Abstract
The objective of this study was to investigate the performance of employing solar photo-Fenton to treat petroleum wastewater from Sohar oil refinery, evaluate this process by a central composite design with response surface methodology and evaluate the relationship among operating variables such as pH, H2O2 dosage, Fe+2 dosage, and reaction time to identify the optimum operating conditions. Quadratic models proved to be significant with very low probabilities (<0.0001) for the following responses: total organic carbon (TOC) and chemical oxygen demand (COD). The optimum conditions were H2O2 dosage (850 mg L−1), Fe+2 dosage (60 mg L−1), pH (3.68) and reaction time (127 min) in this method. The experimental results of the maximum TOC and COD removal rates corresponded good with the predictions, which were 59.3 and 74.7 %, respectively. This method achieved well degradation efficiencies for TOC and COD and reduced the treatment time comparing with the previous work.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large amount of water was used in the petroleum refinery activity and, consequently, significant volumes of wastewater was generated. Recently, one of the major problems facing industrialized nations was contamination of the environment by hazardous chemicals. A wide range of pollutant compounds were detected in petroleum waste water in Sohar Oil Refinery. So, the elimination of these chemicals from petroleum wastewater was presently one of the most important aspects of pollution control in Oman.
Advanced oxidation processes (AOPs) had capability of rapid degradation of recalcitrant pollutants in the aquatic environment. They had shown high efficiency to remove the organic compounds from effluents even when they are present at low concentrations (Silva et al. 2015; Philippopoulos and Poulopoulos 2003; Masomboon et al. 2010; Paz et al. 2013). Remediation of hazardous substances was attributed to hydroxyl radical, which exhibited reactivity toward organic (Hermosilla et al. 2009).
AOPs might be used in wastewater treatment for reduction of overall organic content (COD), color, odor and specific pollutant or sludge treatment (Tony et al. 2012). There were two Fenton reactions; the standard Fenton reaction between Fe+2 ions and hydrogen peroxide (H2O2) and the Fenton-like reaction between Fe+3 ions and hydrogen peroxide (H2O2) (Yeh et al. 2008). Fenton reaction under light such as sunlight or UV was the so-called photo-Fenton and more hydroxyl radicals (·OH) were generated (Torrades et al. 2004).
The Fenton process has three major steps (Eqs. 1–4) (Silva et al. 2015; Krutzler and Bauer 1999);
-
(a)
Production of hydroxyl radicals (·OH).
$${\text{Fe}}^{ + 2} + {\text{ H}}_{ 2} {\text{O}}_{ 2} \to {\text{ Fe}}^{ + 3} + {}^{ \cdot }{\text{OH}}$$(1) -
(b)
Reproduction of Fe+2 ions by energy.
$$\left[ {{\text{Fe}}\left( {{\text{OH}}} \right)} \right]^{{ + 2}}\, \mathop \to \limits^{{h\nu }}\, {\text{Fe}}^{{ + 2}} + {}^{ \bullet }{\text{OH}}$$(2)$$\left[ {{\text{Fe}}\left( {\text{COOCR}} \right)} \right]^{ + 2} \, \mathop \to \limits^{{h\nu }} \, {\text{Fe}}^{ + 2} + {\text{ CO}}_{ 2} + {\text{ R}}$$(3) -
(c)
Degradation of the organic compounds by hydroxyl radicals (·OH).
$${}^{ \cdot }{\text{OH }} + {\text{ RH }} \to {\text{ Oxidation products }} \to {\text{ CO}}_{ 2} + {\text{ H}}_{ 2} {\text{O}}$$(4)
The reactions with hydroxyl radicals could be triggered by several ways depending on the structure of the organic compounds; electron transfer, abstraction of hydrogen atom, and electrophilic (a molecule with a tendency to react with other molecules containing a donatable pair of electrons) addition to compounds containing unsaturated bonds (Silva et al. 2015; Legrini et al. 1993). The major drawbacks of Fenton process were iron complexation by carboxylic intermediates. But these complexes could be photo-activated by photo-Fenton process and additional hydrogen peroxide (HO·) generation (Amor et al. 2015). Therefore, solar photo-Fenton experiments were applied to treat petroleum wastewater.
Solar photo-Fenton is based on using solar radiation to increase production of hydroxyl radicals (·OH) and photoactive complexes through the Fenton process (Amor et al. 2015; Fernandes et al. 2014; Lucas et al. 2012; Pignatello et al. 2006). Using solar energy in AOPs could reduce processing costs and make it more affordable for commercial use (Amor et al. 2015).
