Abstract
Fermentation of Bacillus pumilus (B. pumilus) using different pulp and paper sludges as culture media were performed in this work to produce at lower cost industrial enzymes such as xylanases. Secondary sludge was shown to be a suitable alternative culture medium for B. pumilus growth, while primary sludge may serve as xylanases inducer. Mixing primary (PS) and secondary sludges (SS) at 1PS:2SS (w/w) ratio having 15 g/L total solids concentration resulted in the highest cell concentration of 2 × 108 CFU/mL and the highest xylanase activity of 3.8 IU/mL under shake flask fermentation. Other lignocellulosic biomasses were tested as potential xylanase inducers. Addition of corn stover to SS showed the highest xylanase activity (10.7 IU/mL). When using a 7 L bioreactor, total cell concentration and xylanase activity obtained in the secondary sludge medium supplemented with commercial xylan (2.5 × 109 CFU/mL and 35.5 IU/mL, respectively) and corn stover (3.4 × 109 CFU/mL and 37.8 IU/mL, respectively) were comparative to a semi-synthetic based medium (5.8 × 109 CFU/mL and 47 IU/mL, respectively). The xylanase activity of B. pumilus produced in paper sludge is stable at pH 6–9 at 50 °C that offered a potential application of the enzyme for biobleaching in pulp and paper industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global forest, paper and packaging industry has been under pressure over the last years. The financial situation of the pulp and paper sector was dramatically affected by economic downturns, weak markets, intense competition, environmental regulations, and increasing production costs. The industry must develop new and creative strategies to reduce costs, improve margins and adapt to new regulations. Such strategies could be the application of biotechnology to increase production yields, reduce input cost, energy consumption and pollution generation. One example is the use of enzymes in the pulp and paper mills to mitigate pitch deposits [1, 2] or to convert waste streams into valuable coproducts like C5 sugars or building block molecules [3, 4]. In this study, the potential of the xylanase enzyme to generate interesting coproducts and be part of a value added strategy pulp and paper mills is investigated.
Xylanases are hydrolytic enzymes well known to catalyze the endohydrolysis of 1,4-β-d-xylosidic linkages in xylan of hemicellulose. They find applications in various industrial processes: food (fruit and vegetable processing, brewing, wine production, baking), animal feed, starch, textile, bioremediation. One current application of xylanases in pulp and paper is the prebleaching of kraft pulp to minimize the use of harsh chemicals. Xylanases are also useful for the production of second generation biofuels. They are commonly used as accessory enzymes, to supplement multi-enzyme cocktails, in some biofuel production processes in bioethanol fermentation. They have a synergistic effect with cellulases in the hydrolysis of the lignocellulosic biomass to simple sugars [5–7].
Various microorganisms, including bacteria, fungi, yeasts, and actinomycetes can produce multiple xylanases [5, 6, 8, 9]. Although fungi are considered as host producers of xylolytic enzymes, bacteria like Bacillus sp. have also received attention since these microorganisms can produce xylanases having specific properties. They are cellulase free, neutral or alkaline, and thermo- and halostable. These properties make them suitable for applications in the pulp and paper industry [10–13]. Moreover, Bacillus sp. is fast growing and tolerant to extreme conditions which lead to a high enzyme productivity even under harsh conditions.
Because of potential operating cost reductions related to the use of Bacillus-based xylanase, many researches focused on the utilisation of agricultural and industrial residues as complex culture media instead of expensive semi-synthetic culture media [12, 14–16]. These studies reported high levels of xylanase production with complex culture media that could be comparable to the production of xylanases using commercial xylan as the carbon source. In addition, wastewaters and wastewater sludge have been successfully used as raw materials for Bacillus sp. fermentation at laboratory and pilot scales [17, 18]. Among Bacillus sp. trains, B. pumilus is recognized as a very good producer of xylanases, particularly cellulase-free xylanases for the bio-bleaching of pulps. Enzyme production of B. pumilus using alternative and cheap raw materials such as agricultural residues has been previously demonstrated in several studies [10, 19].
