Abstract
Clayey soils rich in smectite are commonly utilized for the construction of liner materials in engineered landfills owing to their low hydraulic conductivity. However, due to the high swelling potential of such clayey soils, their hydraulic conductivities are adversely influenced by desiccation cracking. In addition to cracks, the hydraulic conductivity of the clayey soils might also be affected by the complex composition of landfill leachate. The current study aims, therefore, to assess the effects of desiccation-induced cracks and leachate infiltration on the hydraulic conductivity of natural and 30% olivine-treated marine clay specimens. Clay specimens were prepared using three compactive energies and subjected to cycles of drying–wetting and hydraulic conductivity testing using tap water and field leachate as the permeants. Test results demonstrate significant improvements in the hydraulic conductivity of the olivine-treated clay samples after cycles of drying–wetting upon permeation with water and leachate. These improvements are attributed to the formation of magnesium–aluminate–hydrate and magnesium–silicate–hydrate gels as confirmed using microstructural analyses. The gels are responsible for minimizing the adverse impact of cracks on the hydraulic conductivity of the treated clay specimens. The findings indicate that olivine can be employed as a suitable additive in decreasing the hydraulic conductivity of soils prone to cracking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Compacted clay liners (CCLs) are integral parts of engineered landfills aimed at preventing/minimizing leachate from leaking out of the landfill into the surrounding environment. They are widely applied as liner materials owing to their low hydraulic conductivity, adequate strength, and good compatibility with the percolating leachate (Li et al. 2013; He et al. 2015, 2017; Ojuri and Oluwatuyi 2018; Yong et al. 2019). Their designs are generally based on the assertion that minimal seepage will occur if the compacted clay has a laboratory-determined hydraulic conductivity of ≤ 1 × 10−7 cm/s (Touze-Foltz et al. 2008; Declan and Paul 2009; Favaretti and Cossu 2018) or ≤ 1 × 10−6 cm/s (MHLGM 2004). However, various failure mechanisms and environmental conditions such as freeze/thaw and dry/wet cyclings, temperature gradients, differential subsidence, intrusion of biota, piping, and desiccation may result in cracks in the CCL (Omidi et al. 1996a, 1996b; Albrecht and Benson 2001; Kalkan 2009; Li et al. 2016). Consequently, the same material in the field may have hydraulic conductivity values of 10 to 10,000 times (1 to 4 order of magnitude) higher than that determined in the laboratory (Daniel 1984), hence increasing the leachate migration rate into the surrounding environment and groundwater. Among the various types of cracks experienced by clay liners, desiccation cracking is a typical crack type influencing liner hydraulic conductivity considerably (Rayhani et al. 2007). Hence, it is a major concern in the design and construction of clay liners and covers, specifically in arid and tropical areas (Rayhani et al. 2008).
In the recent past, various scholars have examined the impact of desiccation cracks on the hydraulic conductivity of CCLs (Omidi et al. 1996a, b; Albrecht and Benson 2001; Rayhani et al. 2007, 2008; He et al. 2015; Li et al. 2016). Omidi et al. (1996a, 1996b) noted a large increase in the hydraulic conductivity of a soil after exposure to two cycles of desiccation. Albrecht and Benson (2001) reported an increase in the hydraulic conductivity as large as three orders of magnitude due to cracking of the specimens. Rayhani et al. (2007) noted an increase in hydraulic conductivity of clayey soils as large as 12–34 times, due to cracking. Rayhani et al. (2008) reported an increase in hydraulic conductivity as large as 5–10 orders of magnitude owing to cracking of specimens. He et al. (2015) found that the mean hydraulic conductivity of a natural clay increased from the order of 10−6–10−4 cm/s due to cracking. Li et al. (2016) reported an increase in the hydraulic conductivity of a clay liner as large as three orders of magnitude due to crack development.
In addition to desiccation cracking, CCLs may also suffer damage from the percolating leachate, which contains high concentration of complex contaminants. When the leachate percolates through the underlying CCL, chemical reactions (e.g. dissolution, ion exchange, precipitation, and biochemical processes) might alter the hydraulic conductivity of the liner material (Yilmaz et al. 2008; Osinubi et al. 2017). Therefore, an enhanced understanding of the influence of leachate on the hydraulic conductivity of CCLs is of utmost importance. Nayak et al. (2007) reported a 50% increase in the hydraulic conductivity of samples mixed with 20% leachate. Aldaeef and Rayhani (2014) recorded a threefold increase in hydraulic conductivity after permeation with synthetic leachate. Xue and Zhang (2014) found that the hydraulic conductivity of a solidified clay increased by one order of magnitude due to the degradation effect of leachate. Razakamanantsoa and Djeran-Maigre (2016) recorded an increase in hydraulic conductivity (more than ten times) for a soil–bentonite mixture after permeation with leachate. Osinubi et al. (2017) noted an increase in hydraulic conductivity by a factor of 1.05 after a lateritic soil was permeated with leachate.
In the recent past, various researchers have made efforts to address the issues of desiccation cracking in liners and covers. Some utilized surface moisture barriers above the soil layer (Albright et al. 2004). Others have considered soil additives to reduce the crack potential and hence decrease the liner hydraulic conductivity. Miller and Rifai (2004) investigated the impact of fibre reinforcement (0.2–2% fibre contents by dry soil weight) on desiccation crack development in compacted clay specimens. Their findings indicate about 50% crack reduction upon the addition of 0.4% and 0.5% fibre contents to the samples. Harianto et al. (2008) examined the influence of fibre additives (0.2–1.2% fibre contents by dry soil weight) on the desiccation crack behaviour of a local soil as a cover material in landfill. Their findings indicate that the volumetric shrinkage strain (VSS) of the compacted soil decreased with increasing fibre content. Likewise, a 78% decrease in the crack intensity factor (CIF) was recorded upon the addition of 0.2% fibre content. Kalkan (2009) investigated the effect of silica fume (5–50% silica fume content by dry soil weight) on desiccation cracks of compacted clayey soils. The results show that silica fume prevented desiccation cracks development on the surface of the compacted specimens. He et al. (2015) investigated the effect of desiccation cracks on the permeability of bentonite-modified clay. Their results indicate that the self-healing ability of bentonite helped in minimizing the adverse effect of cracks on the permeability of the clay. With the dual advantage of additives in minimizing cracks, hence reducing the permeability and simultaneously improving the strength of the soil, there is an increasing interest in the utilization of additives to better improve soil performance as hydraulic barriers.
The literature indicates that clayey soils rich in smectite such as marine clay are commonly employed as containment barriers in landfills and other environmental-related applications due to their high attenuation capacity, low hydraulic conductivity as well as their cost-effective appeal as natural materials (Chalermyanont et al. 2009; Kalkan 2009; Rahman et al. 2013; Emmanuel et al. 2019b, d). However, such soils are extremely plastic and commonly contain clay minerals such as montmorillonite that will tend to expand as they absorb water and shrink if the water is removed (Kalkan 2009, 2011). Hence, when such soils are utilized for compacted liner applications, they may be liable to cracking and deformation. Consequently, these downsides may render them unfit to be employed as liner materials in engineered landfills. Hence, to attain the required hydraulic conductivity values specified by various international organizations and researchers, treatment of clayey soil with various additives is a commonly adopted technique (He et al. 2015).
