Abstract
Microalgae biomasses offer important benefits regarding macromolecules that serve as promising raw materials for sustainable production. In the present study, the microalgae Arthrospira platensis DHR 20 was cultivated in horizontal photobioreactors (HPBR), with and without temperature control, in batch mode (6 to 7 days), with anaerobically digested cattle wastewater (ACWW) as substrate. High dry biomass concentrations were observed (6.3–7.15 g L−1). Volumetric protein, carbohydrate, and lipid productivities were 0.299, 0.135, and 0.108 g L−1 day−1, respectively. Promising lipid productivities per area were estimated between 22.257 and 39.446 L ha−1 year−1. High CO2 bio-fixation rates were recorded (875.6–1051 mg L−1 day−1), indicating the relevant potential of the studied microalgae to mitigate atmospheric pollution. Carbon concentrations in biomass ranged between 41.8 and 43.6%. ACWW bioremediation was satisfactory, with BOD5 and COD removal efficiencies of 72.2–82.6% and 63.3–73.6%. Maximum values of 100, 95.5, 92.4, 80, 98, and 94% were achieved concerning the removal of NH4+, NO3−, Pt, SO42−, Zn, and Cu, respectively. Total and thermotolerant coliform removals reached 99–99.7% and 99.7–99.9%. This microalgae-mediated process is, thus, promising for ACWW bioremediation and valuation, producing a microalgae biomass rich in macromolecules that can be used to obtain friendly bio-based products and bioenergy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In order to escalate milk production, intensive cattle farming has been increasingly applied worldwide [1]. Wastewater generation in intensive farming can reach up to 130 L animal−1 day−1, with this waste containing high nutrient and organic matter concentrations [2, 3]. Cattle wastewater (CWW) displays a BOD5 between 2000 and 30,000 mg L−1, total nitrogen ranging from 200 to 2,055 mg L−1, ammonia between 110 and 1650 mg L−1, and total phosphorus varying from 100 to 620 mg L−1 [4]. These concentrations are alarming and can cause dissolved oxygen depletion in water courses and eutrophication.
However, after undergoing pre-treatments, CWW can become a potential culture medium for microalgae [5,6,7,8]. Besides removing nutrients from wastewater, some microalgae species are also able to assimilate soluble organic carbon contained in substrates through mixotrophy [9]. Chlorella vulgaris, Scenedesmus obliquus, and Arthrospira platensis are recognized as mixotrophic [10]. The mixotrophic mechanism creates an additive and synergistic effect during cultivation, resulting in increased biomass productivity while at the same time promoting wastewater bioremediation [5].
Fossil energy sources like oil, gas, and mineral coal emit approximately 6 billion t of CO2 into the atmosphere [11]. In this context, microalgae exhibit relevant CO2 bio-fixation rates, allowing for increased biomasses, thus ensuring higher culture productivity and growth rates [10], consecutively leading to decreased CO2 emissions. Therefore, microalgae cultivation aiming at waste treatment is favorable in mitigating this environmental problem regarding two aspects, namely the reduction of greenhouse gases (GHG), mainly through CO2 bio-fixation, and wastewater bioremediation.
A general history of the energy matrix reveals that the energy consumed worldwide in recent years was in the order of 14,279,569 ktoe, of which ~ 14% originated from renewable sources, such as solar, hydropower, wind, biomass, and waste [12]. Despite the recent advancements of renewable energy alternatives, their use is still limited concerning their potential [13] and, mainly, the urgent need for a paradigm change in this sector.
Within this scenario of necessary changes, the use of microalgae for the production of 3rd generation biofuels has recently gained more attention from the scientific community. Algal biomasses can be used to produce different biofuels, such as biodiesel/bio-oil, biogas, and bioethanol. Microalgae exhibit high photosynthetic rates compared to higher plants [14], leading to high biomass productivity. In addition, they display the ability to develop in unsuitable agriculture areas [15], avoiding food security-associated conflicts, and can be produced during the wastewater treatment, thus categorized as a nutrient recycling process, requiring no potable water for cultivation [16].
Given the above, new microalgae cultivation techniques are essential in order to increase the productivity of biomasses that contain important macromolecules for the production of friendly bio-based products, focused on bioenergy.
In this context, the aims of the present study were to assess the potential of Arthrospira platensis DRH 20 for the bioremediation of CWW previously treated by an UASB reactor, obtain CO2 biomass bio-fixation rates and culture kinetic parameters at different temperatures, improve the quantitative and qualitative production of the Arthrospira platensis biomass, evaluate macromolecular components, such as lipids, proteins, and carbohydrates contained in the produced biomass, and finally, discuss potential biomass uses for commercial and energy purposes.
Material and Methods
Microalgae Strain
The Arthrospira platensis DHR 20 microalgae used herein were obtained from the Federal Rural University of Rio de Janeiro (UFRRJ) Fermentation Laboratory culture bank, Seropédica campus, RJ, Brazil. Pre-cultivation was performed in a Zarrouk medium in 1-L flasks, illuminated with 150 μmol m−2 s−2 by means of fluorescent white lamps (18 W), at 24.1 °C (± 1.2). Agitation was performed by injecting air from the atmosphere to the bottom of the flasks using an air compressor at a 0.5 L min−1 flow rate. The biomass concentration obtained during the pre-cultivation stage was 0.88 g L−1 (± 0.15), used for the inoculation of horizontal photobioreactors (HPBRs). All procedures were performed according to Mendonça et al. [5].
Wastewater Used as a Culture Medium
The CWW was generated at the experimental UFRRJ “Fazendinha Agroecológica” farm, Seropédica campus, RJ, Brazil (coordinates: 22° 45′ 21″ S; 43° 40′ 28″ W). Cattle in this area are raised in confinement and fed only organic food items, produced on the farm itself, without the use of agrochemicals. Prior to collection, the CWW underwent preliminary treatment in a solid-liquid separator (decanter) and primary anaerobic treatment in a UASB reactor, operated with a hydraulic retention time (HRT) of 7 days. The physicochemical characterization of the anaerobically digested cattle wastewater (ACWW) is presented in Table 1. All analyses were performed according to Standard Methods [17].
Horizontal Photobioreactors (HPBRs) and Experimental Setup
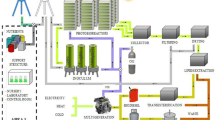
Two identical bench-scale HPBRs, with a usable volume of 7.5 L and usable surface area of 0.08721 m2 (Fig. 1), were used to cultivate the Arthrospira platensis DHR 20 microalgae in ACWW, displaying the characteristics presented in Table 1.
