Abstract
Unconventional and uncontrolled use of agricultural pesticides and their influence in aquatic ecosystems during drainage process causes the accumulation of these toxins in body tissues of fish, and finally, it endangers human health. In order to determine the amount of pollution of Roundup pesticide in aquatics, acute and chronic effects of this poison on gill, kidney, and liver tissues and biochemical activities of cerebral acetyl cholinesterase and liver catalase in rainbow trout (Oncorhynchus mykiss) were investigated. To determine LC50 of Roundup pesticide in rainbow trout, acute doses of Roundup were introduced to fish tanks and fish mortality was recorded for 96 h, and Roundup LC50 was determined using SPSS Probit test. Chronic doses were determined based on the obtained LC50, and the effects of these concentrations were assessed on gill, kidney and liver tissues and cerebral acetyl cholinesterase and liver catalase activities over 28 days. Based on histopathology results, the following changes were observed: adhesion of secondary lamellae, bending of secondary lamellae in gill tissue, glomerular wrinkling, dilatation of Bowman’s capsule space in kidney tissue and necrosis, cellular swelling, and lipid degeneration in liver tissue. Cerebral acetyl cholinesterase and liver catalase activities significantly reduced in groups exposed to Roundup herbicide compared to the control group (p < 0.05). Generally, chronic concentrations of Roundup herbicide cause undesirable tissue and enzymatic changes in antioxidant system of rainbow trout. Therefore, assessment of biochemical factors and histopathological studies can be used as biomarkers in tracing the effects of agricultural toxins on aquatic habitat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The worldwide use of herbicides is estimated at 0.8 kg H−1 (Hajisharafi and Shokouhfar 2009) and is raising annually (Brookesa et al. 2017), and this amount is used in agriculture, aquatic habitats, and forests for pest control and protection of human and animal health. However, increasing and excessive use of herbicides has endangered human health, has reverse effects on non-target creatures and pollutes water, soil, and air. Although aquatic ecosystems are not considered as the target environments for pesticides, these environments and their fauna are inadvertently exposed to negative effects of these toxins, and this has exposed aquatic ecosystems as the largest part of the natural environment to constant threats such as genetic and biodiversity limitations. Studies on toxicity of pesticides in non-target creatures such as fish are used as the basis in determining eco-toxicological hazards of pesticides for aquatic systems (Abdallah et al. 2008).
Because of high level of physiological contact with aquatic environment, aquatics are among creatures that are constantly exposed to various toxins that pollute aquatic ecosystems. Various toxins are introduced into the water resources through irrigation, rain and washing, and pollute the vital tissues (such as liver, kidney and the brain) of the body of aquatic animals (Shankar Murthy et al. 2013). Therefore, aquatic histopathological studies can have a major role in identifying the effects of one or more toxins on aquatics’ body and increase knowledge about the effect of pollutants on fish (Authman et al. 2015).
In addition to histopathological studies, assessment of the activity of certain enzymes, especially antioxidant enzymes, is highly important in determining the effect of toxins on the body of aquatics. Assessment of enzymatic activity in various animal tissues can be applied in the study of metabolic pathways and function of each tissue. Enzymological findings in human and animals are applicable in investigating metabolic disorders and cell and tissue damage caused by toxicity to various compounds (Velmurugan et al. 2008).
Scientifically known as Oncorhynchus mykiss, rainbow trout is a cold-water fish that lives in clear and oxygen-rich waters. This fish belongs to the Salmonidae family and Salmoniformes order (Lee and Donaldson 2001). Because of its high adaptability, it is already found in most freshwaters in the world. Rainbow trout is rich in unsaturated fats, which are essential to healthy nutrition, and therefore has a major role in human nutrition (Guler et al. 2017).
Roundup is one of the most widely used herbicides in the world and commonly known as a non-selective herbicide that is used in agricultural weed control, and resistance to these herbicides has increased due to the development of genetically modified agricultural produce (Cuhra et al. 2015). In aquatic environments, glyphosate has a half-life of 7–14 days (Giesy et al. 2000; Torretta et al. 2018). This toxin may be directly consumed by humans through intake of food, or introduced to human body via accumulation in the food chain. Thus, it is essential that efficacy of various herbicides be properly assessed in different climatic conditions and dominant soil characteristics in different areas to enable managing their use. Efficacy of herbicides is affected by many climatic factors such as intensity of light, temperature, relative humidity and soil characteristics including concentration and types of minerals (Mithila et al. 2008).