According to ASTM D5370, olivine is a sustainable, economical, and widely available pozzolanic material with the ability to improve soil hydro-mechanical properties due to its affinity for the adsorption of CO2 leading to the formation of stable carbonates with hydro-mechanical improvement properties (Emmanuel et al. 2019c, d). Moreover, during the hydration reaction, \({\text{MgO}}\) present in the olivine reacts with soluble \({\text{Al}}_{2} {\text{O}}_{3}\) and \({\text{SiO}}_{2}\) present in the soil to form stable magnesium–silicate–hydrate (M–S–H) and magnesium–aluminate–hydrate (M–A–H) gels, which contributes to the hydro-mechanical properties improvement, durability, and porosity reduction in the stabilized soil. Accordingly, it is well hypothesized that if olivine is used to stabilize the soft marine clay, it may then optimize the hydration reaction leading to hydro-mechanical properties improvement, porosity reduction, as well as less susceptibility to desiccation-induced cracking.
However, using olivine as an additive to suppress desiccation cracking typically encountered in CCLs has not received adequate attention, according to the authors’ best knowledge. Besides, there is a knowledge gap in quantifying the influence of olivine on the hydraulic conductivity of soils. Likewise, the effect of drying–wetting cycles and leachate infiltration on the hydraulic properties of marine clay is not fully understood. Consistent with the above view, the current work aims to assess the effect of desiccation-induced cracks and landfill leachate on the hydraulic conductivity of natural and 30% olivine-treated marine clay.
This research work was carried out at Monash University, Malaysia, from December 2017 to March 2019.
Materials and methods
Materials
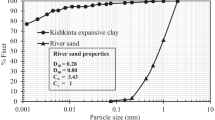
A Malaysian marine clay classified as clay of high plasticity (CH) in accordance with the unified soil classification system (USCS) was used in the current study. The clay specimens were procured approximately 3 m below the ground surface from Jeram situated in the state of Selangor, Malaysia. The basic engineering properties of the clay, including grading, plasticity characteristics, and particle density, determined according to standard procedures stipulated in BS 1377 (1990), are presented in Table 1. The grading curve and X-ray diffraction (XRD) pattern of the clay are depicted in Figs. 1 and 2, respectively. Sample preparation and testing for the XRD analysis followed the procedures reported in Emmanuel et al. (2019d). Emmanuel et al. (2019a) showed that the clay contains 75.84%, 12.09%, and 3.47% of \({\text{SiO}}_{2}\), \({\text{Al}}_{2} {\text{O}}_{3}\), and \({\text{Fe}}_{2} {\text{O}}_{3}\), respectively, as the significant oxides.
The magnesium-rich olivine utilized in the study was procured from Maha Chemicals, Malaysia. Its physico-chemical properties are presented in Table 2, and its mineralogical compositions are depicted in Fig. 2. Upon procuring the olivine, it had a large grain size, thus requiring grinding to reduce the particle size prior to adding to the clay to increase the specific surface area and, consequently, the olivine reactivity. Figure 1 shows the grading curves of the olivine prior to and after grinding.
The leachate used in the study was obtained from the Taman Beringin solid waste transfer station situated in Jinjang Utara, Kuala Lumpur, Malaysia. Taman Beringin station receives municipal solid waste (MSW) from the city of Kuala Lumpur, which is a densely populated city. It is assumed that the composition of the waste is very diverse owing to its inhabitants, and hence, the generated leachate will be representative of the main constituents of a Malaysian MSW, hence the choice of using the leachate. Immediately after collection, the leachate was sieved to remove suspended particles, algae, and any other macroscopic organisms that could contribute to the clogging of the pore spaces during the hydraulic conductivity testing. Figure 3a, b depicts the images of the leachate samples before and after sieving, respectively, observed under the light microscope. After sieving, the leachate was transported to the laboratory for characterization. The characterization was expressed in terms of metals, major cations, anions, environmental qualities, and physical parameters. In all cases, standard laboratory techniques in accordance with the American Public Health Association et al. (1985) were employed. Table 3 lists the physico-chemical compositions of the leachate. Before testing, the leachates were stored in opaque plastic bottles and kept in a refrigerator at an operating temperature range of 7–10 °C. This approach was used to prevent or minimize any biochemical reactions that could lead to bacteria growth and calcite floc formation in the leachate (Frempong and Yanful 2008). Tap water used in the study was the natural drinking water with a pH and electrical conductivity of 7.3 and 94.0 µS/cm, respectively.
Sample preparation
The clay specimens brought from the field were oven-dried for 3 days at 105 °C until constant mass. The dried specimens were then crushed manually into smaller fractions passing through a 2-mm sieve opening. The sieve specimens were then mixed with 30% olivine content by dry clay mass. The natural and treated clay specimens were then subjected to further hydro-mechanical and microstructural investigations. Totally, 30% olivine content was chosen in this study since it was observed as the optimum in improving the hydro-mechanical properties of the clay from previous investigation (Emmanuel et al. 2019c). This improvement was attributed to the excessive formation of M–A–H and M–S–H phases, which bind the soil particles together and harden the soil. The plasticity characteristics of the natural and treated clay samples when mixed with water and leachate are presented in Table 4.
Test method
Compaction test
The moisture–density relationship of the natural and treated clay specimens was determined by employing three compactive energies: reduced, standard, and modified Proctor. Both standard and modified Proctor compaction tests were conducted using the British Standard light and heavy (BS 1377, 1990) specifications, respectively, whereas the reduced Proctor followed the procedure proposed by Daniel and Benson (1990). Modified Proctor represents a reasonable upper limit of compactive effort, standard Proctor represents a medium compactive effort, while the reduced Proctor corresponds to a reasonable minimum level of compaction energy for most earthwork projects. This range of energies was selected to span the range of reasonable compactive efforts anticipated in the field (Daniel and Wu 1993).
Hydraulic conductivity test
To assess the influence of leachate on the hydraulic conductivity of the natural and treated clay samples, two scenarios were considered: samples were saturated and permeated with tap water (TW) [i.e. TW + TW], and samples were saturated with TW and permeated with landfill leachate (LL) [i.e. TW + LL]. Test specimens were prepared by remoulding the clay into permeameter moulds employing the reduced, standard, and modified Proctor efforts and allowed for 7 days of curing in a ~ 20 ± 1 °C temperature-controlled and constant humidity room. After curing, the specimens were subjected to initial hydraulic conductivity testing and series of dry–wet cycles and hydraulic conductivity testing to evaluate the influence of cracks and leachate on the clay hydraulic conductivity. Hydraulic conductivity tests were conducted on the samples using the falling head technique as recommended by Head and Epps (1980). Detailed discussion of the saturation procedure, hydraulic conductivity testing procedures, and termination criteria is reported in our previous studies, Emmanuel et al. (2019c, 2019d).
Drying–wetting procedure for hydraulic conductivity test
The drying–wetting approach by Rayhani et al. (2007, 2008) was adopted in the current study. After determining the initial hydraulic conductivity (Ko), the specimens were extruded from the permeameter and then placed in a tray and kept on a laboratory shelf at room temperature (~ 20 ± 1 °C) for 24 h and allowed to dry slowly. The aim was to mimic the slow drying rate that occurs in the field. After the 24 h of air drying, the specimens were transferred to an oven and dried at 40 °C for another 24 h. The objective was to simulate the gradual increase in temperature that occurs during field conditions. Likewise, the gradual increase in temperature was to minimize the sudden shrinkage/cracking or thermal deformations of the specimens at a later higher temperature. After the 24 h of oven drying at 40 °C, the temperature of the oven was increased to 70 °C, and the samples were dried for another 24 h. The 70 °C drying temperature was used to represent extreme temperatures encountered at the base of landfills (Rowe 1998). After the 24 h, the specimens were removed from the oven, their crack features were measured, and the specimens were submerged in water for saturation. Their hydraulic conductivities were determined depending on the scenario, as previously mentioned. The average of the three hydraulic conductivity test results was reported as cycle 1 (K1). This concluded the first cycle of testing and measurement. The samples were removed from the permeameter and kept in a tray for air drying at a controlled ambient condition (~ 20 ± 1 °C) for 24 h. Later, the specimens were transferred to the oven for the second dry cycle. After 48 h of drying at 70 °C, the specimens were removed from the oven, measured for crack dimensions, and then submerged in water for saturation, and their hydraulic conductivities were determined depending on the scenario as earlier mentioned. The hydraulic conductivity in this cycle was reported as cycle 2 (K2). The same procedure of drying and wetting was repeated for the third cycle after air drying at controlled ambient condition (~ 20 ± 1 °C) for 24 h, and oven drying for 72 h at 70 °C and then submerged in water, and their hydraulic conductivities were determined depending on the scenario as previously mentioned. The hydraulic conductivity in this cycle was reported as cycle 3 (K3). The aim of increasing the drying duration for cycles 2 and 3 was to examine the behaviour of the specimens when subjected to periods of prolonged drying.
