Abstract
In the present study, bulk contents of Ni, Zn, Cu, Pb and Mn in urban area of Tehran city are determined. Subsequently, the chemical bonds of metals with various soil fractions are brought out. Chemical partitioning studies revealed that various percentile of Ni, Zn, Cu, Pb and Mn is found in anthropogenic portion of soils. Zinc, Ni, Cu, Pb and Mn fall within “low pollution” class in accordance with index of pollution (I POLL). The trend of anthropogenic share of studied metals in soils of Tehran is Zn (55 %) > Cu (31 %) > Ni and Pb (30 %) > Mn (12 %). The overall potential of studied plants in metal removal from soil is Salvia > Viola > Portulaca. It should be pointed out that roots have higher potential in metal removal from soil when compared with leaf and stem. Lithogenic portion of metals remains intact before and after pot analysis. Thus, phytoremediation is highly dependent on the chemical bonds of metals. Present study showed that metal contents of loosely bonded ions, sulfide bonds and organometallic bonds are reduced after 90 days of plant cultivation. The overall removal trend of studied metals is Zn (16 %) > Cu (14 %) > Ni (11 %) > Pb (7 %) > Mn (6 %). The obtained results show that the anthropogenic portion of metals is reduced after the phytoremediation practice. For instance, the initial anthropogenic portion of Zn (55 %) is changed to 39 % showing an overall reduction of about 16 %. The anthropogenic portions of Cu, Ni, Pb and Mn are also reduced by 14, 11, 7 and 6 %, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals and organic materials are among essential components in soil. As a result of industrial as well as agricultural activities, the levels of metals and organic materials are increased leading to soil contamination (Subhashini et al. 2013). Subsequently, various soil cleaning techniques (biological, chemical and physical) have been examined by researchers (Sannino et al. 2013, 2016; Kristanti et al. 2011; Niazy et al. 2016). In the recent years, appropriate instrumental methods have been developed for predicting the exact toxicities of metal ion species (Ali and Aboul-Enein 2006). Many researchers opine that organic and inorganic pollutants can be treated by low cost and new generation adsorbents (Ali et al. 2012; Ali 2012, 2014; Daryabeigi Zand and Hoveidi 2016) both from wastewater as well as freshwater. Removal of metal ions from wastewater by inexpensive adsorbents can prevent soil contamination (Ali 2010). Effectiveness of electrocoagulation as well as electrodialysis of contaminated water with arsenic has received ample attention (Ali et al. 2011). It is shown arsenic removal from soils with higher moisture is much feasible (Sultana and Kobayashi 2016). In general, the inexpensive adsorbents are of immense interest to many researchers (Ali 2010) and therefore it is tried to bring out an appropriate protocol for experimental methodologies in this growing field (Ali and Gupta 2006). Immobilization, soil washing and phytoremediation techniques are known as the best available technologies (BATs) for remediation contaminated soils (Scanferla et al. 2012). Some metals possess inauspicious effects on the growth of plants that can be decreased (up to 46 %) by Pseudomonas (Kamran et al. 2016). Chen et al. (2015) found out that metal ATPase (HMA) could regulate concentrations of Cu, Ag, Zn, Cd, Co, Pb and Mn in various tissues of plants. It should be pointed out that the use of endosulfan is banned but it is still being used in several countries for cleaning up hazardous sites (Mitton et al. 2016).
Phytoremediation potential of alfalfa in co-contaminated soil was examined and to some extent increased by citric acid alfalfa (Agnello et al. 2016). Heavy metals and nutrients can be effectively removed by wetland plants possessing high aboveground biomass (Vymazal 2015, Javadi et al. 2010). It is found out that metal accumulation by various plants is not necessarily proportional to growth performance of a particular plant species phytoremediation (Wang et al. 2015). Some analysis also found out that the ability of different plant organs for absorbing metals is not the same (Li et al. 2015). Some researchers have proposed biomass pyrolysis for hyper-accumulation of metals (Dilks et al. 2016). Bettzickiana has been successfully used for phytoremediation of Cd and Pb to lower plant stress (Tauqeer et al. 2015). Some researchers have shown bio-concentration restriction in soil-root transfer at higher zinc concentrations in the soil (Liu et al. 2006).