Since Roundup is a commonly used herbicide in agriculture and its excessive and uncontrolled use in farms poses a serious threat to aquatic and human health, the present study attempted to assess damaging effects of Roundup herbicide on rainbow trout by measuring biochemical factors (cerebral acetyl cholinesterase and liver catalase) and histopathological studies (gills, liver, and kidney tissues) as the most widely used biomarkers in assessing chronic concentrations of pollutants. The present study was designed in October 2017 at the Faculty of Natural Resources of Urmia University of Iran and was implemented at the Artemia and Aquaculture Institute of Urmia University of Iran.
Materials and methods
Fish procurement and adaptation
Following procurement of 630 pieces of rainbow trout (50 ± 2.5 g) (French, Aqualand), the fish were transferred to aquaculture laboratories of Urmia Lake Research Institute of Urmia University, and to adapt to laboratory conditions, they were kept in three 1000-l polyethylene tanks for 10 days. In this period, the fish were fed with growth food of trout (GFT1) commercial extruded nutrition pellets (Faradaneh, Shahrkord, Iran) (Table 1) based on standard feeding table (Hardy 2002). Fish storage tanks contained water in good physicochemical factors (15 ± 2 °C temperature, natural light period, 8 ± 1 mg l−1 oxygen, pH = 7±0.2, and hardness of 100–400 mg l−1 calcium carbonate) and 24-hour aeration.
Determination of Roundup herbicide LC50
The LC50 of Roundup was found statically according to guideline No: 203 of Organization Economic Cooperation Development (OECD) (OECD 2001). At this stage, 360 pieces of rainbow trout were randomly divided into one control and three treatment groups (three replicas for control and each treatment) in a closed-circuit system, supplied with continuous aeration in 300-l fiberglass tanks (each tank containing 150-l water), and were, respectively, exposed to 1 ppm, 5 ppm, and 7 ppm concentrations of Roundup herbicide (Table 2) for 96 h. The water and Roundup herbicide concentrations were replaced in the test medium during the experiment period daily. During the experiment, dead fish in each treatment were counted and recorded and quickly removed from tanks. At the end of the period, LC50 of the herbicide was found by SPSS Probit test.
Chronic toxicity effects of Roundup assay
After determining 96 h LC50 of Roundup, the experiment continued to examine the effect of chronic toxicity of this herbicide in rainbow trout tissues. So, 270 pieces of rainbow trout were divided into a control and two treatment groups (three replicas for control and each treatment) in a closed-circuit system in 300-l fiberglass tanks (each tank containing 150 l water) and were exposed to different amounts of Roundup herbicide for 28 days as follows (OECD 2001):
-
Group one Roundup herbicide concentration of one-fifth of 96 h LC50 (1 ppm)
-
Group two Roundup herbicide concentration of one-tenth of 96 h LC50 (0.5 ppm)
-
Group three Control group
During the study, all tanks were continuously aerated, the light required was supplied by fluorescent lamps, and photoperiod was 10 h light:14 h dark (Bonnet et al. 2007). During the experiment period, the water and Roundup herbicide concentrations were replaced daily. At the end of the experiment, 27 fish from each treatment (nine fish from each replicate) were randomly caught and anesthetized in a solution of clove powder (200 ppm), and then, samples were taken from gills, kidney, and liver tissues for histopathological tests, and after rinsing in saline, immediately placed in formalin 10% solution. Also, cerebral acetyl cholinesterase and liver catalase activities were measured using fish brain and liver samples isolated and kept at − 80 °C.
Histopathological test
Gills, kidney, and liver tissue samples were removed from formalin and dehydrated using a graded series of ethanol (ethanol 60% to absolute alcohol) and clarified using cedar oil (cedarwood oil) and xylol (dimethylbenzene). Samples were molded in liquid paraffin, and 5 micron sections prepared with microtome were placed on slides. Samples were stained hematoxylin–eosin, and then underwent histopathological assessments using an optical microscope equipped with camera. Histological changes of organs were evaluated semiquantitatively based on severity of the lesions. The alterations in gills, kidney, and liver organ were classified in progressive stages of damage to the tissue: stage I, which is normal tissue; stage II, which is severe and impairs the normal functioning of the tissue; and stage III, which is very severe and causes irreparable damage. A value of degree of tissue change (DTC) was calculated for each lesion by the formula: DTC = (1 × SI) + (10 × SII) + (100 × SIII) where I, II and III correspond to the number of alterations of stages I, II and III, respectively. DTC values between 0 and 10 indicate normal functioning of the organ; values between 11 and 50 indicate mild damage to the organ; values between 51 and 100 indicate moderate changes in the organ, and values above 100 indicate sever damage to the organ (Camargo and Martinez 2007).