Crack feature measurement
To assess the effect of drying on crack development, a series of crack evaluations were carried out. Before the start of the final hydraulic conductivity tests, the specimens were removed from the oven after 24, 48, and 72 h of drying and measured for crack dimensions. Surface crack dimensions were measured using a calliper and a ruler. The length, width, and depth of the cracks were the crack features measured. It is worth noting that in some cases, the length and widths of some cracks were difficult to measure using a calliper and ruler due to their irregular shapes, hence thin gauge wires and thread were employed to take the dimension. The crack depths were also determined using the thin gauge wires. The main problem associated with the manual method of crack determination is low accuracy. However, its merits are related to the physical and close contact with the cracks, which helps in preventing false detections. Nonetheless, to overcome the shortcoming of low accuracy in the current study, repetitive measurements were employed to obtain accurate values. This method of crack measurement is consistent with the methods employed by Rayhani et al. (2007, 2008), Harianto et al. (2008).
Crack intensity factor
CIF is used to quantify the magnitude of desiccation cracks developed on the surface of the soil and is defined as the ratio between the crack area and the entire soil surface area, as shown in Eq. (1).
where Ac is the desiccation crack area on the soil surface, while As is the total area of the soil surface. The crack areas were determined using an image pixel method. MATLAB version 2018b was employed to analyse the digital photographs of the desiccating natural and treated clay samples to obtain the crack area.
Scanning electron microscopy (SEM) test
Morphological analysis was performed to assess any compositional changes resulting from the interaction between the clay, olivine, and leachate, as well as to elucidate the mechanisms responsible for the changes in the hydraulic conductivity and crack dimensions. Samples used for the hydraulic conductivity test were cautiously fractured to produce freshly exposed surfaces and then grinded through a 75-µm sieve for the SEM testing. Adhesive carbon tapes were used to mount the samples on aluminium sample holders. The mounted samples were then vacuum-coated with platinum for 40 secs, utilizing a Quorum/Q150RS Sputter Coater. The specimens were then viewed under a Hitachi S-3400N SEM operating at a voltage and current of 10.0 kV and 76.0 µA, respectively.
Statistical analysis
To evaluate the effects of cracks, leachate, as well as the combined effect of cracks and leachate on the hydraulic conductivity of the natural and olivine-treated clay specimens, Two-way analysis of variance (ANOVA) and multiple comparison studies were conducted using SigmaPlot graphing and data analysis (Version 11.0) and Origin (Version 8.0) software packages. The analysis considered Factor A (crack, leachate, and leachate + crack) as the block effect and Factor B (natural and treated clay) as the treatments. Fisher’s least significant difference test was employed to identify significant differences between means. A P value of < 0.05 was considered to indicate significance level.
The detailed testing program, including the different tests, olivine contents, permeants, testing conditions and variables, as well as the number of tests conducted, is summarized in Table 5.
Results and discussion
Compaction characteristics
Figure 4 depicts the compaction curves of the natural and treated clay samples obtained from reduced, standard, and modified compactive energies. As shown in Fig. 4, 30% olivine addition affected both the maximum dry density (MDD) and optimum moisture content (OMC) of the clay. An increase in MDD and a reduction in OMC of the treated clay were noted for all compactive efforts applied. It is also observed that the MDD and OMC for the natural and treated clay samples increase and decrease, respectively, with increasing compactive effort (reduced < standard < modified). The percentage increase in the MDD of the treated clay specimens over the natural clay specimens was approximately 20.4%, 19.6%, and 16.6% when reduced, standard, and modified Proctor energies were applied, respectively.
The increase in MDD is due to the high specific gravity of olivine (2.99) compared to the clay (2.33), which coat the clay to form small aggregates situated between the clay particles, thus causing an increase in MDD (Emmanuel et al. 2019d). Conversely, the decline in the OMC is due to the low moisture empathy of olivine, as demonstrated by a reduction in the plasticity characteristics of the treated samples (see Table 4). The increase in MDD and reduction in the OMC with increasing compactive energy can be explained on the basis that at higher compactive energy, a more parallel orientation of the clay particles is observed, thus providing a dispersed structure with the particles becoming closer and thereby reducing the porosity (Das, 1998). The obtained MDD values indicate that adequate compaction can be attained when olivine is employed to stabilize marine clay for utilization as a liner material in engineered landfills.
Crack pattern, dimension, and analysis
The effect of olivine treatment on the cracking was evaluated as a function of crack reduction. Before the addition of olivine, crack dimensions of the natural clay after series of dry–wet cycles were measured to be used as a baseline data for comparison with the olivine-treated clay samples. Three drying–wetting cycles were employed to ensure the occurrence of cracks with dimensions that can be measured. The average recorded crack dimensions of the natural and olivine-treated samples prepared using the standard Proctor energy for all series of dry–wet cycles when permeated with both water and leachate are presented in Table 6. For the sake of simplicity and clarity, crack dimensions were only reported for samples prepared using the standard Proctor energy. The length of the surface cracks varied from 36 to 95 mm for the natural and treated clay specimens when permeated with water and leachate, while the width of the cracks varied from 0.9 to 4.6 mm, and the depth varied from 3.8 to 11.8 mm. The CIFs of the specimens are also presented in Table 6. The maximum value of the CIF recorded was 6.53% for the natural clay after the third dry–wet cycle upon permeation with water. The number of dry–wet cycles significantly influenced the cracking behaviour of the clay. An increase in the number of dry–wet cycles increases the CIF. This is explained on the basis that increasing drying duration induced suction in the soil. When the suction exceeds the resistance of the soil, cracks developed. Nevertheless, with the addition of olivine, hydration reaction occurred, which led to the generation of the cementitious gels, which turn to fill the voids and cracks, consequently causing a decrease in the CIF.
Figure 5 depicts the photographs of the surface cracking and binary images of some representative samples before and after dry–wet cycle testing. Due to the large number of dry–wet cycle tests performed, the surface cracks and binary images of all the samples could not be presented. Moreover, it is also important to note that the angles at which the surface crack images of the test samples are depicted in Fig. 5 were not the exact angles used for the CIF analysis. A digital camera was fixed approximately 30 cm above the samples to capture the surface image during the drying–wetting cycles, and the images obtained were used to run the analysis. To this, the angles at which the images were taken were consistent for all images. Prior to dry–wet cycle test, little-to-no cracks were observed on the surface of the natural clay specimens permeated with water (Fig. 5a, b) and leachate (Fig. 5c, d). According to Kleppe and Olson’s (1985) crack severity classification, a crack severity number of 0 indicates the absence of cracks, while a crack severity number of 4 describes cracks with widths > 20 mm and with substantial depths. Hence, the natural clay prior to dry–wet cycles test in the present study can be described as having a crack severity number of 0.