The pulp and paper industry generates high amounts of residual materials from various processes. These materials must be disposed at low cost and in an environmentally friendly way [20]. Pulp and paper sludges usually contain high levels of carbohydrates and water that makes them suitable for fermentation into valuable products [21]. In addition, they are continuously available and low cost. However, to make sludge a stable and effective complex culture media for microbial production, modification might be required either by mechanical and chemical pretreatment, nutrient supplementation or solids concentration adjustment [16].
Hence, this research features the use of pulp and paper sludge as an alternative culture medium to produce xylanase enzymes from B. pumilus in submerged culture fermentation. Several treatments were investigated in order to improve B. pumilus growth and xylanase production: sludge mixing, solids concentration adjustment and addition of pretreated lignocellulosic biomass as inducer. Finally, the optimal conditions for enzyme activity (pH and temperature), were determined.
Materials and Methods
Microorganisms
Bacillus pumilus ATCC 7061 was obtained from the American Type Culture Collection (ATCC). The strain was stored at −80 °C and was grown for inocula preparation following ATCC guidelines and Reddy et al. [22].
Fermentation Media
A defined xylan medium (DXM) was used as a reference culture medium containing (g/L): peptone (5), yeast extract (5), K2HPO4 (1), MgSO4 (0.2), and xylan from beechwood (5). The medium was adjusted to pH 7.5 ± 0.1 and sterilized at 121 °C for 30 min.
Different lignocellulosic biomasses used as alternate xylan sources were hydrolysed primary sludge (HPS), untreated (RCS) and pretreated corn stover (PCS), untreated (RF) and pretreated flax (PF), untreated (RR) and pretreated reed canary grass (PR). Corn stover residus, flax (Linum usitatissimum), and reed canary grass feedstocks were supplied by Agrosphère company (QC, Canada), Schweitzer Mauduit (SWM) international (Manitoba, Canada), International Institute for sustainable development (IISD) (Manitoba, Canada), respectively. Theses biomass residues were pre-treated by reactive extruder fractionation at conditions of 5 % NaOH, 200 rotations per minute (rpm), 180 °C. This preprocessing allows essentially burst fibers to release their main components (cellulose, hemicelluloses, proteins) recruited by lignin. Commercial beechwood-extracted xylan from Sigma-Aldrich was also used. All biomass was dried and ground to powder before use. Hemicellulose and xylan content of the biomasses was determined by method of Suliter et al. [23].
Pulp and paper sludge samples were collected at the Kruger Crabtree tissue mill (Crabtree, QC, Canada) wastewater treatment facility. Primary sludge was sampled from primary clarifiers while secondary (biological) sludge was sampled from the activated (excess) sludge treatment system. Sludge properties are presented in Table 1. Sludge samples were pelletized by centrifugation at 3000g for 5 min at 4 °C (Multifuge X3 FR of Thermo Scientific). The pellets were diluted to the desired solids concentration with deionized water. The total solids concentration (TS) of the final sludge samples was measured according to standard methods [24]. Sludge samples were stored for a maximum of 1 week 4 °C to minimize microbial degradation. Before being used as microbial culture media, the sludge samples pH was adjusted to pH 7.5 and they were sterilized at 121 °C for 30 min.
Fermentation Procedure
To prepare an inoculum of growing cells (starter culture), a loopful of B. pumilus from a Tryptic Soya Agar (TSA) plate was transferred into a 500-mL Erlenmeyer flask containing 100 mL of sterilized tryptic soya broth (TSB). The mixture was incubated in a rotary shaker at 250 rpm at 35 °C for 16–18 h until the cell concentration reached 108 CFU/mL.
Shake flask fermentation was carried out in 1-L Erlenmeyer flasks containing 200 mL of DXM and sludge media, namely primary, secondary and mixed sludge. The inoculum was transferred in the flasks (2 % v/v) and incubated in a rotary shaker at 250 rpm and 35 °C for 48 h.