To promote a complete mix in the reactors and prevent self-shading during cultivation, atmospheric air was injected to the bottom of the HPBRs through two fine bubble diffusers (20-μm pore size). Both reactors were operated in batches. Air from the local atmosphere was pumped to the diffusers by a 4-W air pump (Aleas, AP-9804 model, China) at a 1.5-L min-1 flow rate and pressure of 0.002 MPa. The local atmosphere CO2 concentration was 0.0401% (± 0.0001), determined by gas chromatography. The air volume per culture volume of per minute (VVM) was 0.40. To avoid HPBR surface foam formation, 10 mL (10%) of a silicone-based defoamer were added daily [1].
Illumination was maintained constant (24 h day−1 photoperiod) at 265 μmol m−2 s−1, measured using a Lux-Meter-Phywe, Germany. The lamps were positioned horizontally 10 cm above each HPBR (Fig. 1).
One HPBR was operated under two different room temperature ranges, termed R1. The mean fluid temperature was 30 °C (± 2.6 °C) in experiment 1 and 25 °C (± 1.9 °C) in experiment 2. When conducting both experiments, one HPBR (R2) was operated in parallel with heating heated to 35 °C (± 1 °C), considered ideal for the cultivation of the studied species [18]. In both experiments, R2 was considered the control. Both R2 heating and temperature were controlled by a thermostat (Hopar, H386-75 W model, China) directly immersed in the fluid (ACWW + microalgae). To ensure data stability and reliability, each experiment was repeated four times, always in pairs (Fig. 1). All data were collected in triplicate.
Batch Experiment
The growth curves of Arthrospira platensis in ACWW were determined as a function of dry biomass and optical density determined at 670 nm on a 1105 Bel Photonics spectrophotometer (Italy), in triplicates. The time between the reactor inoculation and biomass harvest was defined by culture growth stabilization (steady state).
After measuring the dry biomass in an oven (105 °C), a linear correlation was performed between the dry biomass-Y (g L−1) and optical density (OD670). The biomass calibration equations for experiments 1 (at 30 °C ± 2.6) and 2 (at 25 °C ± 1.9) were YR1 (g L−1) = 1.4932 (OD670) + 3.8299 and YR1 (g L−1) = 1.164 (OD670) + 3.3488, with correlation coefficients of R2 = 0.93 and R2 = 0.95, respectively. The calibration equation determined for R2 (control at 35 °C ± 1) was YR2 (g L−1) = 4.8139 (OD670) − 1.2045, with a correlation coefficient of R2 = 0.97.
Biomass production per area (Pa) was calculated using Eq. 1.
where Pv = volumetric biomass production.
The expected raw oil (total lipid) yield L ha−2year−1 (BP) was calculated using Eq. 2.
CO2 Bio-Fixation
The CO2 bio-fixation rate (RCO2) was calculated through the relationship between biomass productivity and carbon concentration (C%) (Eq. 3). Carbon (C) biomass concentrations were determined by an elemental analysis (Elementar Vario EL III, German).
where P = biomass productivity, mg L−1 day−1; C = carbon concentration in biomass, g g−1; MCO2 = molar mass of CO2, g mol−1; MC = molar mass of carbon, g mol−1.
All procedures were performed according to Duarte et al. [13] and Mendonça et al. [5].
Analytical Methods
Chemical oxygen demand (COD), biochemical oxygen demand (BOD5), total organic carbon (TOC), total solids (TS), total suspended solids (TSS), volatile suspended solids (VSS), ashes (fixed solids - FS), ammoniacal nitrogen (NH4+), total Kjeldahl nitrogen (TKN), pH, electrical conductivity (EC), total phosphorus (Pt), nitrate (NO3-), potassium (K+), copper (Cu), zinc (Zn), calcium (Ca+2), magnesium (Mg+2), sodium (Na+), sulfate (SO42-), and coliforms were determined in triplicate according to standard methods [17].
The treated ACWW biomass was separated using a 0.045-mm-mesh sieve (Granutest, Brazil) and freeze-dried using a Liotop L 101 lyophilizer connected to a pump (Vacuum Technology SRL, Bologna, Italy). After freeze-drying, the biomass was sprayed in a mill (Shymsen, IKA A11 basic, Germany). Subsequently, protein concentrations were quantified by the Kjeldahl method [17]. Carbohydrates were determined according to Dubois et al. [19]. Lipids were quantified by Soxhlet extraction using hexane (130 mL) and ethanol (130 mL), in round-bottom distillation flasks, with a solubilization period for 6 h with hexane and 3 h with ethanol, using the same biomass-containing cartridge. After each extraction, the solvent was evaporated using a rotary evaporator (Buchi Waterbath B-480, Germany) with a thermostatically controlled bath at 50 °C. The pressures used for hexane and ethanol were 500 mbar and 350 mbar, respectively [5].
The local atmosphere CO2 concentration was determined by gas chromatography using a Varian 430-GC cromatograph equipped with a thermal conductivity detector and a Varian Capillary Column SelectTM Permanent Gases/CO2 HR - Malsieve 5 A Parabond Q Tandem #CP7430 column. Helium gas was used as the drag gas (52 mL mn−1). A 0.5-mL air injection for all 18 analyzed samples was used in the chromatograph.
Statistical Analyses
The experimental results were evaluated by growth curve comparisons, and Tukey test was applied to kinetic parameters, biomass production, and pollutant removal. Significant values were obtained when p ≤ 0.05. Prior to performing the parametric tests, the data normality was confirmed by the Shapiro–Wilk test using the PAST software.
Results and Discussion
Specific Growth Rate and Doubling Time
The maximum specific rate growth (μmax) and doubling time (Td) observed in experiment 1 for reactors R1 and R2 were 0.41 day−1 and 1.67 day and 0.20 day−1 and 3.39 day, respectively. In experiment 2, the values for R1 and R2 were 0.27 day−1 and 2.48 day and 0.22 day−1 and 3.22 day, respectively. It is interesting to note that there was little difference in μmax and Td values during the experiments for the heated reactor (R2). In general, the R2 reactor exhibited higher Td compared to the experiments conducted at room temperature. Td was lower at 30 °C in the reactor operated at room temperature (R1), indicating higher culture growth speed at this temperature.