Cracking records for the soil specimens after permeation with water and leachate: a NC + W (prior d/w cycle); b the binary image of a; c NC + L (prior d/w cycle); d the binary image of c; e NC + W (first d/w cycle); f the binary image of e; g NC + W (second d/w cycle); h the binary image of g; i NC + W (third d/w cycle); j the binary image of Fig. 5i; k TC + W (first d/w cycle); l the binary image of k; m TC + W (second d/w cycle); n the binary image of m; o TC + W (third d/w cycle); p the binary image of o; q NC + L (third d/w cycle); r the binary image of q; s TC + L (third d/w cycle); t the binary image of s. NC natural clay, TC treated clay, W water, L leachate, d/w dry–wet
It is worth noting that the surface of the specimen after permeation with tap water (Fig. 5a) was observed to have no deposition of any residues or substances relative to the sample permeated with leachate (Fig. 5c). The clean surface was observed because the permeant was tap water, which is free of salts, metallic compounds, organic, and inorganic compounds. On the other hand, the surface of the specimen after leachate permeation (Fig. 5c) was observed to have some deposition of slightly dark-ash residues with a pungent odour probably resulting from the reduction in sulphate and nitrate salts in the leachate to sulphide and ammonia compounds, respectively (Osinubi et al. 2017). Another interesting observation was that the portion of the clay surface not covered by the depositional residues had a reddish-brown colour relative to the grey colour surface of the clay sample when permeated with water. The reddish-brown colour was probably due to the oxidation of iron and sulphur in the leachate upon interaction with the clay after a prolong period of permeation.
The dry–wet cycle tests cause the specimen surfaces to split into distinct clods by the crack networks. After the first (Fig. 5e, f), second (Fig. 5g, h), and third (Fig. 5i, j) dry–wet cycles of the natural clay specimens upon permeation with water, the cracks were observed to be longer, wider, jagged, and more intensive relative to those of the treated samples after the first (Fig. 5k, l), second (Fig. 5m, n), and third (Fig. 5o, p) dry–wet cycles upon permeation with water. Similar observations were also noted for the leachate-permeated specimens. In the treated samples, the cracks are shorter with a drastic reduction in crack dimensions, as shown in Table 6. Comparing Fig. 5i, o (third dry–wet cycle specimens) and Fig. 5e, k (first dry–wet cycle specimens), it is seen that as the number of dry–wet cycle increases, the number of visible cracks and crack dimension also increases on the specimens.
It is worth noting that the surface cracks in the natural and olivine-treated specimens permeated with water (Fig. 5i, o) were more numerous with wider dimensions (length and width) relative to those permeated with leachate (Fig. 5q, s) after the third cycle of drying–wetting. Nonetheless, the crack depths of the leachate-permeated specimens were higher than those of the water permeated specimens (see Table 6), hence could be a contributing factor for the higher permeability values reported for the leachate-permeated specimens.
Hydraulic conductivity
The mean hydraulic conductivity values of the samples are presented in Table 7. The initial hydraulic conductivity of the natural clay varied from 4.25 × 10−8 to 5.45 × 10−7 cm/s when permeated with water and leachate employing all three compactive energies. Similarly, the mean hydraulic conductivity of the treated clay specimens ranged from 7.29 × 10−9 to 9.76 × 10−8 cm/s when all three compactive efforts were employed. The results show a decrease in the average hydraulic conductivity values of the natural and treated clay specimens with increasing compactive effort. The results also indicate an increase in the hydraulic conductivity values with increasing number of drying–wetting cycles. Figure 6 illustrates the variations in hydraulic conductivity during drying–wetting cycles for the natural and treated clay specimens employing all three compactive energies. As observed from the figure, significant changes in the hydraulic conductivity occurred during the second cycle of drying–wetting. The changes in the hydraulic conductivity for the first and third cycles were relatively small compared to those in the second cycle. This trend was observed for both natural and treated clay specimens upon permeation with water and leachate.
The hydraulic conductivity ratios K1/K0 (ratio of the hydraulic conductivity at the end of cycle one to the initial hydraulic conductivity), K2/K0 (ratio of the hydraulic conductivity at the end of cycle two to the initial hydraulic conductivity), and K3/K0 (ratio of the hydraulic conductivity at the end of cycle three to the initial hydraulic conductivity) were obtained. The cycle effects (K3/K1) for both natural and treated clay specimens permeated with water and leachate, and employing all three compactive efforts were also determined. The observed minimum K1/K0 ratios were 1.93, and 1.53 for the natural clay permeated with leachate when the reduced Proctor energy was employed, and the treated clay permeated with water employing the reduced compactive energy, respectively (see Table 7). The K3/K1 ratio varied from 6.48 to 8.06 for the natural clay specimens, whereas for the treated clay specimens, the K3/K1 ratio varied from 4.73 to 7.91 (see Table 7).
Effects of cracks on the hydraulic conductivity of specimens permeated with water
Figure 6 and Table 7 present the results of the hydraulic conductivity of the natural and treated clay specimens with and without cracks upon permeation with water. The hydraulic conductivity values of the natural clay specimens without cracks ranged from 4.25 × 10−8 to 3.45 × 10−7 cm/s, and the hydraulic conductivity values of the natural clay specimens with cracks after the third cycle of drying–wetting also ranged from 8.89 × 10−7 to 6.07 × 10−6 cm/s. Similarly, the hydraulic conductivity values of the treated samples without cracks ranged from 7.29 × 10 −9 to 5.76 × 10−8 cm/s, the hydraulic conductivity values of the treated samples with cracks after the third cycle of drying–wetting ranged from 8.86 × 10−8 to 6.85 × 10−7 cm/s. Upon the inclusion of olivine to the clay, a fivefold, twofold, and fivefold reduction in the mean hydraulic conductivity values of the samples without cracks were observed when the reduced, standard, and modified compactive energies were employed, respectively. Desiccation-induced cracks caused a 17-fold, 25-fold, and a 15-fold increase in the mean hydraulic conductivity of the natural clay specimens after the third cycle of drying–wetting, when the reduced, standard, and modified compactive energies were employed, respectively. Likewise, for the olivine-treated clay, desiccation-induced cracks caused a tenfold, 11-fold, and 11-fold increase in the mean hydraulic conductivity after the third cycle of drying–wetting, when the reduced, standard, and modified compactive energies were employed, respectively. However, the increase in the hydraulic conductivities of the treated samples due to desiccation cracks was minimal relative to those of the natural clay. This observation demonstrates that olivine can be employed as a suitable additive in decreasing the hydraulic conductivity of soils prone to cracking.
The reduction in the hydraulic conductivity values of the clay upon the addition of olivine may be attributed to the decrease in the dimension of the cracks, as depicted in Table 6 and Fig. 5. The reduction in the cracks was probably due to the hydration reaction (as shown in Eqs. 2-5 and depicted in Fig. 7) between \({\text{MgO}}\) in the olivine and the soluble \({\text{Al}}_{2} {\text{O}}_{3}\) and \({\text{SiO}}_{2}\) in the soil, leading to the formation of M–S–H and M–A–H gels as confirmed by the SEM and XRD analyses. The generated gels fill the voids in the treated samples, hence creating a denser and compact structure for the permeants to flow and consequently reducing the hydraulic conductivity.