Fortunately in the recent years, more attention has been paid to various environmental aspects of urban areas including soil pollution in urban areas of Iran (Mohammadpour Roudposhti et al. 2016a). For instance, a pollution index is developed for agricultural soils adjacent to large cities (Mohammadpour Roudposhti et al. 2016b). Such pollution studies have also been of interest by researchers in the vicinity of urban areas located near industrial zone (Ghaemi et al. 2015). An overview of the spatial distribution and chemical properties of heavy metals through dust fallout in urban area is investigated. It is found out that about 70 % of metals in the deposited particulate matter were from external origin during dust fall (Tabatabaei et al. 2014, 2015).
Tehran is the capital city of Iran with a day population of 14 million. The number of cars and motor vehicles reaches to around 7 million. The overall gasoline consumption by fleet is around 3 billion liters per year. Such huge amount of gasoline consumption leads to severe air pollution that finally finds their way onto the soil. The main objective of present study is to find out the association of metals with various soil phases. It is also aimed to know about the metal bonds and their uptake by plants.
Materials and methods
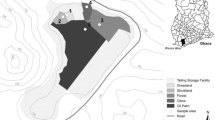
Soil samples from 0 to 55 cm depth were collected at 80 sites within Tehran metropolitan city (Fig. 1). An offset grid pattern is used in the present study (Mohammadpour Roudposhti et al. 2016b). About 80 soil samples (five samples from each grid) were collected. Subsequently, the samples of each grid were mixed together to furnish about 16 composite soil samples (Fig. 1) for further analysis. Soil samples were immediately packed in airtight pre-labeled polyethylene bags and preserved at 4 °C. The samples were oven-dried at 45 °C for about 48 h. Subsequently, they were passed through 63 µm mesh size (Vaezi et al. 2015; Vesali Naseh et al. 2012).
About 0.5 g of each soil sample was digested by 5 mL of HF, and subsequently they were treated with 7 mL of aqua regia (1:3 HCl:HNO3). The volume of solution was brought out to 50 mL by 1 N HCl (Karbassi et al. 2008). Chemical partitioning study was carried out in four sequential steps. In the first step, acetic acid (25 % v/v) was used to dissolved exchangeable/carbonate fraction. In steps 2 and 3, mixture of HOAc (25 % v/v) along with 0.1 M hydroxylamine hydrochloride and 30 % (w/v) H2O2 “extraction with 1 M ammonium acetate” was used to extract metals bonded with sulfides and organics, respectively. In step 4, hot 50 % HCl was used to break down the most resistant metal bonds (Mollazadeh et al. 2013).
In this study, 3 flowering plants (Viola sp., Portulaca and Salvia) were selected for testing their ability to absorb metals from the contaminated soil. These plant species are selected for the present study because of their common use within Tehran city. Once the chemical analysis of soil was carried, 48 pots of 4 kg capacity were used for pot analysis (16 pots for each plant species). Fresh plant samples were collected after 3 months of cultivation by carefully pulling from the soil to avoid damage to the roots. The plant samples were washed with distilled water to remove any foreign particles. Then roots, stems and leaves were separated. The separated parts were oven-dried at 100 °C for about 96 h. Dried samples of different parts of the plants were finely powdered using agate pestle and mortar. Plant samples (0.5 g) of each part (leaves, stems and roots) were digested by 5 mL of concentrated HNO3. After cooling, the solution was filtered with Whatman filter No. 42. It was then made up to 25 mL by distilled water. The metal contents (Ni, Zn, Cu, Pb and Mn) in soil samples as well as plant samples were determined by atomic absorption spectroscopy (Varian AA-30 model). Standard sediment sample (MESS-1) was used to check the accuracy (better than ±6 % for all elements) of the analysis (Biati et al. 2014).
Total organic contents of soil samples were measured by recording the loss on ignition (LOI) through heating the samples for 4 h at 450 °C in a muffle furnace. Phosphorus was measured by extracting solution (0.5 M NaHCO3, pH 8.5); total nitrogen by Kjeldahl digestion; pH was analyzed by glass electrode using a 1:1 soil/water ratio; and electrical conductivity (EC) was measured by conductivity meter in a soil–water extract (1:2 soil/water ratio). Titration method was used to measure soil lime, and Na and K were measured by flame photometer. Total carbon (C) was measured as per the method described by Supaphol et al. (2006). Soil particle size was determined using Bouyoucos hydrometer method. The WinTal software was used to bring out the texture of soil. All reagents used were of analytical grade (Merck). Cation-exchange capacity (CEC) was also measured (Mohammadpour Roudposhti et al. 2016b). The Ca and Mg concentrations were determined by complex-metric titration.