Regarding Td and μmax, only the biomass produced in R1 at 30 °C displayed a significant difference (p ≤ 0.05) in comparison to the same biomass grown at 25 and 35 °C. This indicates that the investigated Arthrospira platensis DHR 20 strain grows favorably under the established R1 conditions of experiment 1. This is an important result, as the ideal temperature for cultivating this species ranged from 34 to 35 °C.
De Mendonça et al. [5] recorded μmax and Td of 0.27 day−1 and 2.5 day under CO2 addition conditions and 0.15 day−1 and 4.7 day without f CO2 addition when cultivating Scenedesmus obliquus in ACWW from a UASB-AF reactor. The best results found by these authors under CO2 addition conditions were very close to those observed in experiment 2 of the present study. During experiment 1 (in R1), the Td for Arthrospira platensis was lower than that reported for Scenedesmus obliquus described by the aforementioned authors.
Zhu et al. [20] cultivated the microalga Chlorella sp. in livestock waste diluted and then filtered in paper filter (to remove non-soluble particles) and recorded μmax between 0.275 and 0.375 day−1 and Td between 2.52 and 2.85 days, values close to those of the present study.
Kim and Kim [6] cultivated Chlorella emersonii in CWW from a tertiary treatment mixed with BG-11 culture medium and obtained μmax = 0.61 day−1 and Td = 1.2 days. The growth rate and doubling time in the present study that most approached the values reported by these authors are those obtained in experiment 1 (R1).
Cardoso et al. [21] cultivated Spirulina sp. in aquaculture wastewater (AWW) and recorded specific growth rates ranging from 0.18 day−1 (raw AWW) to 0.47 day−1 (25% AWW + 75% of Zarrouk medium). The values obtained in the present study were higher than the μmax obtained by the authors for raw AWW and lower than the AWW μmax with the addition of 75% Zarrouk medium.
Based on the results of the aforementioned studies, Arthrospira platensis growth is comparable to those of other microalgae that have already been successfully cultivated in wastewater, especially from cattle farming.
In general terms, Arthrospira platensis (DHR 20) displayed satisfactory growth in ACWW. In comparison to other studies in CWW using other species, Arthrospira platensis was able to adapt and grow satisfactorily in these liquid wastes without the need for either water dilution or the addition of synthetic culture media. This is valuable information, as the wastewater proposed herein can serve as an alternative culture medium for biomass growth and production regarding the studied strain.
Biomass Concentration and Volumetric Productivity
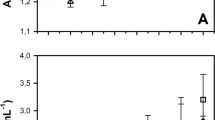
High dry biomass concentrations were achieved (Fig. 2a, b). Maximum concentrations of 7.15 g L−1 and 6.30 g L−1 were recorded in R1, while maximum productivities of 6.55 to 6.6 g L−1 were recorded in R2.
In all experiments, the maximum dry biomass value was reached on the 7th experimental day, except in experiment 1 in R1, where the maximum value was recorded on the 6th day, when Td was also lower. In general terms, the maximum productivity recorded in the present study (7.14 g L−1) was obtained in R1 at an average room temperature of 30 °C. Considering the maximum concentration obtained in R2 heated at 35 °C (6.6 g L−1), 0.54 g L−1 more biomass was produced by the HPBR operated at room temperature (R1) during experiment 1. This indicates that the ideal temperature for this species when cultivated in ACWW is of 30 °C, with an acceptable standard deviation of up to ± 2.6 °C. However, regardless of temperature, the dry biomass concentrations were very similar at the end of each experiment (Fig. 2).
Therefore, under the cultivation conditions proposed herein, high Arthrospira platensis biomass concentrations can be obtained without the need for HPBR heating, which is positive, as no heating systems are required, reducing production costs.
Dry biomass values close to those recorded in the present study were also reported by Hena et al. [22], who used treated dairy farm wastewater as a culture medium (Table 2) and Arthrospira platensis in PBR with an illumination of 300 μmol m−2 s−1.
The maximum volumetric biomass productivities were recorded in experiment 1—R1 (0.664 g L−1 day−1). The second highest productivity was recorded in experiment 2—R2 (0.610 g L−1 day−1). These productivities can be considered high compared to values reported in other studies conducted with agro-industrial wastewater. Values closer to those observed in the present study were obtained with the microalgae Arthrospira platensis (Spirulina platensis), Chroococcus sp. (cyanobacteria), and the green microalgae Chlorella emersonii and Chlorella vulgaris (Table 2).
Zhu et al. [20] obtained a dry biomass concentration of 2.9 g L−1 cultivating Chlorella sp. in diluted and filtered livestock waste. Zhou et al. [28] obtained a dry biomass of 0.81 g L−1 when cultivating Spirulina platensis in saline wastewater. Almomani et al. [27] cultivated Arthrospira platensis in flasks containing sewage treated in a septic tank, obtaining 0.246 g L−1 of dry biomass. The lower biomass concentrations detected in these three studies are attributed to lower NH4+ and P concentrations in the wastewaters used as the culture media compared to the present study.
Yu and Kim [8] recorded 2.6 g L−1 of dry biomass when cultivating Botryococcus braunii in continuous mode in SBR reactor. In the present study, higher values were recorded due to higher nutrient concentrations and illumination rates (Table 2). Furthermore, higher biomass concentrations can be obtained in batch operating modes compared to continuous modes [5].
Hena et al. [23] cultivated a microalgae mix (Chlorella accharophila, Chlamydomonas pseudococcum, Scenedesmus sp., and Neochloris oleoabundans) in CWW treated by activated sludge system and obtained a biomass concentration of 3.02 g L−1.
De Mendonça et al. [5] cultivated the microalga Scenedesmus obliquus in CWW after anaerobic digestion in a hybrid reactor and achieved a volumetric productivity of 0.213–0.358 g L−1 day−1 and maximum dry biomass of 3.7 g L−1. Although Scenedesmus obliquus exhibited exceptional adaptability and growth in ACWW with pollutant and nutrient concentrations similar to those of the present study, the microalga Arthrospira platensis displayed better performance concerning biomass production.
In addition, Arthrospira platensis (present study) achieved a higher volumetric biomass productivity (0.664 g L−1 day−1) when compared to other cyanobacteria, such as Chroococcus sp. (0.558 g L−1 day−1) [24]. These authors obtained a relevant productivity when cultivating this cyanobacterium in CWW without primary treatment. The results reported in the present compared to other studies presented herein indicate that ACWW is an adequate culture medium, as the anaerobic digestion process preserves the nutrients in the solution while at the same time clarifying the effluent, allowing for more light to enter the system. Thus, the CWW pre-treatment in the UASB reactor may have favored the significant dry biomass concentrations and volumetric productivities observed herein.