It is also observed from Fig. 6 and Table 7 that the hydraulic conductivity decreased with an increase in compactive energy because increasing compactive energy reduces the size of the pores, consequently decreasing the hydraulic conductivity. It is worth noting that the variation of the hydraulic conductivity for both the natural and treated clay samples with and without cracks employing all three compactive energies satisfies the minimum recommended specification (≤ × 10−6 cm/s) for bottom liner application recommended by the Ministry of Housing and Local Government, Malaysia (MHLGM 2004).
Effects of leachate on the hydraulic conductivity of the natural and treated clay specimens
Natural clay
The hydraulic conductivity values of the natural clay specimens permeated with leachate are shown in Fig. 6 and Table 7. The hydraulic conductivity values of the natural clay specimens without cracks ranged from 8.43 × 10−8 to 5.45 × 10−7 cm/s, and the average hydraulic conductivity values of the clay specimens with cracks after the third cycle of drying–wetting also ranged from 2.52 × 10−7 to 7.65 × 10−6 cm/s. As shown in Table 7, the hydraulic conductivity values of the samples without cracks upon permeation with leachate are slightly higher than those without cracks when permeated with water, for all compactive efforts employed. Similarly, the hydraulic conductivity values of the crack samples permeated with leachate are also higher than those with cracks permeated with water for all compactive efforts utilized. This finding agrees with those reported by Mosavat and Nalbantoglu (2013), Osinubi et al. (2017). Leachate permeation resulted in approximately 63%, 26%, and 98% per cent increase in the hydraulic conductivity values of the natural clay without cracks when reduced, standard, and modified compactive energies were employed, respectively. Similarly, desiccation-induced cracks caused a 13-fold, 24-fold, and a 23-fold increase in the hydraulic conductivity of the natural clay samples permeated with leachate after the third cycle of drying–wetting, when the reduced, standard, and modified compactive energies were employed, respectively. The increase in the hydraulic conductivity when permeated with leachate is due to the salinity effect of the leachate on the clay. Upon interaction of the high-salinity leachate (TDS = 9660 mg/L) with the clay, shrinkage of the diffuse double layer (DDL) and flocculation of the clay particles occur. The flocculated structure increases the effective pore space, which causes the hydraulic conductivity to increase. It is also stated in the literature that strongly acidic and basic leachate can dissolve clay minerals (Gates and Bouazza, 2010; Liu et al., 2015, 2019), consequently, increasing the effective pore space, which in turn increases hydraulic conductivity. It is worth noting that, although leachate permeation and desiccation cracks caused an increase in the hydraulic conductivity of the natural clay specimens, the specimens still achieved hydraulic conductivity values within the specification recommended by MHLGM (2004) for liner utilization.
Olivine-treated clay
The hydraulic conductivity values of the olivine-treated clay samples permeated with leachate employing all three compactive energies are presented in Fig. 6 and Table 7. Similar to the findings of the olivine-treated clay specimens permeated with water, the hydraulic conductivity of the olivine-treated clay specimens permeated with leachate decreases relative to the natural clay when all three compactive efforts were employed. The hydraulic conductivity values of the treated clay samples without cracks ranged from 9.98 × 10−9 to 9.76 × 10−8 cm/s, and the hydraulic conductivity values of the treated clay samples with cracks after the third cycle of drying–wetting ranged from 3.36 × 10−8 to 1.54 × 10−6 cm/s. Olivine inclusion caused a fivefold, onefold, and sevenfold reduction in the hydraulic conductivity of the samples without cracks when the reduced, standard, and modified compactive energies were employed, respectively. The decrease in hydraulic conductivity is attributed to the formation of the hydration products, which fill the voids, consequently, lowering the hydraulic conductivity. However, desiccation-induced cracks caused a 15-fold, 13-fold, and a 15-fold increment in the hydraulic conductivity of the treated samples after the third cycle of drying–wetting, when the reduced, standard, and modified compactive energies were employed, respectively. It is worth noting that the variation of the hydraulic conductivity of the treated samples with and without cracks employing all three compactive energies upon permeation with leachate satisfies the minimum recommended specification for bottom liner utilization specified by (MHLGM 2004).
It can thus be concluded that leachate and desiccation-induced cracks affected the hydraulic conductivity of both the natural and treated clay specimens. However, the effects of cracks and leachate on the hydraulic conductivity of the treated clay specimens were minimal relative to those of the natural clay specimens. Hence, olivine can be employed as a suitable additive in decreasing the hydraulic conductivity of soils prone to cracking.
Effects of plasticity index on cracking and hydraulic conductivity
Chamberlain and Gow (1979) demonstrated that most intermediate-to-high plasticity clays (CI and CH) experience an increase in hydraulic conductivity with increasing plasticity index (PI) often by two to three orders of magnitude. To evaluate this hypothesis in the current study, the hydraulic conductivity ratios (K1/K0, K2/K0, and K3/K0) obtained for samples remoulded at standard Proctor at the different drying–wetting cycles were plotted against their respective PI values for the natural and treated clay specimens mixed with water and leachate, as depicted in Fig. 8.
As observed in Fig. 8, the hydraulic conductivity changed only slightly for specimens with low plasticity (treated clay mixed with water and leachate), whereas for the natural clay mixed with water and leachate, with PI values ranging from 33 to 44%, the ratios increased by an approximate percentage of 34% to 109% for all three cycles. The trivial variation in the hydraulic conductivity of the treated clay with low PI is analogous to the findings of Yesiller et al. (2000). The maximum values of Kr (3.9, 15, and 25.1) after the first, second, and third dry–wet cycles were measured for the natural clay permeated with leachate. It is worth noting that although low PI values were recorded for the natural clay mixed with leachate relative to the natural clay mixed with water, the natural clay permeated with leachate, however, recorded high hydraulic conductivity values. The high hydraulic conductivities may be due to the salinity effect of the leachate on the clay, as previously discussed.
A reduction in the Atterberg limits (Table 4) of the clay was observed upon saturation with leachate. This decrease is because heavy metals, metal salts, organic and inorganic pollutants, anions, and cations present in the leachate tend to replace the cations of the hydrous layer surrounding the clay particles. Consequently, a reduction in the thickness of the DDL occurs, resulting in shrinkage and flocculation of the clay particles, and hence a reduction in the Atterberg limits (Mosavat and Nalbantoglu 2013; Khodary et al. 2018).
Similarly, the reduction in the Atterberg limits of the treated clay is due to the effect of olivine on the clay. Upon the introduction of olivine to the clay, the olivine dissociates into magnesium and hydroxide ions after dissolving in the leachate. This dissociation results in an ion exchange of Mg2+ ion for the existing cations (e.g. Na+) at the surface of the clay particle. The resulting ion exchange facilitates aggregation of the clay particles and a reduction in the Atterberg limits (Consoli et al. 2010; Harichane et al. 2011). Moreover, the decrease in the Atterberg limits upon the addition of olivine to the clay is due to the effect of flocculation, whereby the repulsion of the negatively charged clay particles is overcome by Van der Waals forces, causing the clay particles to flocculate and give a new edge-to-face orientation (see Fig. 9). The repulsion may also bring the particles into proximity with each other, hence decreasing the thickness of the DDL; consequently, a decrease in the Atterberg limits is observed. In conclusion, the findings are consistent with those of Chamberlain and Gow (1979), who reported that most CI and CH experience an increase in hydraulic conductivity with increasing PI.