Several previous studies reported the enhanced oxidation of contaminants by the photo-Fenton process, for example, Kositzia et al. (2004) showed the solar photo-Fenton process was more efficient for synthetic municipal wastewater in comparison to the photocatalyst of TiO2 process because H2O2 slowly decomposed into oxygen and water. In addition, the abundance, lack of toxicity and ease of removal from water made Fe2+ the most commonly used transition metal for Fenton process (Badawy and Ali 2006).
Moreover, this process had less amount of harmful by-products in comparison to other AOPs. (Demorais and Zamora 2005). The amounts of reagents depended on the concentrations of degradable materials present in the wastewater which were a fundamental factor. But using an efficient radiation system and a suitable design of the photo-Fenton reactor would improve process performance and reduced reagents consumption (Rossiter et al. 2013). Coelho et al. (2006) reported that the maximum DOC removal attained was 87 % by the photo-Fenton process from petroleum refineries wastewater (sourwater). Previous work by Tony et al. (2012) used the photo-Fenton method for the oil-refinery wastewater achieved approximately 50 % COD removal in the optimum concentrations; pH 3, H2O2 (400 mg L−1), and Fe2+ (40 mg L−1). Rossiter et al. (2013) showed that the 53 % COD removal was achieved by the solar photo-Fenton process from petroleum wastewater.
The main aims for this study are as follows:
-
To investigate the performance of solar photo-Fenton in petroleum wastewater treatment.
-
To evaluate the performance of employing the proposed method by a central composite design (CCD) with response surface methodology (RSM) to degradation of TOC and COD from the petroleum wastewater.
-
To evaluate the statistical relationships among operating variables (such as pH, H2O2 dosage, Fe+2 dosage, and reaction time) and the responses, which COD and TOC removal efficiencies were selected as the responses for optimization.
-
To determine the optimum operational conditions of the proposed method.
-
To compare the proposed method with the previous works.
Materials and methods
Wastewater characteristic
The physicochemical characteristics of the petroleum wastewater from Sohar oil refinery (SOR) were summarized in Table 1. Samples of the petroleum waste water were collected on different days. Samples were transferred to the laboratory and stored under refrigeration (4 °C) until use. Samples were characterized before the experiments to obtain their chemical and physical properties. The petroleum wastewater characterization was determined by the quantification of pH, total organic compounds (TOC) and chemical oxygen demand (COD) according to the standard methods for the examination of waste water methodology.
Materials
Samples of the petroleum wastewater were collected from Sohar oil refinery, Oman. Hydrogen peroxide (H2O2) [35 % (v/v)] and Iron sulfate hydrate (FeO12S3) (which was used as source of Fe+2 in the photo-Fenton treatment) are supplied by EMPROVE. Exp. They were used for the solar photo-Fenton process to degradation of TOC and COD. Sulfuric acid (95–97 %) and sodium hydroxide (50 %) were used to adjust the pH to the desired values.
Analytical procedure
A Shimadzu TOC analyzer (LCSH/CSN) was used to measure the TOC for each sample. Chemical oxygen demand (COD) was measured by COD photometer (manufactured by CHEMetrics). COD was estimated before and after treatment. Before each analysis, samples were filtered by filter papers (0.22 um Millipore Durapore membrane, 40 Ashless, Diameter 150 mm). Solar ultraviolet radiation (UV) was measured by a global UV radiometer (KIPP & ZONEN).
Experimental procedure
A sketch of the solar photo-Fenton processes was shown in Fig. 1. It consisted of a glass recirculation tank (5 L), which was subjected to stirring to maintain a well-mixed solution during the experiments, connected to the tubular solar reactor [four tubes (50 cm length × 2 cm inner diameter × 0.1 cm thickness]. The tubular photo reactor operated at a UV-index from 8 to 11 according to exposure category was very high as shown in Fig. 2.
The UV-Index is calculated as follow:
Take the output from the UV-E radiometer according to ISO 17166:1999/CIE S007/E-1998. Transform the output voltage to W/m2 with the instruments sensitivity. Equation (5) allows calculating the amount of UV intensity received on any surface in the same position with regard to the sun by UV-Index (UVI):
where, UVI is the UV-Index, R is the reading (R) in UV radiometer by (W/m2) unit.