Acetyl cholinesterase activity assay
The brain acetyl cholinesterase activity was measured using Ellman et al. (1961) method. Fish brain tissue samples were carefully weighed and 0.2 gr from each sample was added to 2 ml KCl 10% solution in the homogenizer and completely homogenized, and then centrifuged at 10000 rpm for 10 min, and supernatant (brain tissue extract) was separated. Brain tissue extract was poured into a 96-well microplate, 1000 µl of pyrophosphate solution was added to the wells containing the sample or the standard solution, and the resulting set was incubated at 37 °C for 3 min. Next, 250 µl of butyrylthiocholine solution was added to samples, and 2 min later, optical absorption of samples was read four times at one minute intervals using ELISA Reader (DANA, USA) at 405-nm wavelength. According to the guidelines, the brain acetyl cholinesterase activity was found by multiplying mean optical absorption difference between readings by 68500 (Ellman et al. 1961).
Liver catalase activity assay
Liver catalase activity was measured according to Modesto and Martinez method (2010). Samples of liver tissue were weighed and placed in homogenizer, and for every 0.2 g of sample, 2 ml of KCl 10% solution was added, and then fully homogenized. The resulting solution was centrifuged at 5000 rpm for 5 min, and supernatant (liver tissue extract) was separated.
A mixture of 1 ml of liver tissue extract and 0.5 ml of PBS solution (composed of 2.9 gr of \( {\text{Na}}_{2} {\text{HPO}}_{4} + 2{\text{H}}_{2} {\text{O}} \), 0.2 g of \( {\text{KH}}_{2} {\text{PO}}_{4} \), 8 g of NaCl, 0.2 g of KCl, and 1000 ml of distilled water, whose pH is controlled with HCl) was poured in the cuvette as blank (0.5 cm diameter and 3 ml volume), and its optical absorption was read at 0, 10, 20, 40, 60 s and 2 min at 240-nm wavelength using a spectrophotometer. Then, 100 ml of PBS solution was poured into a dark glass vessel, and 340 µl of H2O2 was added; 0.5 ml of this solution was poured into the cuvette (0.5 cm diameter and 3 ml volume), and then, 1 ml of liver tissue extract was added. Changes in absorption of H2O2 were read at 0, 10, 20, 40, 60 s and 2 min at 240-nm wavelength using a spectrophotometer. Based on guidelines, liver catalase activity was measured using the following equation:
where ΔA, dx, and V denote changes in optical absorption, cuvette thickness, and final volume, respectively.
Statistical analysis
Normal distribution of data was assessed using Kolmogorov–Smirnov test. Roundup herbicide LC50 was determined using Probit Analysis Test. Enzyme data were analyzed using one-way variance analysis (ANOVA), and mean values were compared according to Tukey test at 95% CI using SPSS-v20. The results obtained were presented as mean ± SD.
Results and discussion
Acute toxicity and 96 h LC50
In the present study, during adaptation of fish to experimental conditions and 96 h of test, acute toxicity of Roundup herbicides caused no mortality in the control group fish. Therefore, it can be concluded that during 96 h of acute toxicity test, only addition of Roundup herbicide can be the reason for fish mortality. Following the exposure of rainbow trout to 1 ppm, 5 ppm, and 7 ppm concentrations of Roundup for 96 h, mortality for different treatments was recorded, and numerical value of 96 h LC50 was determined to be 4.98 ppm using Probit Analysis Test of statistical software SPSS-v20 (Table 3). Gholami-seyedKalaei et al. (2013) assessed toxicity of malathion, carbaryl, and Roundup in Cyprinus carpio and reported Roundup herbicide 96 h LC50 as 6.75 ppm for Cyprinus carpio. This shows that different fish are affected differently by aquatic pollutants.