The transfer factor (TF) of elements from soil to plant was calculated by the following equation:
where C Plant is the concentration of elements in plants and C Soil is the concentration of elements in soil (Mahmood and Malik 2014).
To assess the intensity of elemental contamination in Tehran’s soils, the pollution index (I poll) was calculated by the following formulae (Karbassi et al. 2008):
where Cn is the total elemental content in soils and Bn is background level (lithogenic portion) of element obtained by chemical partitioning technique (Karbassi et al. 2008). It should be noted that the modified scale (Salehi et al. 2014) was used to bring out soil pollution intensity (Table 1).
Results and discussion
Generally, the soils in Iran are alkaline in nature. The organic content of soils is also very low when compared with many other countries. Table 2 depicts general characteristics of soil samples within Tehran. The higher Na contents of soil might be due to the use of salt during winter time for deicing of the roads. Organic matter has a very high CEC ranging from 250 to 400 meq/100 g (Moore et al. 1998). Generally, higher CEC is indicative of presence of higher amount of clay and organic in the soil. Figure 2 shows the texture of soil in Tehran. The soil classification falls within loam to silt loam. The lower organic matter and also rather low clay fraction of Tehran’s soil may justify the lower CEC values reported in the present investigation.
In spite of various pollution sources, the metal contents of Tehran’s soils show lower values when compared with the values given for mean crust (Table 3). This could be indicative of deviation among the geology of the area of study with the mean crust. Thus, chemical partitioning techniques should be used to bring out the share of lithogenous and non-lithogenous fractions of metals (Table 4).
If the lithogenic fraction (Table 4) is considered as the background values for the areas of study, then it would be obvious that mean metal contents (obtained in the present study) are much higher than the background values (except for Pb). The trend of anthropogenic share of studied metals in soils of Tehran is Zn (55 %) > Cu (31 %) > Ni and Pb (30 %) > Mn (12 %). The chemical partitioning of metals clearly shows the influence of man’s activities on contaminating soils in urban areas. Table 5 shows soil pollution intensity within Tehran city in accordance with I POLL formulae (Karbassi et al. 2008). According to the terminology adopted (Salehi et al. 2014), the contamination class for Ni, Zn, Pb, Cu and Mn falls within low contamination. The low to moderated contamination class has been previously reported for Tehran’s landfill known as Aradkuh (Salehi et al. 2014).
Subsequently, Viola sp., Portulaca and Salvia that are almost of 1-year life span were cultivated on the soils with the above-mentioned characteristics. The aim was to know about the chemical bonds of metals that are being removed by plants. As discussed earlier (Table 4), metals can be found in five different bonds and the bioavailability of each bond differs from each other. Viola sp. is a genus of flowering plants in the violet family Violaceae. It is the largest genus in the family, containing between 525 and 600 species. Most species are found in the temperate Northern Hemisphere; however, some are also found in widely divergent areas such as Hawaii, Australasia and the Andes. Some Viola species are perennial plants, some are annual plants, and a few are small shrubs. Portulaca is the type genus of the flowering plant family Portulacaceae, comprising about 40–100 species found in the tropics and warm temperate regions. They are also known as moss roses. Common Purslane (Portulaca oleracea) is widely considered an edible plant and in some areas an invasive type of weed. Some Portulaca species are used as food plants by the larvae of some Lepidoptera species including the Nutmeg (Hadula trifolii). Salvia is the largest genus of plants in the mint family, Lamiaceae, with nearly 1000 species of shrubs, herbaceous perennials and annuals. Within the Lamiaceae, Salvia is part of the tribe Mentheae within the subfamily Nepetoideae. It is one of several genera commonly referred to as sage. The genus is distributed within three distinct regions of diversity: Central and South America (approx. 500 species); Central Asia and Mediterranean (250 species) and Eastern Asia (90 species).
Table 5 presents the metal removal by Viola sp., Portulaca and Salvia. It is evident that Salvia has the highest potential removal of Ni, Zn and Mn from soil when compared with Viola and Portulaca species. The overall potential of studied plants in metal removal from soil is Salvia > Viola > Portulaca. It should be pointed out that roots have higher potential in metal removal from soil when compared with leaf and stem.