The solution was circulated through the reactor at a flow rate of about 1.5 L/min by means of a peristaltic pump. The Fenton regents were added in a glass recirculation tank during this process.
The pH for petroleum wastewater samples was used between 2 and 6. Several sets of experiments were carried out according to a central composite design (CCD) with response surface methodology (RSM) to determine the COD and TOC removal efficiency under the optimum operational conditions.
At the end of each run, in order to avoid iron precipitation in the reactor, the samples were withdrawn and then kept them at rest for 30 min. By increasing the pH of sample to (8), the major iron ion was precipitated because the removal efficiency of organics by Fenton stopped (Salem et al. 2013).
Tekin et al. (2006) showed that the treatment efficiency of Fenton ceased when the pH was equal to or greater than 7 because the generation of hydroxyl radicals was reduced due to lack of presence of free iron ions. The excess of hydrogen peroxide (H2O2) was removed by adding MnO2 and the suspension was stirred until the formation O2 was finished (no bubbles on surface) (Herrmann and Verlag 1996). The presence of H2O2 normally provokes accurate COD values (Amor et al. 2015).
Results and discussion
Experimental design and the analysis of variance (ANOVA)
Central composite design and response surface methodology were employed in the statistical design of the experiments, data analysis, explaining the optimal conditions of the independent variables and assessment of the relationships among four significant independent variables, which were pH, H2O2 dosage, Fe+2 dosage, and reaction time as shown in Table 2.
The adequacy of the proposed model for degradation of COD and TOC of the petroleum wastewaters by Fenton’s reagent was evaluated at optimum conditions. Each independent variable was varied over three levels according to face centered CCD as −1, 0, and +1, respectively at the determined ranges based on a set of preliminary experiments. The total number of experiments conducted for the four factors according to Eq. (6)
where, k was the number of factors.
The design consisted of 2k factorial points augmented by 2k axial points and six replications for a center point. In this work, the total number of experiments conducted for the four factors were 30 with 16 factorial points, eight axial points and six replications to assess the pure error and got a good estimate. The COD and TOC removal were the dependent variables (responses) during this process. Performance was evaluated by analyzing the COD and TOC removal efficiencies as shown in Table 3.
The behavior of the system was explained through an empirical second-order polynomial model, as shown in Eq. (7): (Montgomery 2008)
where, Y was the response, X i and X j were the variables, β was the regression coefficient, k was the number of factors studied and optimize in the experiment, e i was the random error.
The analysis of variance (ANOVA) was used for graphical analysis of data to obtain the interaction between the process variables and the responses. The quality of the fit polynomial model was expressed by coefficient of determination (R 2). Model terms were evaluated by the P value (probability) with 95 % confidence level.
The analysis of variance (ANOVA) for TOC and COD removal were represented in Table 4. All the response surface quadratic models for parameters in this table were significant at the 5 % confidence level since the P values were less than 0.05. The correlation coefficients (R 2) for the TOC and COD removal rates were 0.983 and 0.986, respectively, which were greater than 0.80, the cut-off for a model with good fit. A high coefficient (R 2) value ensured a satisfactory adjustment of the quadratic model to the experimental data and illustrated good agreement between the calculated and observed results and showed that a desirable and reasonable agreement with the adjusted R 2 (Salem et al. 2013).
If the model terms had the P value (probability) more than 0.05, they were considered limited influence. So, they had to be excluded from the study to improve the models. The model of TOC and COD removal were considered significant using the F test at 5 % significant level (P < 0.05). The “adequate precision” (AP) ratio values higher than four were desirable and confirm that the predicted models could be used to navigate the space defined by the central composite design (CCD) (Noordin et al. 2004). The “adequate precision” (AP) ratio of the models in this study was adequate, which varied between 30.74 and 35.28.
Normal probability
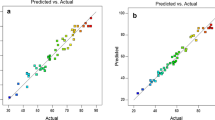
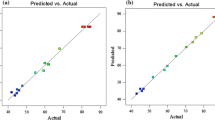
Design Expert 6.0.7 software program (a statistical software package from Stat-Ease Inc) provided plots of normal probability of the studentized residuals and diagnostics to evaluate the models and confirmed that they provided an adequate approximation of the real system. As shown in Fig. 3, the normal probability plots for the studentized residuals for TOC and COD removal efficiency predicted that if the residuals followed a normal distribution, the points fell along a straight line for each case.