Fermentation was conducted in a 7.5-L Labfors III stirred tank bioreactor (Infors HT, Bottmingen, Switzerland) equipped to control fermentation parameters. The Iris 5.2 software allowed automatic set point control and integration of all reaction parameters. The pH electrode was calibrated using pH 4 and 7 buffers. The oxygen probe was calibrated to zero using nitrogen degassed water and 100 % with air saturated water. The culture medium was added to the bioreactor vessel and sterilized at 121 °C for 30 min in a vertical-loading laboratory autoclave. It was then cooled to 35 °C before being inoculated with 2 % (v/v) starter culture. The temperature was regulated at 35 °C with a water circulation pump. The pH was adjusted at 7.5 using 4 N NaOH or 4 N H2SO4 through computer-controlled peristaltic pumps. Mixing speed (200–500 rpm) and aeration rate (1.5–2 L/min) were varied in order to keep dissolved oxygen (DO) values above 30 % saturation, which ensured the oxygen concentration was above the critical level. An anti-foam agent was added automatically (0.1 %, v/v) to control foaming during fermentation. Culture samples were taken at specific intervals during fermentation to evaluate the growth and enzyme production of B. pumilus.
Estimation of Cell and Spore Count
For each experiment, viable cell counts were determined by the plate count technique [17, 22]. Samples were sequentially diluted, plated on TSA and incubated at 35 °C for 24 h. The same method was used for viable spore counts, but samples were heated at 80 °C in a heating bath for 10 min and then chilled on ice for 5 min before being placed on TSA plates and incubated. Counts were reported as colony forming units (CFU) per mL and expressed as total cell or spore counts per mL. The relative standard deviation of cell and spore measurements was respectively 6 and 7 %.
Volumetric Oxygen Transfer Coefficient (k L a)
The volumetric oxygen transfer coefficient k L a was measured in the bioreactor using the dynamic gassing-out method [17]. k L a values were determined during fermentation at different sampling times.
Enzyme Activity Assays
Culture samples were centrifuged at 10,000g and 4 °C for 10 min and the supernatants were used directly to measure xylanase activity. It was determined by measuring the release of reducing sugars from the enzymatic hydrolysis of 0.5 % (w/v) beechwood-extracted xylan (Sigma Chemicals Co.) at 35 °C and pH 7 (50 mM phosphate buffer) for 15 min. Reducing sugars measurement was conducted following the dinitrosalicylic acid (DNS) standard method according to IUPAC [25]. One unit of activity (IU) of xylanase is defined as the amount of enzyme that releases 1 μmol of xylose as reducing sugar equivalent per minute.
Determination of Optimal pH and Temperature
The optimal pH of xylanase activity was determined by measuring enzyme activity at 35 °C in the 5.0–11.0 pH range using different buffers (50 mM): citrate phosphate (pH 5.0 and 6.0), phosphate (pH 6.5; 7.0; 7.5; 8.0), borate (pH 9.0; 9.5; 10.0; 11.0). The optimal temperature of xylanase activity was determined by measuring the enzyme activity at various temperatures ranging from 10 to 80 °C at pH 7.0 (50 mM phosphate buffer).
Results and Discussion
Growth and Xylanase Production in Pulp and Paper Sludge
To investigate the use of pulp and paper sludge as culture media, shake-flask fermentation of B. pumilus in three types of sludge, primary, secondary and primary–secondary mixed sludge, at a solids concentration of 15 g/L and in DXM were carried out. The evolution of the total cell count and the xylanase production of B. pumilus during a 72 h fermentation is illustrated in Fig. 1. B. pumilus was able to grow and utilize the nutrient contained in secondary and mix sludge but not in primary sludge. The trend of B. pumilus growth in secondary and mixed sludge was similar to the DXM medium (Fig. 1a). The enzyme activity profiles (Fig. 1b) show that xylanase production reached a maximum level after 24 h, which seems to coincide with the exponential growth phase of B. pumilus. This was observed in previous studies using agro-industrial residues as substrate for B. pumilus enzyme production [10, 15, 16]. The composition of the sludge samples may have influenced Bacillus growth and enzyme production. Besides high carbohydrate content (cellulose and hemicellulose), low nitrogen content measured in primary sludge (Table 1) did not satisfy the nutrient need of microorganisms. Meanwhile, the secondary sludge generated from the activated sludge treatment contains primary and secondary metabolites of microbial endogenous activities such as amino acids or vitamins and components of dead cells that may stimulate B. pumilus growth and metabolite production. The comparative data on maximum cell concentration, maximum specific growth rate, and maximum xylanase activity in all media are summarized in Table 2. Secondary sludge was the best sludge-based medium for B. pumilus growth with the highest cell concentration at 3.6 × 108 CFU/mL, followed by 1.1 × 108 CFU/mL in mixed sludge, which are less than the cell concentration in DXM (1.2 × 109 CFU/mL). In spite of the lower maximum specific growth rate in mixed sludge (0.25 h−1) than in secondary sludge (0.29 h−1), xylanase activity in mixed sludge (3.8 IU/mL) was about—eight times higher than in secondary sludge (0.5 IU/mL). It could be attributed to xylanase induction by xylan found in primary sludge, which is part of the mixed sludge. Bacillus required that xylan be added as inducer to the substrate for enzyme synthesis as proposed by [12, 26].