No significant differences (p ≥ 0.05) between the experiments and reactors in terms of biomass concentration or volumetric productivity were observed.
The productivity per area (Pa) observed in the present study was relevant compared to synthetic culture media (Table 3). The R1 value (experiment 1) indicates a Pa 2.55-fold higher than that obtained in Zarrouk medium, the traditional Arthrospira culture medium. In this case, the Pa was higher than the synthetic media values reported in the literature in all experiments.
The average productivity values per area were not significantly different (p ≥ 0.05) between reactors in all tests.
CO2 Bio-Fixation Rate
The CO2 bio-fixation and carbon percentages contained in the obtained biomass are presented in Table 4.
Comparing the carbon (C) percentages between the experiments and reactors, concentrations were very similar and lower than the typical concentration detected in microalgae, of 50% [13]. Lower C concentrations in cells can be attributed to two factors. The first is the fact that Arthrospira platensis is an efficient protein accumulator with lower cellular C concentrations, especially when exposed to high substrate N concentrations, as in the present study. This was also reported by de Mendonça et al. [5], who increased N culture supply by changing the operating mode from batches to continuous, with C concentration depletion from 43.9 to 35.7%. Another factor is associated to the low concentration of this element comprises the low CO2 supply in the present study, from only a local atmosphere air application, without any additional CO2 source.
Almomani et al. [27] recorded C concentrations between 46.5 and 55.5% in an Arthrospira platensis biomass with the addition of 10% CO2 to the air injected to flasks containing sewage treated in a septic tank as a culture medium.
High bio-fixation values were observed in all experiments, especially in R1 in experiment 1 (1,051 mg L−1 day−1). These significant values result from the high biomass productivity achieved during this experimental stage. Although HPBRs are not as efficient as airlift PBR, high bio-fixation rates were still possible.
A study conducted with Spirulina sp. LEB 18 cultivated in Zarrouk medium (inorganic) recorded CO2 fixation rates ranging from 165 to 183 mg L−1 day−1 in tubular PBRs and from 110 to 123.8 mg L−1 day−1 in raceway bioreactors [13].
De Mendonça et al. [5] recorded a maximum fixation rate of 547 mg CO2 L−1 day−1 in a Scenedesmus obliquus culture in PBRs with ACWW from a hybrid reactor (UASB-AF) substrate. The bio-fixation rates of the present study were approximately twofold higher than those reported in that study. Thus, Arthrospira platensis exhibits considerable potential to mitigate atmospheric CO2.
In another study carried out with an organic substrate (sewage treated in a septic tank), Almomani et al. [27] recorded a bio-fixation rate of 378 mg L−1 day−1, almost threefold lower than that observed in R1 in experiment 1. Although the authors used the same microalgae species as the present study, volumetric productivities were fourfold lower, explaining the lower bio-fixation rates reported.
The relevant biomass production and CO2 bio-fixation values were also associated with the operational characteristics of the HPBRs, indicating that the adopted agitation and illumination conditions were appropriate for the efficient cultivation of the studied microalgae. According to Mata et al. [34] and Duarte et al. [13], the photobioreactor is key to achieving carbon fixation efficiency, and Ouyang et al. [35] emphasize that, for successful CO2 fixation, adequate illumination is required in the performed experiments. These requirements were met in the present study.
Finally, it is important to note that the bio-fixation rates reported herein are those that would be obtained if all carbon were assimilated by exclusively photoautotrophic nutrition and do not take into account the fraction assimilated from organic carbon (via mixotrophy).
ACWW Bioremediation
The pH values were maintained between 8.5 and 9 during the experiments (Supplementary material). During cultivation, the pH remained basic, a favorable condition for Spirulina platensis growth, which can survive in environments with pH of up to 11 [36].
The obtained NH4+ removal was satisfactory, reaching 98% and 98.6% in R1 in experiment 1 (6 days) and experiment 2 (7 days), respectively. Regarding the heated reactors (R2), removal reached 100% in both experiments at 7 days of operation. Although NH4+ was completely removed in the heated HPBRs, the final concentrations after treatments in the HPBRs operated at room temperature were very low, of 5–7.3 mg L1 (Supplementary material). In Brazil, for example, the maximum NH4+ limit for treated effluent disposal into watercourses is of 20 mg L−1. In this case, R1 reactors would result in the safe disposal of the treated CWW into watercourses at concentrations three- to fourfold lower than those allowed by the Brazilian legislation.
Lv et al. [7] recorded NH4+ removals ranging from 83.16 to 94.27% in a CWW treatment with the microalgae C. vulgaris during 5 days of experiment, close to the values observed in the present study. Hena et al. [23] reported an NH4+ removal of 100% from CWW, as in the present experiment (in R2), but after 10 days of cultivation (Table 5).
Pt removals were higher than 87% in all experimental conditions, reaching a maximum value of 92.4% in R1 (experiment 1). Markou et al. [26] cultivated Arthrospira platensis in the wastewater from an olive oil factory and were able to obtain 100% phosphorus removal after 16 days of cultivation, a further 9 days compared to the present study.
In general, microalgae play a key role in the bioremediation of wastewater containing nutrients and organic matter (Fig. 3).
Regarding NO3− removal, a maximum value of 95.5% was obtained in R2 of experiment 2, and no difference was observed compared to its removal among the other treatments (Fig. 3). Nayak et al. [39] recorded a maximum NO3− removal of 70.2% when cultivating the microalgae Scenedesmus sp. in sewage, slightly lower than that observed in the present study.
Concerning SO42−, removal values above 80% were verified in all experiments (Fig. 3). This indicates that the studied microalgae is able to result in relevant removal values of this anion. Sulfate (SO42−) removal has received significant attention in recent years due to its water resource polluting potential, which can pose environmental degradation risks for both ecosystems and human health. In the present experiment, sulfate values after the HPBR treatment ranged between 3 and 14 mg L−1 (Supplementary material). Both the reactors operated at room temperature (R1) and heated (R2) were able to efficiently remove this molecule.
Molecules containing sulfur (S) participate in the formation of amino acids essential to cell energy metabolism. Sulfur and nitrogen are a relevant part of protein composition, abundant macromolecules in Arthrospira platensis biomass, which explain the high SO42− removal observed herein.
The removals obtained in the present study ranged between 61 and 77% for K+, 66 and 75.6% for Ca2+, 76 and 81% for Mg+2, and 34.2 and 46.5% for Na+. The final concentrations of all analyzed macronutrients are reported in the Supplementary material.