However, some scattering were expected even with normal data; thus, the data could be considered to be normally distributed in the responses of certain models. Empirical relationships among the variables and COD and TOC removal efficiencies after excluding insignificant coefficients were presented by the following polynomial Eqs. (8, 9):
where, A, B, C and D were model terms that represent coded factors of pH, H2O2 dosage, Fe+2 dosage, and reaction time, respectively. Final equations in terms of actual factors were presented by the following Eqs. (10, 11):
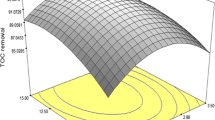
Three-dimensional plots of the regression and optimization process
As shown in Figs. 4 and 5, the response surfaces plots were created by Design Expert 6.0.7 provided a three-dimensional view of the TOC and COD removals surface over the independent variables. The main factors of this process that improved the TOC and COD removal were H2O2 dosages, Fe+2 dosages. Using the high dosages of Fenton reagents to degradation of organic matter was attributed to the nature of petroleum wastewater which had the recalcitrant pollutants and then decreased the process efficiency due to chloride ions, which were able to react with hydroxyl radicals, producing less reactive radicals (Maciel et al. 2004). So the concentration of Fenton’s reagent employed must be enough to overcome the restrictions.
Design Expert 6.0.7 was used to determine optimization of removal efficiency for TOC and COD. The pH, H2O2 dosage, Fe+2 dosages, and reaction time were chosen as “within” the range in the software optimization step. The responses (TOC and COD removals) were defined as “maximum” to achieve the highest performance. The program combined individual desirability into a single number and then search to optimize this function based on the response goal.
Figure 4d showed the COD removal by Fenton oxidation and the corresponding pH variation over time under the optimal conditions a rapid degradation up to 90 min followed a slow degradation. The rapid degradation was attributed to high hydroxyl radical (·OH) concentrations which it exhibited reactivity toward organic compounds as a result of amounts of Fe2+ catalyst in solution that reacts with H2O2. At the second stage, after 90 min, Fe2+ ions were decreased and the excess of H2O2 reacted with hydroxyl radical (·OH) in result to slower rate of production hydroxyl radical (·OH) (Luna et al. 2013).
As shown in Table 5, the model under optimized operational conditions, which were H2O2 dosage (850 mg L−1), Fe+2 dosage (60 mg L−1), pH (3.68) and reaction time (127 min), predicted TOC and COD removal rates approximately 59.3 and 74.7 %, respectively and the desirability function was 0.988.
To confirm these results, an additional experiment under optimized operational conditions was carried out which was revealing agreement with the predicted responses. The near connection between the experimental and the predicted results supported the reliability of the model.
The results of this research were compared with those of other works that treated the wastewater by using the photo-Fenton method, for example, Rossiter et al. (2013) showed that polycyclic aromatic hydrocarbons and aromaticity removal of approximately 92.7 and 96.2 %, respectively, from petroleum-extraction wastewater were obtained by a solar photo-Fenton like process after 420 min and solar photo-Fenton process achieved 53 % COD removal. Tony et al. (2012) obtained 50 % COD removal after using (H2O2/Fe+2/UV) method under optimal conditions; pH 3, H2O2/Fe+2 ratio 10 while the current study revealed that the solar photo-Fenton process in an AOP was more efficient in the petroleum wastewater treatment, achieving a 74.7 % COD removal at conditions; pH 3.68, H2O2/Fe+2 ratio 14.
The maximum of COD removal at pH 3.68 agreed with the results found in the literature for other wastewaters treated by Fenton process, for example, Mohajeri et al. (2010) reported that at pH 3 the COD and color removals were 58 and 78 %, respectively within 120 min of reaction time. Results of Ertugay and Acar (2013) indicated that the highest COD removal was determined 50.7 % at pH 3.
The complexity of intermediate compounds during the reaction and the high initial reaction rate made the study of Fenton process very difficult in industrial wastewater (Lucas and Peres 2009). Comparing this work with the previous works was summarized in the Table 6.
Evaluation of the photolytic process
To evaluate the photolytic effect on the COD reduction, the photocatalytic experiments were carried out under sunlight; the tubular photo reactor operated at a UV-index of 9 (according to Exposure category was very high) as shown in Fig. 6. The results revealed that the photolytic process was less efficient in the petroleum wastewater treatment, achieving a 11.5 % COD removal at pH 3.68 after 180 min of solar irradiation.