Optimising the Mixed Sludge Medium for B. pumilus Growth and Xylanase Production
Mixed sludge supported well the growth and xylanase production of B. pumilus because of its xylan content, originating from the primary sludge, and the nutrients from the organic matter and cell debris of secondary sludge. For this reason, it was proposed to improve the xylan and nutrient content of mixed sludge with different proportions of primary and secondary sludge at different solids concentrations. A set of experiments was conducted at different primary and secondary sludge ratios, which in turn resulted in different solids concentration as shown in Table 3. Increasing primary sludge proportion did not support B. pumilus growth or xylanase production. As explained previously, primary sludge was effective at inducing xylanase production, but it was not adequate for microbial growth due to its lack of nitrogen. Meanwhile, increasing the secondary sludge proportion, to bring more nitrogen and other nutrients, improved the microbial growth as well as xylanase production. However, the growth of B. pumilus decreased gradually followed increase of solids concentration (Table 3). According to previous study, high solids concentration may cause mass transfer limitations during fermentation. Therefore, solids concentration at 15 g/L was used for further studies.
Effect of Xylan Sources on Xylanase Production
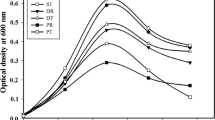
As shown by previous results, adding primary sludge to secondary sludge improves the xylanase production of B. pumilus. In consequence, it was necessary to verify if the xylan content of the primary sludge would trigger the cell growth and the xylanase production of B. pumilus. A set of experiments was conducted in which lignocellulosic residues were supplemented with secondary sludge. Different lignocellulosic biomasses were used as alternate xylan sources including hydrolysed primary sludge, untreated and pretreated corn stover, untreated and pretreated flax, untreated and pretreated reed canary grass those xylan contents are 17.03, 20.25, 22.19, 10.89, 13.17, 10.37 and 16.57 %, respectively. The xylanase activities achieved during B. pumilus fermentation of secondary sludge supplemented with these different lignocellulosic residues are presented in Fig. 2. Higher xylanase activities were obtained in all the sludge supplemented with these residues than that in secondary sludge, thus indicating the presence of xylan in these residues could induce—xylanase production in B. pumilus. The addition of residual corn stover gave the highest xylanase activity at 10.7 IU/mL, followed by pre-treated corn stover (8.0 IU/mL). These highest activities were observed from 24 to 36 h of fermentation. It is also observed in Fig. 2 that the supplementation of untreated corn stoves has more impact on xylanase activities than pretreated ones. In the present case, the pretreatment not only removes lignin in biomass but also reduces about 70 % of the hemicelluloses contained in corn stove, leading to the reduction of intact xylan in the pretreated residues. It may explain why higher xylanase activities were observed when using untreated corn stover as sludge culture medium supplements. Even untreated corn stover gave xylanase activity (10.7 IU/mL) approximately to commercial xylan (9.8 IU/mL). Thus corn stover could be an alternative to commercial xylan to induce xylanase production in B. pumilus using complex and inexpensive culture medium such as secondary sludge, which could offset the cost of enzyme production. Consequently, untreated corn stover powder and beechwood-extracted xylan were chosen as xylanase substrates for subsequent experiments in the bioreactor. With controlled pH, agitation and aeration, it was expected to obtain higher total cell count and xylanase activity.