Once the pot analysis was completed (Table 6); the metal contents of soils remaining in pots (bulk as well as chemical partitioning) were measured. This was done to know the amount of depletion of total metal contents as well as individual fractions. A comparison between Tables 4 and 7 shows that lithogenic portion of metals remains intact before and after pot analysis. On the other hand, the metal contents of loosely bonded ions, sulfide bonds and organometallic bonds are reduced after cultivation of plants for a duration of 90 days. The obtained results show that the anthropogenic portion of metals is reduced for all studied metals {Zn (39 %) > Pb (23 %) > Ni (19 %) > Cu (17 %) > Mn (6 %)}. It is obvious that initial anthropogenic portion of Zn (55 %) is about 39 % after the phytoremediation practice. In other words, about 16 % of anthropogenic portion of Zn is removed by phytoremediation. The overall removal trend of studied metals is Zn (16 %) > Cu (14 %) > Ni (11 %) > Pb (7 %) > Mn (6 %). It is interesting to note that loosely bonded ions are the main contributors to the phytoremediation process (except for Cu). It should be noted that sulfide and organic bonds of Cu play a major role in phytoremediation processes. In general, the trend of various bonds in contribution of metals to the phytoremediation process can be summarized as loosely bonded ions > sulfide bonds > organometallic bonds.
Conclusion
Phytoremediation has received ample attention as an environmentally friendly technique for treating contaminated soils. This is especially true in the urban areas where people are more concerned with environmental issues. Many plants have been studied worldwide. The potential of many plants in removal of metals from contaminated soils has been extensively discussed. The importance of CEC, pH, soil grain size, organic matters and other factors is brought out. However, the previous studies do not discuss about role of various metal bond in phytoremediation processes. In the present study, chemical partitioning technique was used to know which metal bond is more affected by phytoremediation processes. The results revealed that lithogenic portion of metals (most resistant and within lattice bonds) does not contribute any metal to the plants. The results also showed that loosely bonded ions as well as sulfides bonds are the most contributor to the phytoremediation plants. Though organometallic bond of metals do contribute to the processes of phytoremediation but their share is negligible when compared with loosely bonded ions and sulfide bonds.
The results of present investigation showed that metal contents in Tehran’s soil in spite of various human activities are not higher than the mean crust. However, chemical partitioning studies revealed that various percentile of Ni, Zn, Cu, Pb and Mn are found in anthropogenic portion of soils. The trend of anthropogenic share of studied metals in soils of Tehran is Zn (55 %) > Cu (31 %) > Ni and Pb (30 %) > Mn (12 %) when compared to total metal contents of the soil. The overall potential of studied plants in metal removal from soil is Salvia > Viola > Portulaca. It should be pointed out that roots have higher potential in metal removal from soil when compared with leaf and stem. Since CEC of soil types differs from place to place and also considering the role of enzymes in the processes of CEC, we suggest further studies of present kind to better know about the role of metals associated with various soil phases in phytoremediation processes.
References
Agnello AC, Huguenot D, van Hullebusch ED, Esposito G (2016) Citric acid-and tween 80-assisted phytoremediation of a co-contaminated soil: alfalfa (Medicago sativa L.) performance and remediation potential. Environ Sci pollut Res Int. doi:10.1007/s11356-015-5972-7
Ali I (2010) The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater. Sep Purif Rev 39(3–4):95–171. doi:10.1080/15422119.2010.527802
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112(10):5073–5091. doi:10.1021/cr300133d
Ali I (2014) Water treatment by adsorption columns: evaluation at ground level. Sep Purif Rev 43(3):175–205. doi:10.1080/15422119.2012.748671