The combined action of solar radiation and H2O2
After the evaluation of the photolytic process, some experiments were carried out by adding 0.85 g L−1 Hydrogen peroxide (H2O2) [35 % (v/v)], which was the optimum H2O2 concentration as determined previously, to evaluate the effect of the Hydrogen peroxide (H2O2) with solar radiation. The results revealed that 23 % of COD was removed within 180 min at pH 3.68. The degradation was attributed to the photochemical cleavage of H2O2 by solar light absorption resulting to increase rate of production hydroxyl radical (·OH) (Oliveira et al. 2012; Parson 2004).
Evaluation of the Fenton process
To evaluate the performance of employing the Fenton process in the absence of solar irradiation to treat petroleum wastewater, The dark Fenton experiments were carried out with adding 0.85 g L−1 Hydrogen peroxide (H2O2) [35 % (v/v)] and 0.06 g L−1 Iron sulfate hydrate (FeO12S3) at pH 3.68.
Results of this process indicated that the COD removal was decreased to 61 % at pH 3.68 after 180 min, due to the lower generation of hydroxyl radical (·OH).
Conclusion
The general results of this work indicated that the solar photo-Fenton was a practical method to treat petroleum wastewaters, allowing achieved well removal of TOC and COD. Quadratic models for the following two responses proved to be significant with very low probabilities (<0.0001): Chemical oxygen demand (COD), total organic carbon (TOC). The obtained optimum conditions included H2O2 dosage (0.85 g L−1), Fe+2 dosage (0.06 g L−1), pH (3.68) and reaction time (127 min). TOC and COD removal rates were 59.3 and 74.7 %, respectively. The predictions correspond well with experimental results. The current study revealed that the solar photo-Fenton process in an AOP was well efficient in the petroleum wastewater treatment, achieving a 74.7 % COD removal at conditions; pH = 3.68, H2O2/Fe+2 ratio = 14.
References
Amor C, Torres-Socias ED, Peres J A, Maldonado M I, Oller I, Malato S, Lucas M S (2015) Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J Hazard Mater 286:261–268. http://www.sciencedirect.com/science/article/pii/S0304389414010206
Badawy MI, Ali MEM (2006) Fenton’s per-oxidation and coagulation processes for the treatment of combined industrial and domestic wastewater. J Hazard Mater 136:961–966. http://www.sciencedirect.com/science/article/pii/S0304389406000641
Chu L, Wanga J, Dong J, Liu H, Sun X (2012) Treatment of coking wastewater by an advanced Fenton oxidation process using iron powder and hydrogen peroxide. Int Chemosphere J 86:409–414. http://www.sciencedirect.com/science/article/pii/S0045653511010393
Coelho A, Antonio V, Castro Dezotti M, Sant GL, Anna J (2006) treatment of petroleum refinery sour water by advanced oxidation processes. J Hazard Mater 137:178–184. http://www.sciencedirect.com/science/article/pii/S0304389406000781
Cristovao R O, Goncalves C, Botelho CM, Martins RJE, Boaventur RAR (2014) Chemical oxidation of fish canning wastewater by Fenton’s reagent. J Environ Chem Eng 2(4):2372–2376. http://www.sciencedirect.com/science/article/pii/S2213343713002704
Demorais JL, Zamora PP (2005) Use of advanced oxidation processes to improve the biodegradability of mature landfill leachates. J Hazard Mater 123:181–186. http://www.sciencedirect.com/science/article/pii/S0304389405001603
Dincer AR, Karakaya N, Gunes E, Gunes Y (2008) Removal of COD from oil recovery industry wastewater by the advanced oxidation processes (AOP) based on H2O2. Glob N J 10:31–38. http://journal.gnest.org/sites/default/files/Journal%20Papers/31-38_479_DINCER_10-1.pdf
Ertugay N, Acar FN (2013) Removal of COD and color from Direct Blue 71 azo dye wastewater by Fenton’s oxidation: kinetic study. Arab J Chem (in press). http://www.sciencedirect.com/science/article/pii/S1878535213000415