Xylanase activity in pulp and paper secondary sludge (SS) based medium supplemented with lignocellulosic residues; hydrolyzed primary sludge (HPS), untreated and extrusion-pretreated corn stover (RCS and PCS), untreated and extrusion-pretreated flax (RF and PF), and untreated and extrusion-pretreated reed canary grass (RR and PR) and commercial beechwood-extracted xylan (SSX)
Bioreactor Production of Xylanase
Batch fermentations of B. pumilus in DXM, xylan-added secondary sludge media supplemented with xylan (SSX) and corn stover (SSC) were conducted into a 7.5 L bioreactor with a work volume of 5 L. Total solids concentration (TS) were adjusted to 15 g/L. The evolution of the volumetric mass transfer coefficient (k L a), oxygen uptake rate (OUR), and oxygen transfer rate (OTR) along with the total cell count, spore count, and xylanase activity during 60 h fermentation are illustrated in Figs. 3, 4 and 5 for DXM, SSX and SSC, respectively. The profiles were similar from one medium to another, except for the spore production that was triggered earlier in SSX and SSC media. The highest value of k L a occurred at 12 h on DXM and at 15 h on SSX and SSC and then decreased continuously to the end of fermentation (Figs. 3b, 4b, 5b, respectively). The decline of this parameter could be explained by a decrease in oxygen demand of B. pumilus when substrate exhaustion occurred [17]. The exponential phase of B. pumilus in DXM occurred during the first 9–10 h in DXM and 18–20 h in both SSX and SSC. The production of spores was observed sooner in SSX and SSC (at 5 h of fermentation) than DXM (at 9 h of fermentation). Some sludge components may trigger spore production earlier compared to a synthetic or semi-synthetic culture medium. Xylanase activities in the three culture media show a maximum enzyme level at 24 h for SSX and DXM and at 30 h for SSC when the total cell count cell concentration was maximal and decreased after 36 h of fermentation when sporulation was at its maximum in all media.
Table 4 summarizes the maximum growth and enzyme production of B. pumilus fermentation in the bioreactor. Compared to results obtained in shake flask fermentation (Table 1), the growth and xylanase production of B. pumilus were found to be much higher in the bioreactor in both synthetic (DXM) and sludge-based (SSX) media. It was attributed to a better mass transfer in the bioreactor due to the control of aeration and mixing. High growth and enzymatic activity were achieved in both SSX and SSC media. The highest values of k L a and specific growth rate calculated in both SSX and SSC are 56 and 0.49 h−1, respectively. These values were higher in DXM with 68 h−1 for k L a and 0.69 h−1 for the maximum specific growth rate. Lower values of k L a in sludge media compared to a synthetic soluble medium are attributed to the complexity of the media in terms of nutrient accessibility and rheological properties [27–29]. Moreover, the better growth and xylanase activity observed in SSC (3.4 × 109 CFU/mL of cell count and 37.8 IU/mL of xylanase activity) compared to that in SSX (2.5 × 109 CFU/mL of cell count and 35.5 IU/mL of xylanase activity) demonstrated that corn stover is a good alternative to the costly commercial xylan used to induce xylanase production.
Xylanase Activity at Different pH and Temperature
The impact of the culture medium on xylanase properties has been studies. It is expected that complex culture media such as wastewater sludge can improve enzyme tolerance to pH and temperature variation. The fermented broths of DXM, SSX and SSC were used to determine the pH and temperature optimum for their xylanase activities. There are no xylanase activity was observed at pH 5 and 10 for all three media (DXM, SSX and SSC) and the enzyme activities were observed in a pH range from 6 to 9 as shown in Fig. 6a. The results showed that, xylanase produced from sludge had highest activity at pH 6–7 and slightly reduce at higher pH, these result accorded to the optimal pH for B. pumilus is 7–7.5. The alkalothermophilic properties of xylanase produced by B. pumilus were reported in previous studies [30, 31]. The optimal temperature for xylanase activity was 50 °C (Fig. 6b) and the enzyme activity in range temperature from 30 to 55 °C. These results suggest that sludge based media have no influence on the pH and temperature tolerance of xylanase produced by B. pumilus as expected. In addition, B. pumilus xylanase produced from sludge was determined to be almost cellulase free in our other results (data not shown), which could be interesting for the use of these enzymes in the pulp and paper industry.