Ali I, Aboul-Enein HY (2006) Instrumental methods in metal ions speciation: chromatography, capillary electrophoresis and electrochemistry. Taylor and Francis Ltd, New York. ISBN 0-8493-3736-4
Ali I, Gupta VK (2006) Advances in water treatment by adsorption technology. Nat Lond 1:2661–2667. doi:10.1038/nprot.2006.370
Ali I, Khan TA, Asim M (2011) Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sep Purif Rev 40(1):25–42. doi:10.1080/15422119.2011.542738
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manage 113:170–183. doi:10.1016/j.jenvman.2012.08.028
Biati A, Karbassi AR, Keyhani Z (2014) Origination and assessment of metal pollution in Qarechay River bed sediments. Environ Monit Assess 186(7):4289–4297. doi:10.1007/s10661-007-0102-8
Chen F, Huber C, May R, Schroder P (2015) Metabolism of oxybenzone in a hairy root culture: perspective for phytoremediation of a widely used sunscreen agent. J Hazard Mater 306:230–236. doi:10.1016/j.jhazmat.2015.12.022
Daryabeigi Zand A, Hoveidi H (2016) Plant-aid remediation of hydrocarbon-contaminated sites. Pollution 2(3):233–246. doi:10.7508/pj.2016.03.001
Dilks RT, Monette F, Glaus M (2016) The major parameters on biomass pyrolysis for hyper accumulative plants—a review. Chemosphere 146:385–395. doi:10.1016/j.chemosphere.2015.12.062
Ghaemi Z, Karbassi AR, Moattar F, Hassani AH, Khorasani N (2015) Evaluating soil metallic pollution and consequent human health hazards in the vicinity of an industrialized zone, case study of Mubarakeh steel complex, Iran. J Environ Health Sci Eng 13(75):1–9. doi:10.1186/s40201-015-0231-x
Javadi E, Moattar F, Karbassi AR, Monavari SM (2010) Removal of lead, cadmium and manganese from liquid solution using water lily (Nymphaea alba). J Food Agri Environ 8(3–4):1220–1225. http://world-food.net/removal-of-lead-cadmium-and-manganese-from-liquid-solution-using-water-lily-nymphaea-alba/
Kamran MA, Eqani SA, Bibi S, Xu RK, Amna MMF, Katsoyiannis A, Bokhari H, Chaudhary HJ (2016) Bioaccumulation of nickel by E. sativa and role of plant growth promoting rhizobacteria (PGPRs) under nickel stress. Ecotox Environ Safe 126:256–263. doi:10.1016/j.ecoenv.2016.01.002
Karbassi AR, Monavari SM, Nabi Bidhendi GhR, Nouri J, Nematpour K (2008) Metal pollution assessment of sediment and water in the Shur river. Environ Monit Assess 147(1–3):107–116. doi:10.1007/s10661-007-0102-8
Kristanti RA, Hadibarata T, Tadashi T, Yasuhiro T, Kazuhiro M (2011) Bioremediation of crude oil by white rot fungi Polyporus sp. S133. J Microbiol Biotechnol 21(9):995–1000. doi:10.4014/jmb.1105.05047
Li J, Yu H, Luan Y (2015) Meta-analysis of the copper, zinc, and cadmium absorption capacities of aquatic plants in heavy metal-polluted water. Int J Environ Public health 12(12):12958–12973. doi:10.3390/ijerph121214959
Liu DH, Wang M, Zou JH, Jiang WS (2006) Uptake and accumulation of cadmium and some nutrient ions by roots and shoots of maize (Zea mays L.). Pak J Bot 38:701–709. http://www.pakbs.org/pjbot/PDFs/38(3)/PJB38(3)701.pdf
Mahmood A, Malik RN (2014) Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab J Chem 7:91–99. doi:10.1016/j.arabjc.2013.07.002
Mitton FM, Gonzalez M, Monswrrat JM, Miglioranza KS (2016) Potential use of edible crops in the phytoremediation of endosulfan residues in soil. Chemosphere 148:300–306. doi:10.1016/j.chemosphere.2016.01.028
Mohammadpour Roudposhti Gh, Karbassi AR, Baghvan A (2016a) Origin and distribution of metals in agricultural soils. Global J Environ Sci Manage 2(2):145–156. doi:10.7508/gjesm.2016.02.005
Mohammadpour Roudposhti Gh, Karbassi AR, Baghvan A (2016b) A pollution index for agricultural soils. Arch Agron Soil Sci 62(10):1411–1424. doi:10.1080/03650340.2016.1154542
Mollazadeh N, Moattar F, Karbassi AR, Khorasani N (2013) Distribution of metals, chemical partitioning, pollution and origins in riverbed sediment. World Appl Sci J 21(5):674–680. doi:10.5829/idosi.wasj.2013.21.5.722
Moore G, Dolling P, Porter B, Leonard L (1998) Soil acidity. In: Moore G (ed) Soil guide. A handbook for understanding and managing agricultural soils. Agriculture Western Australia Bulletin No. 4343. https://www.agric.wa.gov.au/sites/gateway/files/Soil%20Guide%20-%20a%20handbook.pdf