Gill tissue histopathology
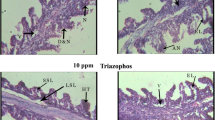
Microscopic examination of gills tissue in the control group fish (treatment three) 28 days after the experiment revealed no structural changes or abnormality (Fig. 1a). Meanwhile, gills of fish receiving 1 ppm of Roundup (treatment one) showed the following changes: adhesion and bending of secondary lamellae (Fig. 1b). Gills of fish receiving 0.5 ppm of Roundup (treatment two) showed changes such as separation of epithelium from the basement membrane in secondary lamellae, and clubbing of secondary lamellae (Fig. 1c). Accumulation of pollutants in the gills of fish increases osmotic pressure, and more water enters the gills; and to compensate for this, the number of cells increases in gill filaments and lamellae and the lamella adheres together to reduce epithelium contact with water, so that osmotic pressure can be maintained by reducing pollutants entering the gills (Bernet et al. 2004). Therefore, gills dysfunction due to pollutants is significantly associated with the fish health, and gills are considered a key indicator of water pollution. According to the present study results, chronic toxicity with Roundup herbicide caused bending of secondary lamellae, dilatation of blood vessels of gill filaments, separation of epithelium from basement membrane, clubbing and adhesion of secondary lamellae in rainbow trout gill tissue (Fig. 1, Table 4). Braz-Mota et al. (2015) exposed Colossoma macropomum to Roundup herbicide, and their results showed liver and gill disorders, DNA damage, and inhibition of brain cholinergic activity in this fish due to exposure to Roundup herbicide. They reported that fish exposed to concentrations of 50% and 75% of 96 h LC50 showed histopathological changes in gill tissue such as epithelial hyperplasia and hypertrophy of gill filaments, epithelial separation from basement membrane, aneurysm and rupture of epithelial lamellae, and mitochondrial-rich cell proliferation. The above study results are largely similar to those of the present study (Table 4). Rocha et al. (2015) studied proteomic response and histopathology of gills of guppy fish exposed to Roundup herbicide and reported that exposure to a concentration of 1.82 ppm changes the curvature of gill lamellae, and in some areas, hypertrophy and hyperplasia in gill lamella epithelium. In the present study, exposure of rainbow trout to Roundup herbicide concentrations of 1 ppm and 0.5 ppm caused similar damage to gill tissue (Fig. 1, Table 4).
Histopathological changes in rainbow trout gill exposed to chronic toxicity of Roundup herbicide. A section of gills with normal secondary lamellae L, in the control group (a); adhesion of secondary lamellae La, and bending of secondary lamellae Lb in gills of fish receiving 1 ppm of Roundup herbicide (b); and separation of epithelium from the basement membrane in secondary lamellae Lc, and clubbing of secondary lamellae Ld in gills of fish receiving 0.5 ppm of Roundup herbicide (c) (mag × 40; H and E staining)
Kidney tissue histopathology
Microscopic examination of kidney tissue in the control group fish (treatment three) 28 days after the experiment revealed no structural changes or abnormality (Fig. 2a), while in fish receiving 1 ppm of Roundup herbicide (treatment one) tissue changes appeared as dilatation of Bowman’s capsule space, and glomerular wrinkling (Fig. 2b). In fish receiving 0.5 ppm of Roundup herbicide (treatment two), tissue changes occurred as glomerular cell swelling, and dilatation of Bowman’s capsule space (Fig. 2c). Kidneys are constantly exposed to toxins due to their key role in maintaining hemostasis of the fish body, receiving large amounts of blood, and excreting metabolites resulting from toxins; therefore, they are considered an important target organ for many environmental pollutants (Au 2004). According to the present study results, the effects of chronic toxicity of Roundup herbicide on rainbow trout kidney tissue caused such complications as glomerular wrinkling, dilatation of Bowman’s capsule space, cloudy swelling in proximal tubule, and dilatation of glomerular space (Fig. 2, Table 4). Jiraungkoorskul et al. (2002) studied histopathological effects of Roundup herbicide on the Nile Tilapia (Oreochromis niloticus) and reported that exposure to 46.9 ppm, 44.4 ppm, 40 ppm, and 36.8 ppm concentrations of Roundup herbicide after 24, 48, 72, and 96 h caused changes such as dilatation of Bowman’s capsule space, glomerular shrinkage, pyknosis, separation of epithelium from tubules, broadening of vacuoles, necrosis in some cells, and cellular swelling in kidney tissue. In the present study, Roundup herbicide caused similar damage to kidney tissue in rainbow trout (Fig. 2, Table 4). In their in vitro and field study, Samanta et al. (2016) investigated the effects of Roundup herbicide on the kidneys of Heteropneustes fossilis and reported that in vitro conditions, Roundup herbicide causes necrosis in epithelial cells, fragmentation of endoplasmic network, damage to glomerular, distal and proximal tubules, vacuolation of epithelial cells in kidney tubules, and loss of kidney’s hematopoietic tissue. These results are largely similar to those obtained in the present study.