Effects of shrinkage index on cracking and hydraulic conductivity
Desiccation cracks begin to develop in clayey soils when the water content decreases beyond its shrinkage limit. Consequently, the difference in the water content between the plastic limit and the shrinkage limit (PL–SL), termed the shrinkage index (SI), is a vital indicator of soil cracking behaviour and potential variations in the hydraulic conductivity (Rayhani et al. 2007, 2008). Desiccation cracks may also occur before the specimen reaches its shrinkage limit due to factors such as flaws, displacement boundary conditions, environmental conditions, specimen dimensions, soil texture, and soil structure (Peron et al. 2009). This paper, however, focused on the first aspect (i.e. when the water content decreases beyond its shrinkage limit), which was the major observation throughout the testing. Figure 10 presents the variation of the shrinkage index with the hydraulic conductivity ratios (K1/K0, K2/K0, and K3/K0) obtained for the natural and treated clay samples remoulded at standard Proctor permeated with water and leachate at the different drying–wetting cycles. As observed in Fig. 10, the shrinkage index for the tested samples varied from 2.99 for the treated clay to 10.85% for the natural clay. Kr increased with an increase in shrinkage index, which may be attributed to the high void ratio of the natural clay specimens (Rayhani et al. 2007). Comparing Figs. 8 and 10, it is seen that the shapes of Kr versus the plasticity index (LL–PI) and Kr versus the shrinkage index (PL–SL) are analogous, indicating a good correlation between shrinkage index and the plasticity of the studied clay.
Statistical analysis
To evaluate the effects of cracks, leachate, as well as the combined effect of leachate and crack on the hydraulic conductivity of the natural and olivine-treated clay specimens, two-way ANOVA and multiple mean comparison studies were conducted. The analysis considered Factor A (crack, leachate, and leachate + crack) as the block effect and Factor B (natural and treated clay specimens) as the treatments. Table 8 shows the results of the descriptive statistics for Factors A and B, while Table 9 presents the ANOVA results. A P value of < 0.05 was considered to indicate the significance level. The significance column portrays the result of the comparison (i.e. “Yes” if the result is statistically significant, and “No” otherwise).
To ensure a good model, a test for significance of the model and individual model coefficients was conducted. Generally, the significant factors are ranked based on the F value or P value (“Prob > F” value) at 95% confidence level, as shown in Table 9. The larger the F value and smaller the P value, the more significant is the corresponding coefficient. It is seen from Table 9 that the model F-value is 3.71382, and the corresponding P value is very low (0.03729), indicating that the model is significant. There is only a 3.73% chance that an F value this large could occur due to noise. The calculated P value (P = 0.029) for Factor B indicates that the mean hydraulic conductivity values for both the natural and treated clay samples are significantly different from each other. There is only a 2.9% chance that an F value (6.191) this large could occur due to noise. On the other hand, at the 0.05 level, there is no statistically significant difference in the means of Factor A. That is, the effects of all parameters of Factor A on the mean hydraulic conductivity of the natural and treated clay samples are not significantly different from each other (P = 0.105).
Since ANOVA analysis indicates the significant difference due to the treatments, a least square difference multiple means comparisons study was conducted using Fisher’s least significant difference technique, as depicted in Table 10. As observed in Table 10, the mean difference between leachate vs. crack is not statistically significant. This indicates that the effects of leachate vs. crack on the hydraulic conductivity of the natural and treated clay specimens are not significantly different. Likewise, the mean difference of leachate + crack vs. crack is not significant at the 0.05 level. On the other hand, the mean difference of leachate + crack vs. leachate is significant at the 0.05 level. It can also be observed from Table 10 that the mean difference of the hydraulic conductivity for the natural clay vs. treated clay is significant at the 0.05 level. This indicates that the effect of Factor A (cracks, leachate, and leachate + crack) on the hydraulic conductivity of the natural and treated clay samples is significantly different. It can thus be concluded that crack, leachate, and leachate + crack are significant factors that affect the hydraulic performance of the natural and olivine-treated clay samples, with their effects not significantly different from each other. However, the hydraulic performance of both natural and treated clay samples behaves differently under the influence of these factors.
Microstructural study
Microstructural analysis was conducted to assess any compositional changes resulting from the interaction between the clay, olivine, and leachate, as well as to elucidate the mechanisms responsible for the changes in the hydraulic conductivity and crack dimensions. Microstructural investigations were conducted on the natural clay permeated with water and leachate, as well as the treated clay permeated with water and leachate. For the sake of completeness, microstructural investigation was also conducted on the pure olivine.
XRD test
The XRD diffractogram of the natural clay permeated with water (natural clay + TW), natural clay permeated with leachate (natural clay + LL), treated clay permeated with water (30% olivine-treated clay + TW), treated clay permeated with leachate (30% olivine-treated clay + LL), and the pure olivine are depicted in Fig. 2. Quartz and montmorillonite were found to be the main minerals in the natural clay permeated with water and leachate. Minor reflections of kaolinite, illite, and feldspar were also seen. The appearance of two new low-intensity phases at 2θ angles of 32.43° and 64.13° can be seen for both the natural and treated samples permeated with leachate. Both reflections are characteristic of hercynite (FeAl2O4). This is consistent with the observations of Frempong and Yanful (2005), who reported the appearance of hercynite phases after leachate permeation on tropical soils. Forsterite, fayalite, and quartz were found to be the significant minerals in the diffractogram of the pure olivine, with minor reflections of feldspar and kaolinite.
Albeit no significant changes are noted in the diffractograms of the natural clay permeated with water and leachate in comparison with the treated clay permeated with water and leachate, the appearance of several new phases for the treated clay samples is observed due to the formation of new hydration products (M–S–H and M–A–H) at 2θ angles, of 42.18°, 46.55°, 61.28°, and 65.06°. These hydration products are responsible for the reduction in the crack dimensions as well as a decrease in the hydraulic conductivity by filling the inter-aggregate pores. The absence of these hydration products in the natural clay proves that the improvement in the clay hydraulic property is attributed mainly to the hydration reaction.
Various researchers, including Kayabali (1997); Cazaux and Didier (2000); Gates et al. (2009), indicated that minerals of the smectite groups are suitable to be utilized for the construction of hydraulic barriers owing to their potential to self-heal cracks, consequently reducing the permeability of the liner material. Met et al. (2005) also indicated that illite is a desirable clay mineral to be employed in barrier construction due to its ability to remain inactive in the presence of leachate. Hence, based on the identified minerals and the formation of the hydration products, the treated clay is expected to exhibit low-to-moderate shrinkage upon drying, low hydraulic conductivity, and adequate strength, when utilized in the field for bottom liner application.
SEM analysis
The SEM images of the natural clay permeated with water (natural clay + TW), natural clay permeated with leachate (natural clay + LL), treated clay permeated with water (30% olivine-treated clay + TW), and treated clay permeated with leachate (30% olivine-treated clay + LL) are presented in Fig. 11a–d. As shown in Fig. 11a, the natural clay permeated with water has a dense and compact structure with smaller voids, hence providing a more convoluted flow path for the permeant to flow. The micrograph agrees with the low primary hydraulic conductivity values obtained. The morphology of the natural clay permeated with leachate (Fig. 11b) appeared to be flocculated and loose with more visible pores varying considerably in size, which may be due to the dissolution of clay minerals by the acidic leachate as postulated by Shivaraju et al. (2011) and Harun et al. (2013). The image also agrees with the slight increase in the hydraulic conductivity values relative to the natural clay permeated with water.
The SEM images of the treated clay permeated with water and leachate are presented in Fig. 11c, d, respectively. Both images depict binding and coating of the clay particles by the hydration gels (M–S–H and M–A–H), which results in the formation of a compacted and densely packed morphology. The gels are also responsible for the decrease in the crack dimensions and reduction in the hydraulic conductivity values.