The micronutrients Zn and Cu, despite being present at low concentrations in the ACWW (Table 1), were satisfactorily assimilated by the investigated microalgae. Removal efficiencies above 98% and 94% were noted for Zn and Cu, respectively. Cu plays a key role in photosynthesis and an important role in nitrogen fixation, while Zn is an enzymatic activator and growth promoter. The presence of both is crucial for the development not only of terrestrial crops, but also of microalgae.
In terms of organic matter, COD removals for reactors R1 and R2 were 59.6 and 72.3% in experiment 1 and 63.3 and 73.6% in experiment 2, respectively. In parallel, BOD5 removals were equal to 75.3 and 82% in reactors R1 and R2 of experiment 1 and to 78.7 and 82.6% in reactors R1 and R2 of experiment 2 (Fig. 3 and Table 5). Thus, COD and BOD5 removals were higher in the HPBRs with heating. TOC removals between 59.3 and 73.3 were obtained. A comparison of COD and TOC removals with those obtained in other studies is displayed in Table 5. Figure 3a and b display the efficiencies of organic matter, macro and micronutrient, solids, and coliform removals from the ACWW at the end of the experiments.
The total and thermotolerant coliform removals were 99–99.7% and 99.7–99.9%, respectively (Fig. 3). Gupta et al. [40] reported total coliform removals between 99.93 and 99.97% from sanitary sewage when cultivating the microalgae Scenedesmus obliquus. Therefore, relevant microorganism reductions can be achieved during microalgae cultivation. The elimination of bacteria belonging to the coliform group may be associated to the fact that several metabolites displaying bactericidal activities are excreted from these microalgae [41].
Values higher than 77 and 88% were obtained for average TS and VS removals in the experiments. In the heated reactors (R2), solid removals were higher (Fig. 3). In the same figure, TSS and VSS removals greater than 80% are observed in both experiments, indicating that the adopted filtration mechanism was efficient to reduce the solid loads contained in the wastewater along with the produced biomass. Fine-mesh filtration (0.045-mm-mesh sieve) is considered efficient for separation of Scenedesmus obliquus biomasses, while also not requiring the use of electricity as in the case of centrifuge separation, leading to production process savings.
Although the experiments conducted with heating at 35 °C led to greater organic pollutant and nutrient removals, no significant difference (p ≥ 0.05) between the reactors operated at room temperature was observed.
Macromolecular Composition of Biomass and Bioproducts
In terms of macromolecule production, no significant differences (p ≥ 0.05) between the experiments or reactors were verified. Concerning macromolecular composition, protein concentrations were the most abundant, as expected. The maximum value was recorded in R1, of 45% (Fig. 4a).
Ogbonda et al. [42] studied the relationship between temperature and both biomass production and amino acid biosynthesis in Spirulina sp. and concluded that the highest amounts of proteins were obtained at 30 °C, corroborating the results reported in the present study. Biomasses with significant protein concentrations can be used for animal feeding as a complementary protein source or for agricultural use as nitrogen biofertilizers.
Morais et al. [43] cultivated Spirulina sp. at different glycerol concentrations and obtained a protein percentage of 69.78% (0.05 mol L−1 of glycerol), carbohydrate percentage of 12.41% (0.01 mol L−1 of glycerol), and lipid percentage of 13.34% (0.01 mol L−1 of glycerol).
Ash concentrations (fixed solids) were above 5% in experiment 1 and below 5% in experiment 2, without great differences (Fig. 4a, b).
When heated, the HPBRs (R2) enabled the production of higher carbohydrate concentrations by the microalgae. The maximum obtained value was of 24% (Fig. 4a). The obtained carbohydrate values are considered relevant and are associated with two factors: (1) The adoption of constant illumination (24 h photoperiod, at 265 μmol m−2 s−1), as increased lighting hours is a driving force that intensified carbohydrate synthesis [44]; (2) The high CO2 bio-fixation rates, along with the species’ ability to perform mixotrophy, resulting in carbohydrate production. Biomasses containing relevant concentrations of this macromolecule can be used in the production of biofuels such as biobutanol, bioethanol, biohydrogen, and methane/biomethane [45]. Furthermore, according to Molinuevo-Salces et al. [46], higher carbohydrate accumulations seem to be achieved in batch operations, the operating mode adopted in the present study. As the batch process with wastewater progresses, nitrogen compound concentrations decrease (and are no longer replaced), approaching zero in the last days, when the culture begins a more intense lipid accumulation process. Microalgae tend to accumulate lipids under stress conditions, such as nutrient limitations. Lipid microalgae accumulation depends on several conditions, such as lack of nitrogen and phosphate, high salinity, light intensity, and temperature, in addition to the carbon source concentration [47].
Lipid concentrations were higher in the unheated reactors (R1) in both experiments, with maximum values of 16.3% in experiment 1 and 15.7% in experiment 2 (Fig. 4). These values are considered high for this species, since the literature reports lipid concentrations ranging from 4 to 16.6% in Arthrospira platensis [34].
De Jesus et al. [48] cultivated Arthrospira platensis outdoors in both northern and southern Brazil in Zarrouk synthetic medium and obtained a maximum lipid percentage of 12%, close to that of the experiment conducted herein.
In this context, ACWW was proven a valuable culture medium to maximize lipid production in S. platensis. This points to the potential use of this species in biodiesel production, which is often neglected by the scientific community. The high dry biomass production of Arthrospira platensis (6.3 to 7.15 g L−1 day−1), when grown in ACWW compensates its lower lipid concentrations when compared to other species. For example, Chlorella sp., a microalgae exhibiting potential for biodiesel production, can reach lipid concentrations between 10 and 48% of its biomass, but reaching productivity values between 0.02 and 2.5 g L−1 day−1 [34]. Comparing the data obtained by the aforementioned authors with those of the present study, Chlorella sp. reaches an average lipid productivity of 0.0421 g L−1 day−1, whereas Arthrospira platensis DHR 20 reaches productivities between 0.061 and 0.108 g L−1 day−1 (Table 5). On the other hand, Kuo et al. [49] cultivated Chlorella sp. in swine wastewater and recorded a volumetric lipid productivity of 0.155 g L−1 day−1, close to that obtained in R1 (experiment 1).