Fernandes L, Lucas MS, Maldonado MI, Oller I, Sampaio A (2014) Treatment of pulp mill wastewater by Cryptococcus podzolicus and solar photo-Fenton. Chem Eng J 245:158–165. http://www.sciencedirect.com/science/article/pii/S1385894714001910
Hermosilla D, Cortijo M, Huang CP (2009) Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes. Sci Total Environ 407:3473–3481. http://www.sciencedirect.com/science/article/pii/S0048969709001569
Herrmann WA, Verlag GT (1996) Synthetic methods of organometallic and inorganic chemistry. Catalysis 10:140–141. https://books.google.com.my/books?id=hgUqZkG23PAC&pg=PR6&lpg=PR6&dq=Synthetic+Methods+of+Organometallic+and+Inorganic+Chemistry:+Catalysis,&source=bl&ots=4xEQGIJnTp&sig=WQvet-5PXeNtYqlJ2ZclOwNGwbo&hl=en&sa=X&redir_esc=y#v=onepage&q=Synthetic%20Methods%20of%20Organometallic%20and%20Inorganic%20Chemistry%3A%20Catalysis%2C&f=false
Hussain S, Shaikh S, Farooqui M (2013) COD reduction of waste water streams of active pharmaceutical ingredient Atenolol manufacturing unit by advanced oxidation-Fenton process. J Saudi Chem Soc 17(2) 199–202. http://www.sciencedirect.com/science/article/pii/S1319610311000597
Kang YW, Hwang KY (2000) Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res 34:2786–2790. http://www.sciencedirect.com/science/article/pii/S0043135499003887
Karthikeyan S, Titus A, Gnanamani A, Mandal AB, Sekaran G (2011) Treatment of textile wastewater by homogeneous and heterogeneous Fenton oxidation processes. Des J 2:438–445. http://www.sciencedirect.com/science/article/pii/S001191641100720X
Kositzia M, Poulios I, Malato S, Caceres J, Campos A (2004) Solar photocatalytic treatment of synthetic municipal wastewater. J Water Res 38:1147–1154. http://www.sciencedirect.com/science/article/pii/S004313540300664X
Krutzler T, Bauer R (1999) Optimization of a photo-Fenton prototype reactor. Chemosphere 38:2517–2532. http://www.sciencedirect.com/science/article/pii/S0045653598004603
Legrini O, Oliveros E, Braun AM (1993) Photochemical processes for water treatment. Chem Rev 93:671–698. doi:10.1021/cr00018a003
Lucas MS, Peres JA (2009) Removal of COD from olive mill wastewater by Fenton’s reagent: kinetic study. J Hazard Mater 168:1253–1259. http://www.sciencedirect.com/science/article/pii/S0304389409003847
Lucas MS, Peres JA, Amor C, Prieto-Rodriguez L, Maldonado MI, Malato S (2012) Tertiary treatment of pulp mill wastewater by solar photo-Fenton. J Hazard Mater 225:173–181. http://www.sciencedirect.com/science/article/pii/S0304389412004955
Luna MDG, Luna BRM, Chia-Chi S, Ming-Chun L (2013) Kinetics of acetaminophen degradation by Fenton oxidation in a fluidized-bed reactor. Chemosphere 90:1444–1448. http://www.sciencedirect.com/science/article/pii/S0045653512011265
Maciel R, Sant’Anna JGL, Dezotti M (2004) Phenol removal from high salinity effluents using Fenton’s reagent and photo-Fenton reactions. Chemosphere 57:711–719. http://www.sciencedirect.com/science/article/pii/S0045653504006198
Martinez NSS, Fernandez JF, Segura XF, Escola ASF (2003) Pre-oxidation of an extremely polluted industrial wastewater by the Fenton’s reagent. J Hazard Mater B 101:315–322. http://www.sciencedirect.com/science/article/pii/S0304389403002073
Martins RC, Rossi AF, Rosa M (2010) Fenton’s oxidation process for phenolic wastewater remediation and biodegradability enhancement. J Hazard Mater 180:716–721. http://www.sciencedirect.com/science/article/pii/S0304389410005443
Masomboon N, Chen CW, Anotai J, Lu MC (2010) A statistical experimental design to determine o-toluidine degradation by the photo-Fenton process. Chem Eng J 159,:116-122. http://www.sciencedirect.com/science/article/pii/S138589471000207X
Mohajeri S, Hamidi AA, Isa MH, Bashir MJK, Mohajeri L (2010) Influence of Fenton reagent oxidation on mineralization and decolorization of municipal landfill leachate. J Environ Sci Health A 45:692–698. doi:10.1080/10934521003648883