Histopathological changes in rainbow trout kidney exposed to chronic toxicity of Roundup herbicide. Glomerulus G, Bowman’s capsule B, Bowman’s capsule space K, proximal tubule P, and distal tubule D in all control group fish (a). Dilatation of Bowman’s capsule space Bs, and glomerular wrinkling Gh in all fish receiving 1 ppm of Roundup herbicide (b). Glomerular cell swelling Gt, and dilatation of Bowman’s capsule space Bs in fish receiving 0.5 ppm of Roundup herbicide (c) (mag × 40; H and E staining)
Liver tissue histopathology
Microscopic examination of liver tissue in the control group fish (treatment three) 28 days after the experiment revealed no structural changes or abnormality (Fig. 3A), while in fish receiving 1 ppm of Roundup herbicide (treatment one), tissue changes appeared as lipid degeneration in cytoplasm and pyknosis (Fig. 3b). In the liver of fish receiving 0.5 ppm of Roundup herbicide (treatment two), tissue changes were also in the form of lipid degeneration in cytoplasm and pyknosis (Fig. 3c). Liver is prone to chemical damage due to its key metabolic features, bile production, vitellogenesis, and biological transfers such as secretion of organic and non-organic pollutants and high sensitivity to pollutants; it is therefore an appropriate organ for studying the effect of environmental stimuli in animals. Hence, change in the structure of liver has a major role in the evaluation of fish health (Boran et al. 2010). According to the present study results, chronic toxicity of Roundup herbicide causes complications such as: cloudy swelling of cytoplasm, lipid degeneration in cell cytoplasm, necrosis, pyknosis of nucleus, and vascular hyperemia in liver tissue in rainbow trout (Fig. 3, Table 4). Do Carmo Langiano and Martinez (2008) studied the effect of toxicity of Roundup herbicide on Prochilodus lineatus fish and reported that 24-h exposure of fish to 10 ppm concentration of Roundup herbicide caused many pathological changes in the liver, and the most common of these changes included cytoplasmic and hepatocyte nucleus changes, decreased bile, hyperemia, increased blood flow in the liver, cytoplasmic vacuolation of hepatocytes, and hypertrophy of hepatocytes. As shown in Fig. 3 and Table 4, in the present study, 1 ppm and 0.5 ppm concentrations of Roundup herbicide caused many liver tissue damages in rainbow trout (Table 4). In their in vitro study, Samanta et al. (2016) investigated toxic effects of Roundup herbicide on liver, gill, and kidney of Heteropneustes fossilis fish and reported that this herbicide causes pyknosis of nucleus of liver cells, cytoplasmic vacuolation of liver cells, fat deposition in hepatocytes, necrosis of liver cell nucleus, and liver cell swelling. These results largely concur with the present study results. Table 4 shows the comparison of severity of tissue damages in gills, kidney, and liver in different treatments.