De Mendonça et al. [5] cultivated Scenedesmus obliquus in PBR with ACWW from a hybrid reactor as a culture medium and recorded a maximum dry biomass lipid accumulation of 29%, while the maximum volumetric productivity was 0.064 g L−1 day−1, 1.7-fold lower than that reported in the present study. Kumar et al. [50] cultivated Ascochloris sp. in dairy wastewater and obtained a lipid productivity of 0.094 g L−1 day−1, close to the maximum detected in the present study.
Another hypothesis to justify a higher lipid production is that the biomasses in microalgal-based wastewater treatment comprise a mixture of algae, bacteria, zooplankton, and detritus (ALBAZOD). In this case, part of the assessed biomass was probably not composed of Arthrospira platensis DHR 20, contributing to a higher lipid content.
Therefore, when grown in ACWW (after a UASB reactor treatment) under the conditions proposed in the present study, Arthrospira platensis DHR 20 exhibits the potential for significant lipid production, and low cellular lipid concentrations are compensated by high biomass productivity.
Lipid productivities per area between 5.24 and 9.29 g m−2 day−1 were observed herein (Table 6). These values allowed for the calculation of an expected total crude oil production from biomass per hectare between 22,257 and 39,446 L ha−1 year−1 (Table 6). This is considered promising, especially compared to terrestrial plant oil sources, like sunflower (1,190 L ha−1 year−1), canola (1,892 L ha−1 year−1), coconut (2,689 L ha−1 year−1), and oil palm (12,000 L ha−1 year−1) [34, 45].
Based on the observed productivities, it would be possible to obtain 7.864 to 9.900 gallons ha−1 year−1 of total lipids (Table 6). As a realistic projection, considering that 68% of total lipids are saponifiable [51] and considering 2% transesterification process losses [45], about 5.201 to 6.540 gallons per hectare per year of biodiesel can be produced via Arthrospira platensis DHR 20 biomasses.
It is a noteworthy that, as certain essential amino acids, some lipids contained in Arthrospira platensis biomasses are also essential, including a-linolenic acid and linoleic acid, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), omega-3, omega-6, and other long-chain essential polyunsaturated fatty acids [52, 53]. The potential use of microalgae as a lipid supplement in the feeding of lactating cows was assessed by Stamey et al. [54], who reported fatty acid profile changes in milk, especially due to increased omega-3 contents, indicating that the lipid traces contained in microalgae are also beneficial when used in ruminant feeding. This is a valuable feature, as the proximity of the microalgae production units to intensive cattle production facilities leads to transportation and logistics savings and ease in obtaining effluents for cultivation. In addition, the biogas generated during the primary wastewater treatment process (by anaerobic digestion) can be converted into energy for biomass drying and separation without additional electricity costs.
The carbohydrate and protein productivities (volumetric and per area) contained in the dry Arthrospira platensis biomass are presented in Table 7.
Carbohydrate productivities were similar in all experiments, and the lowest value was obtained in the R1 cultivation in experiment 2 (Table 7).
The highest productivity macromolecular compounds were proteins. In terms of volumetric productivity, values between 0.232 and 0.299 g L−1 day−1 indicate potential dry biomass use as a cattle protein supplement, for example. Production values per area reached up to 25.75 g m−2 day−1 (Table 7).
Despite the operational simplicity of the HPBRs used in the present study, they were efficient for Arthrospira platensis cultivation, converting potential environmental threats into an opportunity to obtain high economic value bioproducts.
Finally, the use of microalgae biomass grown in anaerobic cattle wastewater will soon become a valuable animal feed source and biofertilizer production, as well as biofuel, especially biodiesel production. It is important to highlight that, with the use of ACWW as a culture medium, about 35% of biomass production costs can be saved [5]. It is also important to point out that, due to the presence of various bioactive compounds contained in Arthrospira platensis biomasses , further studies are required concerning medicinal purposes to combat and prevent autoimmune, degenerative, and infectious diseases caused by viruses, parasitic bacteria or fungi, such as lupus, Alzheimer’s disease, HIV/AIDS, polio, influenza, Zika virus, malaria, Ebola virus [55], and COVID-19. These authors suggest that further research should be carried out to improve both upstream and downstream processes to produce bioactive compounds from microalgae biomasses and their conversion for low-cost commercialization in different industrial and agricultural sectors mainly using wastewater as culture medium.
Conclusions
Arthrospira platensis (DHR 20) cultivation in HPBR displays high potential for dry biomass production, with the highest value obtained at 30 °C. Relevant protein, carbohydrate, and lipid concentrations were recorded, displaying the high potential of this species to produce macromolecules exhibiting relevant economic value. Heating the HPBRs did not significantly alter protein, carbohydrate, and lipid production, but the doubling time was shorter at 30 °C, indicating a higher culture growth rate. High CO2 bio-fixation rates were observed, indicating a relevant potential of the studied microalgae in mitigating air pollution. Macro- and micronutrients contained in the ACWW were satisfactorily assimilated by the assessed microalgae, resulting in improved and intensified biomass production. An intensive biomass production lead to considerable thermotolerant and total coliforms reductions. Finally, the macromolecular Arthrospira platensis composition displays the potential to produce important bioproducts such as biodiesel, bioethanol, nitrogen biofertilizers, and animal feed, displaying environmental and economic importance, reducing the pressure on raw materials and contributing to an increasingly green and circular bioeconomy.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