Montgomery DC (2008) Design and analysis of experiments, 7th ed. https://books.google.com.my/books?hl=en&lr=&id=kMMJAm5bD34C&oi=fnd&pg=PA1&dq=Design+and+Analysis+of+Experiments,+7th+edition&ots=Kmm7Nw5Nvw&sig=bKSSgVSKxanwbuM8JHjiRJx4Vv0&redir_esc=y#v=onepage&q&f=false
Noordin VC, Venkatesh S, Sharif S, Elting AA (2004) Application of response surface methodology in describing the performance of coated carbide tools when turning AISI 1045 steel. J Mater Pro Tech 145:46–58. http://www.sciencedirect.com/science/article/pii/S0924013603008616
Oliveira A, Saggioro EM, Moreira JC, Ferreira LFV, Pavesi T (2012) Solar photochemistry for environmental remediation—advanced oxidation processes for industrial wastewater treatment. INTECH Open Access Publisher. http://cdn.intechopen.com/pdfs-wm/34626.pdf
Oliveira C, Alves A, Madeira LM (2014) Treatment of water networks (waters and deposits) contaminated with chlorfenvinphos by oxidation with Fenton’s reagent. Chem Eng J 241:190–199. http://www.sciencedirect.com/science/article/pii/S1385894713016070
Parson S (2004) Advanced oxidation processes for water and wastewater treatment. IWA, 86–109. http://www.cabdirect.org/abstracts/20043078106.html
Paz DS, Foletto EL, Bertuol DA, Jahn SL, Collazzo GC, Silva SS, Chiavone-Filho O, Nascimento CAO (2013) CuO/ZnO coupled oxide films obtained by the electrodeposition technique and its photocalytic activity in phenol degradation under solar irradiation. Water Sci Tech 68:1031–1036. http://www.iwaponline.com/wst/06805/wst068051031.htm
Philippopoulos CJ, Poulopoulos SG (2003) Photo-assisted oxidation of an oily wastewater using hydrogen peroxide. J Hazard Mater 98:201–210. http://www.sciencedirect.com/science/article/pii/S0304389402003576
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–84. doi:10.1080/10643380500326564
Rossiter O, Rochaa S, Renato Dantas F, Marta MM, Duarte B (2013) Solar photo-Fenton treatment of petroleum extraction wastewater. J Desalin Water Treat 51:5785–5791. doi:10.1080/19443994.2013.792136
Salem SA, Hamidi AA, Mohd NA, Bashir MJK (2013) Optimization of semi-aerobic stabilized leachate treatment using ozone/Fenton’s reagent in the advanced oxidation process. J Environ Sci Health Part A 48:720–729. doi:10.1080/10934529.2013.744611
Silva SS, Chiavone-Filho O, Neto ELB, Foletto EL (2015) Oil removal from produced water by conjugation of flotation and photo-Fenton processes. J Environ Manag 147:257–263. http://www.sciencedirect.com/science/article/pii/S0301479714004356
Tekin H, Bilkay O, Ataberk SS, Balta TH, Ceribasi IH (2006) Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J Hazard Mater 136:258–265. doi:10.1080/10934529.2013.744611
Tony MA, Patrick J, Purcell Yaqian Z (2012) Oil refinery wastewater treatment using physicochemical, Fenton and photo-Fenton oxidation processes. J Environ Sci Health A 47:435–440. doi:10.1080/10934529.2012.646136
Torrades J, Garcia M, Garcia HJA, Domenech X, Peral J (2004) Decolorization and mineralization of commercial reactive dyes under solar light assisted photo-Fenton conditions. Sol Energy 77:573–581. http://www.sciencedirect.com/science/article/pii/S0038092X04001094
Yeh CKJ, Hsu CY, Chiu CH, Huang KL (2008) Reaction efficiencies and rate constants for the goethite-catalyzed Fenton-like reaction of NAPL-form aromatic hydrocarbons and chloroethylenes. J Hazard Mater 151:562–569. http://www.sciencedirect.com/science/article/pii/S0304389407008618
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aljuboury, D.A., Palaniandy, P., Aziz, H.B.A. et al. Evaluation of the solar photo-Fenton process to treat the petroleum wastewater by response surface methodology (RSM). Environ Earth Sci 75, 333 (2016). https://doi.org/10.1007/s12665-015-5192-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-5192-y