Histopathological changes in rainbow trout liver exposed to chronic toxicity of Roundup herbicide. Hepatocytes Hc and cirrhosis S1 in the control group fish (a). Lipid degeneration in cytoplasm V and pyknosis P in all fish receiving 1 ppm of Roundup herbicide (b). Lipid degeneration in cytoplasm V and pyknosis P in all fish receiving 0.5 ppm of Roundup herbicide (c) (mag × 40; H and E staining)
Cerebral acetyl cholinesterase and liver catalase activities
Cerebral acetyl cholinesterase and liver catalase activities in treatment 1 and 2 in chronic toxicity significantly decreased compared to the control (P < 0.05). It should be noted that cerebral acetyl cholinesterase activity in fish receiving treatment one (2275 ± 73 U L−1) and liver tissue catalase activity in fish receiving treatment two (4.07 ± 0.83 U ml−1) showed significantly greater decrease compared to the control group (P < 0.05) (Table 5). Acetyl cholinesterase (ACHE) is an enzyme that breaks down acetylcholine into choline and acetic acid. Acetylcholine is found in central and peripheral nervous system, neuromuscular synapses, and red blood cells. Inhibition of acetyl cholinesterase leads to increased acetylcholine in synaptic space (O’brien 1976) and may overstimulate cholinergic nerves, which can lead to behavior change, tremor, loss of balance, and ultimately death of the aquatic creature (Uner et al. 2006). In the present study, cerebral acetyl cholinesterase activity of rainbow trout exposed to 1 ppm and 0.5 ppm concentrations of Roundup herbicide decreased compared to control treatment (Table 5), such that 28 days after experiment, in 1 ppm treatment, activity of this enzyme showed greater decrease. Gholami-Seyedkolaei et al. (2013) studied the effect of Roundup herbicide on common carp (Cyprinus carpio) by assessing acetyl cholinesterase activity, blood, and serum biochemical parameters and reported that acetyl cholinesterase activity reduced in fish exposed to concentrations of 1.4 ppm of this herbicide. These results agree with those of the present study. Braz-Mota et al. (2015) reported inhibition of cerebral acetyl cholinesterase activity in Amazon bony fish (Colossoma macropomum) exposed to 1.2 and 2.3 of 96-h LC50 concentrations of Roundup herbicide for 96 h.
Harmful effects of free radicals are neutralized through protective system of cellular antioxidants including catalase (CAT), and superoxide dismutase (SOD) (Limon-Pacheco and Gonsebatt 2009). Superoxide dismutase (SOD) converts superoxide radical into H2O2, and catalase (CAT) reacts to and neutralizes toxicity of H2O2 by turning it into water and oxygen molecules (Mates et al. 1999). The imbalance between production of free radicals and antioxidant system in the body is referred to as oxidative stress, which is the cause of many diseases (Limon-Pacheco and Gonsebatt 2009). In the present study, liver catalase activity reduced compared to control treatment in rainbow trout exposed to 1 ppm and 0.5 ppm concentrations of Roundup herbicide for 28 days, such that catalase activity in fish treated with 0.5 ppm Roundup showed greater decrease after 28 days exposure. In a study conducted by Do Carmo Langiano and Martinez (2008), Roundup concentration of 10 ppm reduced liver catalase activity compared to the control group in Prochilodus lineatus fish, which largely agrees with the present study results.
Lushchak et al. (2009) investigated low toxicity Roundup herbicide that causes oxidative stress in goldfish (Carassius auratus) and reported increased liver catalase activity in fish exposed to Roundup concentrations of 2.5 ppm and 5 ppm compared to the control group. Cell exposure to oxidative stress induces antioxidant enzymes (catalase CAT and superoxide dismutase SOD). CAT and SOD enzymes act in synergy and form a set of mutual protective enzymes, and increased activity of these enzymes indicates compatibility of cell or tissue with the stress (John et al. 2001). In the present study, liver catalase activity reduced compared to the control group. According to the results of the present study, following the Roundup acute toxicity test for rainbow trout, 96 h LC50 was 4.97 ppm (Table 3), and in chronic concentration, maximum concentration causing chronic toxicity, tissue damage (Table 4), negative effect on cerebral acetyl cholinesterase, and liver catalase activities (Table 5) was 1 ppm.
Conclusion
Uncontrolled use of various agricultural toxins introduces large amounts of these toxins to land and aquatic ecosystems and affects wide ranges of plants and animals living in them. Since most aquatic creatures like fish are cold-blooded and have an extensive physiological contact with their habitat, they are very sensitive to pollutions caused by agricultural toxins and suffer from acute or chronic toxicity, depending on the amount of pollution. Therefore, according of the results of the present study, concentrations of Roundup lower than acute that are considered ineffective and permissible for non-target species can induce chronic toxicity in gill, kidney, and liver tissues of rainbow trout and change cerebral acetyl cholinesterase and liver catalase activities in this fish. The above tissue and enzymatic changes can be used as biomarkers for habitat pollution of fish and other aquatics with glyphosate. Therefore, it is suggested to apply histopathological and enzymatic studies of the antioxidant system of fish to achieve the correct and safe pattern application of herbicides in agricultural fields and less damage to aquatic ecosystems.