Conclusion
Laboratory investigations were conducted to study the influence of desiccation-induced cracks and leachate infiltration on the hydraulic conductivity of natural and 30% olivine-treated marine clay specimens prepared using reduced, standard, and modified compactive efforts. Specimens were subjected to three cycles of drying–wetting and hydraulic conductivity testing using tap water and leachate as the permeants. Two-way ANOVA analysis was employed to better asses the influence of cracks, leachate, and leachate + cracks on the hydraulic conductivity of the specimens. Changes occurring in the untreated and treated clay specimens after permeation with leachate were examined by employing microstructural analyses. The following conclusions can be drawn based on the findings:
The index properties of the clay improved upon the addition of olivine and interaction with the leachate, as reflected by a decrease in the plasticity characteristics, resulting in a change in the unified soil classification system from clay of high plasticity for the natural clay mixed with water to clay of intermediate plasticity for the 30% olivine-treated clay mixed with leachate.
The MDD and OMC of the natural and treated clay specimens increased and decreased, respectively, with increasing compactive energy. The MDD of the treated clay specimens was found to be higher than that of the natural clay specimens for all compactive efforts employed.
The crack dimensions were observed to increase with an increase in the number of dry–wet cycles. However, upon the addition of olivine to the clay, the crack dimensions were significantly reduced. This was mainly due to the formation of the hydration products (M–S–H and M–A–H), which helped to fill the voids and heal/seal the cracks.
The inclusion of olivine to the clay before cycles of drying–wetting caused a fivefold and sevenfold reduction in the average hydraulic conductivity values when permeated with water and leachate, respectively, employing the modified compactive effort.
When tap water was employed as the permeating fluid, desiccation cracks caused an increase in the mean hydraulic conductivity values: a 15-fold increase for the natural clay and an 11-fold increase for the treated clay when the modified compactive effort was employed. Similarly, when leachate was adopted as the permeating fluid, a 23-fold increase for the natural clay and a 14-fold increase for the treated clay were recorded.
Desiccation cracks, leachate, and leachate + cracks are important factors that affect the hydraulic conductivity of both the natural and treated clay samples. In the statistical sense, the effect of all three parameters on the mean hydraulic conductivity of the natural and treated clay samples is not significantly different from each other. However, the hydraulic conductivity values obtained for both the natural and treated clay samples are significantly different from the statistical viewpoint.
SEM and XRD analyses were useful for assessing the compositional changes resulting from the interaction of the clay with olivine and leachate, as well as to elucidate the mechanisms responsible for the reduction in the hydraulic conductivity and crack dimensions, which was due to the formation of M–A–H and M–S–H gels.
Summarily, the findings suggest the potential application of olivine as a suitable additive in improving the hydraulic performance of soils prone to cracking, especially for application in landfill liners and covers.
Future research work
It is important to note that the inclusion of olivine greatly helped in sealing/healing the cracks and reducing the crack dimensions, consequently decreasing the hydraulic conductivity of the treated samples. However, the desiccation cracks could not be completely healed by the hydration products of olivine. Hence, the durability of the tested marine clay treated with 30% olivine against drying–wetting cycles and leachate infiltration is not guaranteed. Therefore, to ensure durability of the tested marine clay, future research works on utilizing olivine still as a sole additive but introducing a new technique for improving the quantity and/or quality of the hydration products form or utilizing olivine in combination with other additives so as to completely heal or seal the desiccation cracks are highly recommended. Furthermore, enhanced understanding of drying–wetting test procedures is required to facilitate a more objective selection of drying–wetting techniques by design engineers and to enable more meaningful comparisons of laboratory-determined data and field studies. Finally, digital image analyses are recommended for the determination of the crack features, and comparison should be made with the manual method for accuracy check.
References
Albrecht BA, Benson CH (2001) Effect of desiccation on compacted natural clays. J Geotech Geoenviron Eng 127(1):67–75
Albright WH, Benson CH, Gee GW, Roesler AC, Abichou T, Apiwantragoon P, Lyles BF, Rock SA (2004) Fieldwater balance of landfill final covers. J Environ Qual 33(6):2317–2332
Aldaeef AA, Rayhani MT (2014) Hydraulic performance of compacted clay liners (CCLs) under combined temperature and leachate exposures. Waste Manag 34(12):2548–2560
America Public Health Association, Greeberg AE, Trussell RR, Clesceri LS, Franson MAH, American Water Works Association, Water Pollution Control Federation (1985) Standard methods for the examination of water and wastewater, 16th edn. American Public Health Association, Washington, DC, p 1268
BS 1377 (1990) Methods of testing soils for civil engineering purposes. British Standards Institution, London
Cazaux D, Didier G (2000) Field evaluation of hydraulic performances of geosynthetic clay liners by small and large-scale tests. Geotext Geomembr 18(2–4):163–178
Chalermyanont T, Arrykul S, Charoenthaisong N (2009) Potential use of lateritic and marine clay soils as landfill liners to retain heavy metals. Waste Manag 29(1):117–127
Chamberlain EJ, Gow AJ (1979) Effect of freezing and thawing on the permeability and structure of soils. Eng Geol 13(1–4):73–92
Consoli NC, JrLDS Lopes, Prietto PDM, Festugato L, Cruz RC (2010) Variables controlling stiffness and strength of lime-stabilized soils. J Geotech Geoenviron Eng 137(6):628–632
Daniel DE (1984) Predicting hydraulic conductivity of clay liners. J Geotech Eng 110(2):285–300
Daniel DE, Benson CH (1990) Water content-density criteria for compacted soil liners. J Geotech Eng 119(12):1811–1830
Daniel DE, Wu YK (1993) Compacted clay liners and covers for arid sites. J Geotech Eng 199(2):223–237
Das BM (1998) Principles of geotechnical engineering, 4th edn. PWS Publishing Company, New York, p 712
Declan O, Paul Q (2009) Geotechnical engineering and environmental aspects of clay liners for landfill projects. Tech Pap 3:1–11
Emmanuel E, Lau CC, Anggraini V, Pasbakhsh P (2019a) Stabilization of a soft marine clay using halloysite nanotubes: a multi-scale approach. Appl Clay Sci 173:65–78
Emmanuel E, Anggraini V, Gidigasu SSR (2019b) A critical reappraisal of residual soils as compacted soil liners. SN Appl Sci. https://doi.org/10.1007/s42452-019-0475-7
Emmanuel E, Anggraini V, Raghunandan ME, Asadi A, Bouazza A (2019c) Improving the engineering properties of a soft marine clay with forsteritic olivine. Eur J Environ Civ Eng. https://doi.org/10.1080/19648189.2019.1665593
Emmanuel E, Anggraini V, Asadi A, Raghunandan ME (2019d) Interaction of landfill leachate with olivine-treated marine clay: suitability for bottom liner application. Environ Technol Innov. https://doi.org/10.1016/j.eti.2019.100574