de Souza DS, Maciel AM, Otenio MH, de Mendonça HV (2020) Optimization of ozone application in post-treatment of cattle wastewater from organic farms. Water Air Soil Pollut 231:362. https://doi.org/10.1007/s11270-020-04736-2
de Mendonça HV, Ometto JPHB, Otenio MH (2017) Production of energy and biofertilizer from cattle wastewater in farms with intensive cattle breeding. Water Air Soil Pollut 228:72. https://doi.org/10.1007/s11270-017-3264-1
de Mendonça HV, Ometto JPHB, Otenio MH, dos Reis AJD, Marques IPR (2017) Bioenergy recovery from cattle wastewater in an UASB-AF hybrid reactor. Water Sci Technol 76:2268–2279. https://doi.org/10.2166/wst.2017.325
Cheng DL, Ngo HH, Guo WS, Chang SW, Nguyen DD, Kumar SM (2019) Microalgae biomass from swine wastewater and its conversion to bioenergy. Bioresour Technol 275:109–122. https://doi.org/10.1016/j.biortech.2018.12.019
de Mendonça HV, Ometto JPHB, Otenio MH, Marques IPR, dos Reis AJD (2018) Microalgae-mediated bioremediation and valorization of cattle wastewater previously digested in a hybrid anaerobic reactor using a photobioreactor: comparison between batch and continuous operation. Sci Total Environ 633:1–11. https://doi.org/10.1016/j.scitotenv.2018.03.157
Kim JY, Kim HW (2017) Photoautotrophic microalgae screening for tertiary treatment of livestock wastewater and bioresource recovery. Water 9:192. https://doi.org/10.3390/w9030192
Lv J, Liu Y, Feng J, Liu Q, Nan F, Xie S (2018) Nutrients removal from undiluted cattle farm wastewater by the two-stage process of microalgae-based wastewater treatment. Bioresour Technol 264:311–318. https://doi.org/10.1016/j.biortech.2018.05.085
Yu JU, Kim HW (2017) Enhanced microalgal growth and effluent quality in tertiary treatment of livestock wastewater using a sequencing batch reactor. Water Air Soil Pollut 228:357. https://doi.org/10.1007/s11270-017-3547-6
Sun N, Wang Y, Li Y et al (2008) Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta). Process Biochem 43:1288–1292. https://doi.org/10.1016/j.procbio.2008.07.014
Zhai J, Li X, Li W, Rahaman MH, Zhao Y, Wei B, Wei H (2017) Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol Eng 108:83–92. https://doi.org/10.1016/j.ecoleng.2017.07.023
Mondal M, Goswami S, Ghosh A, Oinam G, Tiwari ON, Das P, Gayen K, Mandal MK, Halder GN (2017) Production of biodiesel from microalgae through biological carbon capture: a review. 3Biotech 7:99. https://doi.org/10.1007/s13205-017-0727-4
International Energy Agency (2020) Data and statistics. https://www.iea.org/dataand-statistics/?country¼WORLD&fuel¼Energy_supply& indicator¼TPESbySource. Accessed 8 Oct 2020
Couto E, Calijuri ML, Assemany P (2020) Biomass production in high rate ponds and hydrothermal liquefaction: Wastewater treatment and bioenergy integration. Sci Total Environ 724:138104. https://doi.org/10.1016/j.scitotenv.2020.138104
Chisti Y (2013) Constraints to commercialization of algal fuels. J Biotechnol 167:1–14. https://doi.org/10.1016/j.jbiotec.2013.07.020
Savage PE, Hestekin JA (2013) A perspective on algae, the environment, and energy. Environ Prog Sustain Energy 33:877–883. https://doi.org/10.1002/ep.11847
de Mendonça HV, Assemany P, Abreu M et al (2020) Microalgae in a global world: new solutions for old problems? Renew Energy 165:842–562. https://doi.org/10.1016/j.renene.2020.11.014
American Public Health Association (2012) Standard methods for the examination of water and wastewater. APHA, Washington
Kumar M, Kulshreshtha J, Singh GP (2011) Growth and biopigment accumulation of cyanobacterium Spirulina Platensis at different light intensities an temperature. Braz J Microbiol 42:1128–1135
Dubois M, Gilles KA, Hamilton PA et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Zhu LD, Li ZH, Guo DB, Huang F, Nugroho Y, Xia K (2016) Cultivation of Chlorella sp. with livestock waste compost for lipid production. Bioresour Technol 223:296–300. https://doi.org/10.1016/j.biortech.2016.09.094
Cardoso LG, Duarte JH, Andrade BB, Lemos PVF, Costa JAV, Druzian JI, Chinalia FA (2020) Spirulina sp. LEB 18 cultivation in outdoor pilot scale using aquaculture wastewater: High biomass, carotenoid, lipid and carbohydrate production. Aquaculture 525:735272. https://doi.org/10.1016/j.aquaculture.2020.735272
Hena S, Znad H, Heong KT, Judd S (2017) Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res 128:267–277. https://doi.org/10.1016/j.watres.2017.10.057
Hena S, Fatimah S, Tabassum S (2015) Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour Ind 10:1–14. https://doi.org/10.1016/j.wri.2015.02.002
Prajapati SK, Choudhary P, Malik A, Vijay VK (2014) Algae mediated treatment and bioenergy generation process for handling liquid and solid waste from dairy cattle farm. Bioresour Technol 167:260–268. https://doi.org/10.1016/j.biortech.2014.06.038
Qin L, Shu Q, Wang Z, Shang C, Zhu S, Xu J, Li R, Zhu L, Yuan Z (2014) Cultivation of chlorella vulgaris in dairy wastewater pretreated by UV irradiation and sodium hypochlorite. Appl Biochem Biotechnol 172:1121–1130. https://doi.org/10.1007/s12010-013-0576-5
Markou G, Chatzipavlidis I, Georgakakis D (2012) Cultivation of Arthrospira (Spirulina) platensis in olive-oil mill wastewater treated with sodium hypochlorite. Bioresour Technol 112:234–241. https://doi.org/10.1016/j.biortech.2012.02.098
Almomani F, Judd S, Bhosale RR, Shurair M, Aljaml K, Khraisheh M (2019) Intergraded wastewater treatment and carbon bio-fixation from flue gases using Spirulina platensis and mixed algal culture. Process Saf Environ Prot 124:240–250. https://doi.org/10.1016/j.psep.2019.02.009
Zhou W, Li Y, Gao Y, Zhao H (2017) Nutrients removal and recovery from saline wastewater by Spirulina platensis. Bioresour Technol 245:10–17. https://doi.org/10.1016/j.biortech.2017.08.160
Grobbelaar JU (2009) From laboratory to commercial production: a case study of a spirulina (arthrospira) facility in Musina, South Africa. J Appl Phycol 21:523–527. https://doi.org/10.1007/s10811-008-9378-5
Morais MG, Radmann EM, Andrade MR, Teixeira GG, Brusch LRF, Costa JAV (2009) Pilot scale semicontinuous production of Spirulina biomass in southern Brazil. Aquaculture 294:60–64. https://doi.org/10.1016/j.aquaculture.2009.05.009
Vonshak A, Laorawat S, Bunnag B, Tanticharoen M (2014) The effect of light availability on the photosynthetic activity and productivity of outdoor cultures of Arthrospira platensis (Spirulina). J Appl Phycol 26:1309–1315. https://doi.org/10.1007/s10811-013-0133-1