References
Abdallah L, Yu Q, Han H, Owen M, Powles S (2008) Distinct non-target site mechanisms endow resistance to glyphosate, ACCase and Als-inhibiting herbicides in multiple herbicide-resistant Lolium rigidum. Planta 230:713–723. https://doi.org/10.1007/s00425-009-0981-8
Au D (2004) The application of histo-cytopathological biomarkers in marine pollution monitoring: a review. Mar Pollut Bull 48:817–834. https://doi.org/10.1016/j.marpolbul.2004.02.032
Authman MMN, Zaki SM, Khallaf EA, Abbas HH (2015) Use of fish as bio-indicator of the effects of heavy metals pollution. Aquac Res Dev 6:328. https://doi.org/10.4172/2155-9546.1000328
Bernet D, Schmidt-Posthaus H, Wahli T, Burkhardt-Holm P (2004) Evaluation of two monitoring approaches to assess effects of waste water disposal on histological alternations in fish. Hydrobiologia 52(1):53–66
Bonnet E, Montfort J, Esquerre D, Hugot K, Fostier A, Bobe J (2007) Effect of photoperiod manipulation on rainbow trout (Oncorhynchus mykiss) egg quality: a genomic study. Aquaculture 268(1–4):13–22. https://doi.org/10.1016/j.aquaculture.2007.04.027
Boran H, Altinok I, Capkin E (2010) Histopathological changes induced by maneb and carbaryl on some tissues of rainbow trout, Oncorhynchus mykiss. Tissue Cell 42:158–164. https://doi.org/10.1016/j.tice.2010.03.004
Braz-Mota S, Sadauskas-Henrique H, Duarte RM, Val AL, Almeida-Val VM (2015) Roundup exposure promotes gills and liver impairments, DNA damage and inhibition of brain cholinergic activity in the Amazon teleost fish Colossoma Macropomum. Chemosphere 135:53–60. https://doi.org/10.1016/j.chemosphere.2015.03.042
Brookesa G, Taheripourb F, Tyner WE (2017) The contribution of glyphosate to agriculture and potential impact of restrictions on use at the global level. Biotechnol Agric Food Chain 8:216–228. https://doi.org/10.1080/21645698.2017.1390637
Camargo MMP, Martinez CBR (2007) Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotropical Ichthyol 5(3):327–336
Cuhra M, Traavik T, Dando M, Primicerio R, Holderbaum DF, Bohn T (2015) Glyphosate-residues in roundup-ready soybean impair daphnia magna life-cycle. J Agric Chem Environ 4:24–30. https://doi.org/10.4236/jacen.2015.41003
Do Carmo Langiano V, Martinez CB (2008) Toxicity and effects of a glyphosate-based herbicide on the neotropical fish Prochilodus lineatus. Comp Biochem Physiol Part C Toxicol Pharmacol 147:222–231. https://doi.org/10.1016/j.cbpc.2007.09.009
Ellman GL, Courtney KD, Andresjr V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Gholami-seyedKalaei SJ, Shiri N, Mirvaghefi A, Rafiei Gh, Makhdomi Ch (2013) Toxicity of malathion, carbaryl, and glyphosate in common juvenile carp (Cyprinus carpio). J Vet Res 68(3):257–267
Gholami-Seyedkolaei SJ, Mirvaghefi A, Farahmand H, Kosari AA (2013) Effect of a glyphosate-based herbicide in Cyprinus carpio: assessment of acetylcholinesterase activity, hematological responses and serum biochemical parameters. Ecotoxicol Environ Saf 98:135–141. https://doi.org/10.1016/j.ecoenv.2013.09.011
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for Roundup herbicide. Rev Environ Contam Toxicol 167:35–120
Guler GO, Zengin G, Cakmak YS, Aktumsek A (2017) Comparison of fatty acid compositions and ω3/ω6 ratios of wild brown trout and cultured rainbow trout. Turk J Fish Aquat Sci 17:1179–1187. https://doi.org/10.4194/1303-2712-v17_6_11
Hajisharafi GHH, Shokouhfar AR (2009) Replace herbicide sugarcanes to reduce consumption and optimal use of pesticide in agro industrial sugarcane khuzestan. J Crop Physiol 1:49–57 (in Persian)
Hardy RW (2002) Nutrient requirement and feeding of fish for aquaculture. CABI Publishing, Walling Ford, pp 184–202
