils across China and associated health risks. Environ Pollut 195:16–23
Organization WH (2004) Guidelines for drinking-water quality: recommendations. World Health Organization, Geneva
Pandey SK, Kim K-H, Brown RJ (2011) A review of techniques for the determination of polycyclic aromatic hydrocarbons in air. TrAC, Trends Anal Chem 30(11):1716–1739
Primo O, Rivero MJ, Ortiz I (2008) Photo-Fenton process as an efficient alternative to the treatment of landfill leachates. J Hazard Mater 153(1–2):834–842
Psillakis E, Goula G, Kalogerakis N, Mantzavinos D (2004) Degradation of polycyclic aromatic hydrocarbons in aqueous solutions by ultrasonic irradiation. J Hazard Mater 108(1–2):95–102
Ranc B, Faure P, Croze V, Simonnot M (2016) Selection of oxidant doses for in situ chemical oxidation of soils contaminated by polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 312:280–297
Rubio-Clemente A, Torres-Palma RA, Peñuela GA (2014) Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: a review. Sci Total Environ 478:201–225
Selvaraj KK, Sundaramoorthy G, Ravichandran PK, Girijan GK, Sampath S, Ramaswamy BR (2015) Phthalate esters in water and sediments of the Kaveri River, India: environmental levels and ecotoxicological evaluations. Environ Geochem Health 37(1):83–96
Sha Y, Xia X, Yang Z, Huang GH (2007) Distribution of PAEs in the middle and lower reaches of the Yellow River, China. Environ Monit Assess 124(1–3):277–287
Souza J, Martínez-Huitle C, Ribeiro da Silva D (2011) Electrochemical treatment for removing petroleum polycyclic aromatic hydrocarbons (PAHs) from synthetic produced water using a DSA-type anode: preliminary. Sustain Environ Res 21:329–335
Umar M, Aziz HA, Yusoff MS (2010) Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Manage 30(11):2113–2121
Wang P, Wang S, Fan C (2008) Atmospheric distribution of particulate-and gas-phase phthalic esters (PAEs) in a Metropolitan City, Nanjing East China. Chemosphere 72(10):1567–1572
Yap CL, Gan S, Ng HK (2011) Fenton based remediation of polycyclic aromatic hydrocarbons-contaminated soils. Chemosphere 83(11):1414–1430
Yaqub A, Isa MH, Kutty SRM, Ajab H (2014) Electrochemical degradation of PAHs in produced water using Ti/Sb2O5-SnO2-IrO2 anode. Electrochemistry 82(11):979–984
Zheng Z, Zhang H, He P-J, Shao L-M, Chen Y, Pang L (2009) Co-removal of phthalic acid esters with dissolved organic matter from landfill leachate by coagulation and flocculation process. Chemosphere 75(2):180–186
Acknowledgement
The authors would like to express their sincere gratitude to Unversiti Teknologi PETRONAS for funding this project under YUTP grant (0153AA-E15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Samareh Mirkia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singa, P.K., Isa, M.H., Lim, J.W. et al. Photo-Fenton process for removal of polycyclic aromatic hydrocarbons from hazardous waste landfill leachate. Int. J. Environ. Sci. Technol. 18, 3515–3526 (2021). https://doi.org/10.1007/s13762-020-03010-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-03010-6