Conclusions
Pulp and paper sludge can be used as a complex and inexpensive culture media for the xylanase production of B. pumilus. It is recommended to mix primary and secondary sludge at ratio of 1:2 at 15 g/L TS to get high cell counts and xylanase activity. Supplementation of pulp and paper sludge media with lignocellulosic residues such as raw and ground corn stover increases xylanase activity. Compared to commercial xylan, untreated powder corn stover could be a cost effective inducer for xylanase production. A fermentation time of 30 h is proposed to recover the enzyme from the fermented broth or even use the broth as accessory enzymes for bioethanol production. In fact, it would be interesting to verify if the fermented broth can be used as a supplement for multi-enzyme cocktails in the saccharification process for bioethanol production.
References
Farrell, R.L., Hata, K., Wall, M.B.: Solving pitch problems in pulp and paper processes by the use of enzymes or fungi. In: Eriksson, K.-E. L., et al. (eds.) Biotechnology in the Pulp and Paper Industry, pp. 197–212. Berlin: Springer (1997)
Gutiérrez, A., José, C., Martínez, A.T.: Microbial and enzymatic control of pitch in the pulp and paper industry. Appl. Microbiol. Biotechnol. 82, 1005–1018 (2009)
Nghiem, N.P., Montanti, J., Johnston, D.B., Drapcho, C.: Fractionation of corn fiber treated by soaking in aqueous ammonia (SAA) for isolation of hemicellulose B and production of C5 sugars by enzyme hydrolysis. Appl. Biochem. Biotechnol. 164, 1390–1404 (2011)
Petersen, M.Ø., Larsen, J., Thomsen, M.H.: Optimization of hydrothermal pretreatment of wheat straw for production of bioethanol at low water consumption without addition of chemicals. Biomass Bioenergy 33, 834–840 (2009)
Beg, Q.K., Kapoor, M., Mahajan, L., Hoondal, G.S.: Microbial xylanases and their industrial applications: a review. Appl. Microbiol. Biotechnol. 56, 326–338 (2001)
Collins, T., Gerday, C., Feller, G.: Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 29, 3–23 (2005)
Paës, G., Berrin, J., Beaugrand, J.: GH11 xylanases: structure/function/properties relationships and applications. Biotechnol. Adv. 30, 564–592 (2012)
Polizeli, M.L.T.M., Rizzatti, A.C.S., Monti, R., Terenzi, H.F., Jorge, J.A., Amorim, D.S.: Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol. 67, 577–591 (2005)
Shallom, D., Shoham, Y.: Microbial hemicellulases. Curr. Opin. Microbiol. 6, 219–228 (2003)
Battan, B., Sharma, J., Dhiman, S.S., Kuhad, R.C.: Enhanced production of cellulase-free thermostable xylanase by Bacillus pumilus ASH and its potential application in paper industry. Enzyme Microbiol. Technol. 41, 733–739 (2007)
Duarte, M.C.T., da Cristina, S.E., de Bulhões Gomes, I.M., Ponezi, AlN, Portugal, E.P., Vicente, J.R., Davanzo, E.: Xylan-hydrolyzing enzyme system from Bacillus pumilus CBMAI 0008 and its effects on Eucalyptus grandis kraft pulp for pulp bleaching improvement. Bioresour. Technol. 88, 9–15 (2003)
Pham, P.L., Taillandier, P., Delmas, M., Strehaiano, P.: Production of xylanases by Bacillus polymyxa using lignocellulosic wastes. Ind. Crops Prod. 7, 195–203 (1998)
Subramaniyan, S., Prema, P.: Minireview. Cellulase-free xylanases from Bacillus and other microorganisms. FEMS Microbiol. Lett. 183, 1–7 (2000)
Kapilan, R., Arasaratnam, V.: Paddy husk as support for solid state fermentation to produce xylanase from Bacillus pumilus. Rice Sci. 18, 36–45 (2011)
Kapoor, M., Nair, L.M., Kuhad, R.C.: Cost-effective xylanase production from free and immobilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochem. Eng. J. 38, 88–97 (2008)
Poorna, C.A., Prema, P.: Production and partial characterization of endoxylanase by Bacillus pumilus using agro industrial residues. Biochem. Eng. J. 32, 106–112 (2006)