John S, Kale M, Rathore N, Bhatnagar D (2001) Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem 12:500–504. https://doi.org/10.1016/S0955-2863(01)00160-7
Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, Pokethitiyook P (2002) Histopathological effects of roundup, a glyphosate herbicide, to Nile tilapia (Oreochromis niloticus). Sci Asia 28:121–127
Lee CS, Donaldson EM (2001) General discussion on “Reproductive biotechnology in finfish aquaculture”. Aquaculture 197:303–320
Limon-Pacheco J, Gonsebatt ME (2009) The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res/Gen Toxicol Environ Mutagen 674:137–147. https://doi.org/10.1016/j.mrgentox.2008.09.015
Lushchak V, Kubrak OI, Storey JM, Storey KB, Lushchak VI (2009) Low toxic herbicide roundup induces mild oxidative stress in goldfish tissues. Chemosphere 76:932–937. https://doi.org/10.1016/j.chemosphere.2009.04.045
Mates JM, Perez-Gomez C, De Castro IN (1999) Antioxidant enzymes and human diseases. Clin Biochem 32:595–603. https://doi.org/10.1016/S0009-9120(99)00075-2
Mithila J, Swanton C, Blackshaw R, Cathcart R, Hall JC (2008) Physiological basis for reduced glyphosate efficacy on weeds grown under low soil nitrogen. Weed Sci 56:12–17. https://doi.org/10.1614/WS-07-072.1
Modesto KA, Martinez CB (2010) Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78:294–299. https://doi.org/10.1016/j.chemosphere.2009.10.047
O’brien R (1976) Acetylcholinesterase and its inhibition. Insecticide biochemistry and physiology. Plenum Press, New York, pp 271–296
OECD (Orgaziation Economic Cooperation Development) (2001) Guideline for testing of chemicals. No. 210. Section 2. Effect on biotic system direction. OECD Publishing, Paris, pp 1–39
Rocha TL, Dos Santos APR, Yamada AT, De Almeida Soares CM, Borges CL, Bailao AM, Saboia-Morais SMT (2015) Proteomic and histopathological response in the gills of Poecilia reticulata exposed to glyphosate-based herbicide. Environ Toxicol Pharmacol 40:175–186. https://doi.org/10.1016/j.etap.2015.04.016
Samanta P, Mukherjee AK, Pal S, Kole D, Ghosh AR (2016) Toxic effects of glyphosate-based herbicide, Excel Mera 71 on gill, liver, and kidney of Heteropneustes fossilis under laboratory and field conditions. J Microsc Ultrastruct 3:147–155. https://doi.org/10.1016/j.jmau.2016.01.002
Shankar Murthy K, Kiran BR, Venkateshwarlu M (2013) A review on toxicity of pesticides in Fish. Int J Open Sci Res 1(1):15–36
Torretta V, Katsoyiannis IA, Viotti P, Rada EC (2018) Critical review of the effects of glyphosate exposure to the environment and humans through the food supply chain. Sustainability 10(4):950. https://doi.org/10.3390/su10040950
Uner N, Oruc EO, Sevgiler Y, Sahin N, Durmaz H, Usta D (2006) Effects of Diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol 21:241–245. https://doi.org/10.1016/j.etap.2005.08.007
Velmurugan B, Selvanayagam M, Cengiz EI, Uysal E (2008) Levels of transaminases, alkaline phosphatase, and protein in tissues of Clarias gariepienus fingerlings exposed to sublethal concentrations of cadmium chloride. Environ Toxicol 23:672–678. https://doi.org/10.1002/tox.20372
Acknowledgements
The authors thank the Artemia and Aquaculture Institute of Urmia University, Iran, for their support and cooperation in this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Shahid Hussain.
Rights and permissions
About this article
Cite this article
Meshkini, S., Rahimi-Arnaei, M. & Tafi, A.A. The acute and chronic effect of Roundup herbicide on histopathology and enzymatic antioxidant system of Oncorhynchus mykiss. Int. J. Environ. Sci. Technol. 16, 6847–6856 (2019). https://doi.org/10.1007/s13762-018-2095-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-2095-y