Toyoshima M, Aikawa S, Yamagishi T, Kondo A, Kawai H (2015) A pilot-scale floating closed culture system for the multicellular cyanobacterium Arthrospira platensis NIES-39. J Appl Phycol 27:2191–2202. https://doi.org/10.1007/s10811-014-0484-2
Matos ÂP, da Silva T, Sant’Anna ES (2020) The feasibility of using inland desalination concentrate (DC) as an alternative substrate for Spirulina platensis mass cultivation. Waste Biomass Valorization. https://doi.org/10.1007/s12649-020-01233-9
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232. https://doi.org/10.1016/j.rser.2009.07.020
Ouyang Y, Zhao Y, Sun S, Hu C, Ping L (2015) Effect of light intensity on the capability of different microalgae species for simultaneous biogas upgrading and biogas slurry nutrient reduction. Int Biodeterior Biodegrad 104:157–163. https://doi.org/10.1016/j.ibiod.2015.05.027
Venkataraman LV (1997) Spirulina platensis (Arthrospira): physiology, cell biology and biotechnologym, edited by Avigad Vonshak. J Appl Phycol 9:295–296. https://doi.org/10.1023/A:1007911009912
Ding J, Zhao F, Cao Y, Xing L, Liu W, Mei S, Li S (2015) Cultivation of microalgae in dairy farm wastewater without sterilization. Int J Phytoremediation 17:222–227. https://doi.org/10.1080/15226514.2013.876970
K S, M P (2018) Nutrients uptake from anaerobically digested distillery wastewater by Spirulina sp. under xenon lamp illumination. J Water Process Eng 25:295–300. doi:https://doi.org/10.1016/j.jwpe.2018.08.014
Nayak M, Karemore A, Sen R (2016) Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res 16:216–223. https://doi.org/10.1016/j.algal.2016.03.020
Gupta PL, Lee SM, Choi HJ (2015) A mini review: photobioreactors for large scale algal cultivation. World J Microbiol Biotechnol 31:1409–1417. https://doi.org/10.1007/s11274-015-1892-4
Kümmerer K (2008) Pharmaceuticals in the environment: sources, fate, effects and risks, First ed Berlim. https://doi.org/10.1007/978-3-540-74664-5
Ogbonda KH, Aminigo RE, Abu GO (2007) Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bioresour Technol 98:2207–2211. https://doi.org/10.1016/j.biortech.2006.08.028
de Morais EG, Druzian JI, Nunes IL et al (2018) Glycerol increases growth, protein production and alters the fatty acids profile of Spirulina (Arthrospira) sp LEB 18. Process Biochem 76:40–45. https://doi.org/10.1016/j.procbio.2018.09.024
González-Fernández C, Ballesteros M (2012) Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol Adv 30:1655–1661. https://doi.org/10.1016/j.biotechadv.2012.07.003
dos Santos MGB, Duarte RL, Maciel AM, Abreu M, Reis A, de Mendonça HV (2020) Microalgae biomass production for biofuels in Brazilian scenario: a critical review. Bioenergy Res 14:23–42. https://doi.org/10.1007/s12155-020-10180-1
Molinuevo-Salces B, Mahdy A, Ballesteros M, González-Fernández C (2016) From piggery wastewater nutrients to biogas: microalgae biomass revalorization through anaerobic digestion. Renew Energy 96:1103–1110. https://doi.org/10.1016/j.renene.2016.01.090
Singh P, Guldhe A, Kumari S, Rawat I, Bux F (2016) Combined metals and EDTA control: an integrated and scalable lipid enhancement strategy to alleviate biomass constraints in microalgae under nitrogen limited conditions. Energy Convers Manag 114:100–109. https://doi.org/10.1016/j.enconman.2016.02.012
de Jesus CS, Uebel LS, Costa SS et al (2018) Outdoor pilot-scale cultivation of Spirulina sp. LEB-18 in different geographic locations for evaluating its growth and chemical composition. Bioresour Technol 256:86–94. https://doi.org/10.1016/j.biortech.2018.01.149
Kuo CM, Chen TY, Lin TH, Kao CY, Lai JT, Chang JS, Lin CS (2015) Cultivation of Chlorella sp. GD using piggery wastewater for biomass and lipid production. Bioresour Technol 194:326–333. https://doi.org/10.1016/j.biortech.2015.07.026
Kumar AK, Sharma S, Patel A, Dixit G, Shah E (2019) Comprehensive evaluation of microalgal based dairy effluent treatment process for clean water generation and other value added products. Int J Phytoremediation 21:519–530. https://doi.org/10.1080/15226514.2018.1537248
Lu W, Liu S, Lin Z, Lin M (2020) Enhanced microalgae growth for biodiesel production and nutrients removal in raw swine wastewater by carbon sources supplementation. Waste Biomass Valorization. https://doi.org/10.1007/s12649-020-01135-w
Torres-Tiji Y, Fields FJ, Mayfield SP (2020) Microalgae as a future food source. Biotechnol Adv 41:107536. https://doi.org/10.1016/j.biotechadv.2020.107536
Swanson D, Block R, Mousa SA (2012) Omega-3 fatty acids EPA and DHA: Health. J Adv Nutr 3:1–7. https://doi.org/10.3945/an.111.000893.Omega-3
Stamey JA, Shepherd DM, de Veth MJ, Corl BA (2012) Use of algae or algal oil rich in n-3 fatty acids as a feed supplement for dairy cattle. J Dairy Sci 95:5269–5275. https://doi.org/10.3168/jds.2012-5412
Tang DYY, Khoo KS, Chew KW, Tao Y, Ho SH, Show PL (2020) Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour Technol 304:122997. https://doi.org/10.1016/j.biortech.2020.122997
Acknowledgements
The authors thank the National Council for Scientific and Technological Development-CNPq for granting a scientific initiation scholarship (Code-PVT870-2019).
Funding
This study was funded by AGEVAP-Association for the Management of Waters in the Water Basin of Rio Paraíba do Sul–Brazil (Call 16/2019), project number 003.028.001.2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Highlights
• Cultivation of microalgae Spirulina platensis DHR 20 in anaerobic cattle wastewater was investigated in HPBR.
• High concentrations of dry biomass were obtained between 6.3 and 7.15 g L−1.
• Volumetric productivities in proteins, carbohydrates, and lipids of 0.299, 0.135, and 0.108 g L−1 day−1 were recorded.
• Organic pollutants, macro, and micronutrient have been efficiently removed.
• The macromolecules produced can be used for obtaining friendly bio-based products and bioenergy
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
de Souza, D.S., Valadão, R.C., de Souza, E.R.P. et al. Enhanced Arthrospira platensis Biomass Production Combined with Anaerobic Cattle Wastewater Bioremediation. Bioenerg. Res. 15, 412–425 (2022). https://doi.org/10.1007/s12155-021-10258-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10258-4