Vu, K.D., Tyagi, R.D., Valéro, J.R., Surampalli, R.Y.: Batch and fed-batch fermentation of Bacillus thuringiensis using starch industry wastewater as fermentation substrate. Bioprocess Biosyst. Eng. 33, 691–700 (2010)
Yezza, A., Tyagi, R.D., Valéro, J.R., Surampalli, R.Y., Smith, J.: Scale-up of biopesticide production processes using wastewater sludge as a raw material. J. Ind. Microbiol. Biotechnol. 31, 545–552 (2004)
Nagar, S., Gupta, V.K., Kumar, D., Kumar, L., Kuhad, R.C.: Production and optimization of cellulase-free, alkali-stable xylanase by Bacillus pumilus SV-85S in submerged fermentation. J. Ind. Microbiol. Biotechnol. 37, 71–83 (2009)
Gavrilescu, D.: Energy from biomass in pulp and paper mills. Environ. Eng. Manag. J. 7, 537–546 (2008)
Lynd, L.R., Lyford, K., South, C.R., van Walsum, G., Levenson, K.: Evaluation of paper sludges for amenability to enzymatic hydrolysis and conversion to ethanol. TAPPI J. 84, 50 (2001)
Reddy, C.A., Breznak, J.A., Marzluf, G.A., Schmidt, T.M., Snyder, L.R.: Methods for General and Molecular Microbiology. ASM Press, Washington, DC (2007)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D.: Determination of Structural Carbohydrates and Lignin in Biomass. [National Renewable Energy Laboratory Technical Report NREL/TP-510-42618] (2008)
APHA, AWWA, WPCF: Standard methods for examination of water and wastewaters. In: 20th Edition American Public Health Association, Washington, US. Section 2540B, pp. 216–218 (1999)
Ghose, T.K., Bisaria, V.S., IUPAC (International Union of Pure and Applied Chemistry): Measurement of hemicellulase activities. Part 1: xylanases. Pure Appl. Chem. 59, 1739–1752 (1987)
Schneider, G., Strehaiano, P., Taillandier, P.: Improvement of a fed-batch process for high level xylanase production by a Bacillus strain. J. Chem. Technol. Biotechnol. 76, 456–460 (2001)
Marques, S., Alves, L., Roseiro, J.C.: Gírio conversion of recycle paper sludge to ethanol by SHF and SSF using Pichia stipitis. Biomass Bioenergy 32, 400–406 (2008)
Verma, M., Brar, S.K., Tyagia, R.D., Valéroa, J.R., Surampalli, R.Y.: Wastewater sludge as a potential raw material for antagonistic fungus (Trichoderma sp.): role of pre-treatment and solids concentration. Water Res. 39, 3587–3596 (2005)
Michelin, M., de Oliveira Mota, A.M., de Lourdes Polizeli, M., de Teixeira, M., da Pereira, S.D., Vicente, A.A., Teixeira, J.A.: Influence of volumetric oxygen transfer coefficient (k L a) on xylanases batch production by Aspergillus niger van Tieghem in stirred tank and internal-loop airlift bioreactors. Biochem. Eng. J. 80, 19–26 (2013)
Degrassi, G., Vindigni, A., Venturi, V.: A thermostable α-arabinofuranosidase from xylanolytic Bacillus pumilus: purification and characterisation. J. Biotechnol. 101, 69–79 (2003)
Wang, C.-Y., Chan, H., Lin, H.-T., Shyu, Y.-T.: Production, purification and characterisation of a novel halostable xylanase from Bacillus sp. NTU-06. Ann. Appl. Biol. 156, 187–197 (2009)
Acknowledgments
The authors are sincerely thankful to the Natural Sciences and Engineering Research Council of Canada (Grant No. 371821) for financial support. The views and opinions expressed in this article are those of authors. We would like to extend our thanks to Mr. Brice Ouedraogo for experimental assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, T.T., Pham, T.T.H., Adjallé, K. et al. Strategies for Using Pulp and Paper Sludges as Culture Media for Xylanase Production with Bacillus pumilus . Waste Biomass Valor 6, 1103–1113 (2015). https://doi.org/10.1007/s12649-015-9404-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-015-9404-1