Niazy Z, Hassanshahian M, Ataei A (2016) Isolation and characterization of diesel-degrading pseudomonas strains from diesel-contaminated soils in Iran (Fars province). Pollution 1(1):67–75. doi:10.7508/pj.2016.01.007
Salehi F, Abdoli MA, Baghdadi M (2014) Sources of Cu, V, Cd, Cr, Mn, Zn, Co, Ni, Pb, Ca and Fe in soil of Aradkooh landfill. Int J Environ Res 8(3):543–550. https://ijer.ut.ac.ir/article_748_a58e7ee0ed46390bb637f85b9c77016b.pdf
Sannino F, Spaccini R, Savy D, Piccolo A (2013) Remediation of highly contaminated soils from an industrial site by employing a combined treatment with exogenous humic substances and oxidative biomimetic catalysis. J Hazard Mater 261:55–62. doi:10.1016/j.jhazmat.2013.06.077
Sannino F, Nuzzo A, Ventorino V, Pepe O, Piccolo A (2016) Effective degradation of organic pollutants in aqueous media by microbial strains isolated from soil of a contaminated industrial site. Chem Biol Technol Agric 3:2. doi:10.1186/s40538-016-0052-x
Scanferla P, Marcomini A, Pellay R, Girotto P, Zavan D, Fabris M, Collina A (2012) Remediation of a heavy metals contaminated site with a botanical garden: monitoring results of the application of an advanced S/S technique. Chem Eng Tech 28:235–240. doi:10.3303/CET1228040
Subhashini V, Swamy A, Krishna RH (2013) Pot experiment: to study the uptake of Zinc by different plant species in artificially contaminated soil. World J Environ Eng 1(2):27–33. doi:10.12691/wjee-1-2-3
Sultana R, Kobayashi K (2016) Adsorption of arsenic on soil under different soil moisture conditions. Pollution 2(2):211–220. doi:10.7508/pj.2016.02.009
Supaphol S, Panichsakpatana S, Trakulnaleamsai S, Tungkananuruk N, Roughjanajirapa P, O’Donnell AG (2006) The selection of mixed microbial inocula in environmental biotechnology: example using petroleum contaminated tropical soils. J Microbiol Method 65:432–441. doi:10.1016/j.mimet.2005.09.001
Tabatabaei T, Karbassi AR, Moatar F, Monavari SM (2014) Geospatial patterns and background levels of heavy metal in deposited particulate matter in Bushehr, Iran. Arab J Geosci 8(4):2081–2093. doi:10.1007/s12517-013-1241-6
Tabatabaei T, Karbassi AR, Moatar F, Monavari SM (2015) Multivariate geostatistical analysis in assessment of aerosols (case study: Bushehr). J Remote Sens GIS Nat Res 5(4):35–46. http://en.journals.sid.ir/ViewPaper.aspx?ID=461043
Tauqeer HM, Ali S, Rizwan M, Ali Q, Saeed R, Iftikhar U, Ahmad R, Farid M, Abbas GH (2015) Phytoremediation of heavy metals by Alternanthera bettzickiana: growth and physiological response. Ecotox Environ Safe 126:138–146. doi:10.1016/j.ecoenv.2015.12.031
Vaezi AR, Karbassi AR, Valavi Sh, Ganjali MR (2015) Ecological risk assessment of metals contamination in the sediment of the Bamdezh wetland, Iran. Int J Environ Sci Technol 12:951–958. doi:10.1007/s13762-014-0710-0
Vesali Naseh MR, Karbassi AR, Ghazaban F, Baghvand A (2012) Evaluation of heavy metal pollution in Anzali wetland, Guilan, Iran. Iran J Toxicol 5(15):565–576. http://ijt.arakmu.ac.ir/browse.php?a_code=A-10-2-82&slc_lang=fa&sid=1
Vymazal J (2015) Concentration is not enough to evaluate accumulation of heavy metals and nutrients in plants. Sci Total Environ 544:495–498. doi:10.1016/j.scitotenv.2015.12.011
Wang WW, Wu YJ, Akbar S, Jia XQ, He ZH, Tian XJ (2015) Effect of heavy metals combined stress on growth and metals accumulation of three Salix species with different cutting position. Int J Phytoremed 18(8):761–767. doi:10.1080/15226514.2015.1131237
Acknowledgments
Author wishes to thank authorities of Municipality of Tehran for the financial assistance (Grant No. 5664-111). We also thank the authorities of Department of Environment for laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour
Rights and permissions
About this article
Cite this article
Karbassi, S., Malek, M., Shahriari, T. et al. Uptake of metals by plants in urban areas. Int. J. Environ. Sci. Technol. 13, 2847–2854 (2016). https://doi.org/10.1007/s13762-016-1110-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1110-4