Favaretti M, Cossu R (2018) Mineral liners. In: Solid waste landfilling. Concepts, processes, technologies. Chapter 7.2, pp. 289–312. https://doi.org/10.1016/b978-0-12-407721-8.00016-4
Frempong EM, Yanful EK (2005) Geoenvironmental assessment of two tropical clayey soils for use as engineered liner materials. In: Waste containment and remediation, pp 1–10. https://doi.org/10.1061/40789(168)17
Frempong EM, Yanful EK (2008) Interactions between three tropical soils and municipal solid waste landfill leachate. J Geotech Geoenviron Eng 134(3):379–396
Gates WP, Bouazza A (2010) Bentonite transformations in strongly alkaline solutions. Geotext Geomembr 28(2):219–225
Gates WP, Bouazza A, Churchman GJ (2009) Bentonite clay keeps pollutants at bay. Elements 5(2):105–110
Harianto T, Hayashi S, Du YJ, Suetsugu D (2008) Effects of fiber additives on the desiccation crack behavior of the compacted Akaboku soil as a material for landfill cover barrier. Water Air Soil Pollut 194(1–4):141–149
Harichane K, Ghrici M, Kenai S (2011) Effect of the combination of lime and natural pozzolana on the compaction and strength of soft clayey soils: a preliminary Study. Environ Earth Sci 66(8):2197–2205
Harun NS, Ali ZR, Rahim AS, Lihan T, Idris RMW (2013) Effects of leachate on geotechnical characteristics of sandy clay soil. AIP Conf Proc 1571(1):530–536
He J, Wang Y, Li Y, Ruan XC (2015) Effects of leachate infiltration and desiccation cracks on hydraulic conductivity of compacted clay. Water Sci Eng 8(2):151–157
He Y, Cui YJ, Ye WM, Conil N (2017) Effects of wetting-drying cycles on the air permeability of compacted Téguline clay. Eng Geol 228:173–179. https://doi.org/10.1016/j.enggeo.2017.08.015
Head KH, Epps R (1980) Manual of soil laboratory testing, vol 1(2). Pentech Press, London
Kalkan E (2009) Influence of silica fume on the desiccation cracks of compacted clayey soils. Appl Clay Sci 43(3–4):296–302
Kalkan E (2011) Impact of wetting–drying cycles on swelling behavior of clayey soils modified by silica fume. Appl Clay Sci 52(4):345–352
Kayabali K (1997) Engineering aspects of a novel landfill liner material: Bentonite amended natural zeolite. Eng Geol 46(2):105–114
Khodary SM, Negm AM, Tawfik A (2018) Geotechnical properties of the soils contaminated with oils, landfill leachate, and fertilizers. Arab J Geosci 11(13):2–17
Kleppe JH, Olson RE (1985) Dessication cracking of soil barriers. In: Johnson AI, Frobel RK, Cavalli NJ, Pettersson CB (eds) Hydraulic barriers in soil and rock, ASTM STP 874. ASTM, West Conshohocken, pp 263–275
Li J, Xue Q, Wang P, Liu L (2013) Influence of leachate pollution on mechanical properties of compacted clay: a case study on behaviors and mechanisms. Eng Geol 167:128–133. https://doi.org/10.1016/j.enggeo.2013.10.013
Li JH, Li L, Chen R, Li DQ (2016) Cracking and vertical preferential flow through landfill clay liners. Eng Geol 206:33–41. https://doi.org/10.1016/j.enggeo.2016.03.006
Liu Y, Bouazza A, Gates WP, Rowe RK (2015) Hydraulic performance of geosynthetic clay liners to sulfuric acid solutions. Geotext Geomembr 43(1):14–23
Liu Y, Gates WP, Bouazza A (2019) Impact of acid leachates on microtexture of bentonites used in geosynthetic clay liners. Geosynthetics International 26(2):136–145
Met I, Akgün H, Türkmenoğlu AG (2005) Environmental geological and geotechnical investigations related to the potential use of Ankara clay as a compacted landfill liner material. Turk Environ Geol 47(2):225–236
Miller CJ, Rifai S (2004) Fiber reinforcement for waste containment soil liners. J Environ Eng 130(8):891–895
Ministry of Housing and Local Government, Malaysia (MHLGM) (2004) The study on save closure and rehabilitation of landfill sites in Malaysia. Final Rep 5:222
Mosavat N, Nalbantoglu Z (2013) The impact of hazardous waste leachate on performance of clay liners. Waste Manag Res 31(2):194–202
Nayak S, Sunil BM, Shrihari S (2007) Hydraulic and compaction characteristics of leachate-contaminated lateritic soil. Eng Geol 94(3–4):137–144
Ojuri OO, Oluwatuyi OE (2018) Compacted sawdust ash–lime-stabilised soil-based hydraulic barriers for waste containment. Proc Inst Civ Eng-Waste Resour Manag 171(2):52–60
Omidi G, Prasad TV, Thomas JC, Brown KW (1996a) The influence of amendments on the volumetric shrinkage and integrity of compacted clay soils used in landfill liners. Water Air Soil Pollut 86(1–4):263–274
Omidi GH, Thomas JC, Brown KW (1996b) Effect of desiccation cracking on the hydraulic conductivity of a compacted clay liner. Water Air Soil Pollut 89(1–2):91–103
Osinubi KJ, Oluremi JR, Eberemu AO, Ijimdiya ST (2017) Interaction of landfill leachate with compacted lateritic soil–waste wood ash mixture. Proc Inst Civ Eng-Waste Resour Manag. https://doi.org/10.1680/jwarm.17.00012
Peron H, Hueckel T, Laloui L, Hu LB (2009) Fundamentals of desiccation cracking of fine-grained soils: experimental characterisation and mechanisms identification. Can Geotech J 46(10):1177–1201
Rahman Z, Yaacob WZW, Rahim SA, Lihan T, Idris WMR, Sani WM (2013) Geotechnical characterisation of marine clay as potential liner material. Sains Malays 42(8):1081–1089
Rayhani MH, Yanful EK, Fakher A (2007) Desiccation-induced cracking and its effect on the hydraulic conductivity of clayey soils from Iran. Can Geotech J 44(3):276–283
Rayhani MH, Yanful EK, Fakher A (2008) Physical modeling of desiccation cracking in plastic soils. Eng Geol 97(1–2):25–31
Razakamanantsoa AR, Djeran-Maigre I (2016) Long term chemo-hydro-mechanical behavior of compacted soil bentonite polymer complex submitted to synthetic leachate. Waste Manag 53:92–104. https://doi.org/10.1016/j.wasman.2016.04.023
Rowe RK (1998) Geosynthetics and the minimization of contaminant migration through barrier systems beneath soil waste. In: Keynote lecture in proceeding of the 6th international conference on geosynthetics, Atlanta, pp 27–103
Shivaraju R, Ravishankar BV, Nanda HS (2011) Engineering properties of soil contaminated with dyeing effluent. In: Proceedings of Indian geotechnical conference, pp 788–790
Touze-Foltz N, Lupo J, Barroso M (2008) Geoenvironmental applications of geosynthetics. In: Keynote lecture, proceedings EuroGeo4, pp 1–98
Xue Q, Zhang Q (2014) Effects of leachate concentration on the integrity of solidified clay liners. Waste Manag Res 32(3):198–206
Yesiller N, Miller C, Inci G, Yaldo K (2000) Desiccation and cracking behavior of three compacted landfill liner soils. Eng Geol 57(1–2):105–121
Yilmaz G, Yetimoglu T, Arasan S (2008) Hydraulic conductivity of compacted clay liners permeated with inorganic salt solutions. Waste Manag Res 26(5):464–473
Yong LL, Emmanuel E, Purwani R, Anggraini V (2019) Geotechnical assessment of Malaysian residual soils for utilization as clay liners in engineered landfills. Int J GEOMATE 16(58):20–25
Acknowledgements
The first author, gratefully, acknowledges Monash University, Malaysia, for supporting his graduate studies. The authors also express thanks to the Abunde Sustainable Engineering Group for its valued suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Emmanuel, E., Anggraini, V. Effects of desiccation-induced cracking and leachate infiltration on the hydraulic conductivity of natural and olivine-treated marine clay. Int. J. Environ. Sci. Technol. 17, 2259–2278 (2020). https://doi.org/10.1007/s13762-019-02606-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02606-x